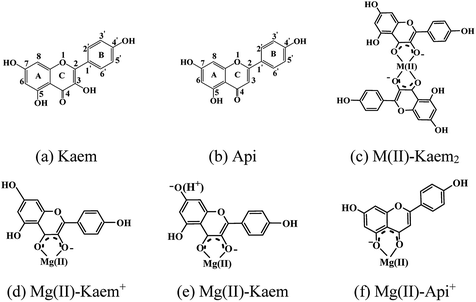
Open Access Article
Open Access ArticleAlkaline earth metal ion coordination increases the radical scavenging efficiency of kaempferol†
Ling-Ling Qian a,
Yao Lu
a,
Yao Lu a,
Yi Xua,
Zhi-Yin Yanga,
Jing Yanga,
Yi-Ming Zhou
a,
Yi Xua,
Zhi-Yin Yanga,
Jing Yanga,
Yi-Ming Zhou a,
Rui-Min Han
a,
Rui-Min Han *a,
Jian-Ping Zhang
*a,
Jian-Ping Zhang a and
Leif H. Skibsted
a and
Leif H. Skibsted b
b
aDepartment of Chemistry, Renmin University of China, Beijing, China 100872. E-mail: rmhan@ruc.edu.cn; Fax: +86-10-6251-6444; Tel: +86-10-6251-6604
bDepartment of Food Science, University of Copenhagen, Rolighedsvej 30, DK-1958 Frederiksberg C, Denmark
First published on 14th August 2020
Abstract
Flavonoids are used as natural additives and antioxidants in foods, and after coordination to metal ions, as drug candidates, depending on the flavonoid structure. The rate of radical scavenging of the ubiquitous plant flavonoid kaempferol (3,5,7,4′-tetrahydroxyflavone, Kaem) was found to be significantly enhanced by coordination of Mg(II), Ca(II), Sr(II), and Ba(II) ions, whereas the radical scavenging rate of apigenin (5,7,4′-trihydroxyflavone, Api) was almost unaffected by alkaline earth metal (AEM) ions, as studied for short-lived β-carotene radical cations (β-Car˙+) formed by laser flash photolysis in chloroform/ethanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and for the semi-stable 2,2-diphenyl-1-picrylhydrazyl radical, DPPH˙, in ethanol at 25 °C. A 1
3) and for the semi-stable 2,2-diphenyl-1-picrylhydrazyl radical, DPPH˙, in ethanol at 25 °C. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex was found to be in equilibrium with a 1
1 Mg(II)–Kaem complex was found to be in equilibrium with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 complex, while for Ca(II), Sr(II) and Ba(II), only 1
2 Mg(II)–Kaem2 complex, while for Ca(II), Sr(II) and Ba(II), only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM(II)–Kaem complexes were detected, where all complexes showed 3-hydroxyl and 4-carbonyl coordination and stability constants of higher than 109 L2 mol−2. The 1
2 AEM(II)–Kaem complexes were detected, where all complexes showed 3-hydroxyl and 4-carbonyl coordination and stability constants of higher than 109 L2 mol−2. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2 complex had the highest second order rate constant for both β-Car˙+ (5 × 108 L mol−1 s−1) and DPPH˙ radical (3 × 105 L mol−1 s−1) scavenging, which can be attributed to the optimal combination of the stronger electron withdrawing capability of the (n − 1)d orbital in the heavier AEM ions and their spatially asymmetrical structures in 1
2 Ca(II)–Kaem2 complex had the highest second order rate constant for both β-Car˙+ (5 × 108 L mol−1 s−1) and DPPH˙ radical (3 × 105 L mol−1 s−1) scavenging, which can be attributed to the optimal combination of the stronger electron withdrawing capability of the (n − 1)d orbital in the heavier AEM ions and their spatially asymmetrical structures in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM–Kaem complexes with metal ion coordination of the least steric hindrance of two perpendicular flavone backbones as ligands in the Ca(II) complex, as shown by density functional theory calculations.
2 AEM–Kaem complexes with metal ion coordination of the least steric hindrance of two perpendicular flavone backbones as ligands in the Ca(II) complex, as shown by density functional theory calculations.
Introduction
Flavonoids are among the most investigated phytochemicals due to their high abundance and structural diversity.1 These compounds are supplied to animals and humans supplied by fruit and vegetable intake, and are associated with well-documented benefits including, anticancer, anti-inflammation, antiatherosclerotic and antioxidant properties.2–4 The complexation of flavonoids with metal ions has been reported to significantly enhance the antioxidant activities of most of these complexes,5–17 while for some other combinations of metal ions and flavonoids, their antioxidant activities are unchanged or even decrease.18–22 The binding of metal ions with flavonoids and the type of metal–flavonoid complexes formed depend on solvent, pH, concentration and the nature of both the metal ions and flavonoids, which complicates the understanding of the molecular mechanism behind the effect that the metal ions have on the activities of the flavonoids.5,6,18,19A remarkable increase in the radical scavenging efficiency of flavonoids by the coordination of the transition metal ions Zn(II) and Cu(II) has been demonstrated and quantitatively assigned to specific species in equilibrium in solution in our recent studies.5,6 For both Zn(II) and Cu(II), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal–flavonoid complexes were found to have a higher radical scavenging ability than 1
1 metal–flavonoid complexes were found to have a higher radical scavenging ability than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes as a result of electron withdrawal from the phenolic group of the flavonoid by metal ions.5,6 For 1
2 complexes as a result of electron withdrawal from the phenolic group of the flavonoid by metal ions.5,6 For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal complexes with two flavonoid ligands in a symmetrical structure, the electron withdrawal by transition metal ions from the ligands is balanced, and the radical scavenging activity decreases compared to that of the asymmetric 1
2 metal complexes with two flavonoid ligands in a symmetrical structure, the electron withdrawal by transition metal ions from the ligands is balanced, and the radical scavenging activity decreases compared to that of the asymmetric 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes.5,18 In contrast, the effect that the binding of the ions of main group elements to flavonoids has on the radical scavenging activity of flavonoids and on the structure–activity is less clear.23
1 complexes.5,18 In contrast, the effect that the binding of the ions of main group elements to flavonoids has on the radical scavenging activity of flavonoids and on the structure–activity is less clear.23
In the present study, we extend the investigation of the binding of metal ions to flavonoids and systematically explore the interactions of alkaline earth metal (AEM) ions with flavonoids and the effect on the radical scavenging efficiency of binding of AEMs to flavonoids. Ca, Sr and Ba have recently been found to mimic transition metals, showing unusual structures and reactivities.24–26 Mg and Ca are ubiquitous and essential to living organisms, playing vital biological roles.27,28 Sr also plays an important role in aquatic life and has found some use in medicine for improving bone strength,29 whereas Ba is toxic to living organisms. Both Sr and Ba are included in the present study in order to systematically elucidate the effect that AEM ions have on the radical scavenging activities of flavonoids. Kaem (3,5,7,4′-tetrahydroxyflavone) and Api (5,7,4′-trihydroxyflavone apigenin, with a 3-hydroxyl group less than kaempferol, Scheme 1), were compared for their chelation of AEM ions and for the effect of AEM ion binding on their radical scavenging efficiencies. The finding of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2 complex as the most efficient radical scavenger should be of more general interest for the use of flavonoids as additives and antioxidants in food systems with high calcium content, such as dairy products.
2 Ca(II)–Kaem2 complex as the most efficient radical scavenger should be of more general interest for the use of flavonoids as additives and antioxidants in food systems with high calcium content, such as dairy products.
Experimental
Chemicals
Kaempferol (Kaem, >98%) and apigenin (Api, >98%) from Huike Plant Exploitation Inc (Shaanxi, China), magnesium acetate Mg(CH3COO)2 (>98%) and calcium acetate Ca(CH3COO)2·H2O (>99%) from Titan Scientific (Shanghai, China), strontium acetate Sr(CH3COO)2·1/2H2O (>99%) and barium acetate Ba(CH3COO)2 (>99%) from Shandong Xiya Chemical Co. Ltd., and 1,1-diphenyl-2-picrylhydrazyl radical (DPPH˙) from ZhongShengRuiTai Technology Inc (>97%, Beijing, China) were used as received. All-trans-β-carotene (β-Car, >93%), ferrocene (>98%) and NaClO4 (>98%) from Sigma-Aldrich (St. Louis, MO, USA) and spectrophotometric grade ethanol (99.9%, Fine Chemical Industry Research Institute, Tianjin, China) were used as received. Chloroform (>99.0%, Beijing Chemical Works, Beijing, China) was purified before use by passing it through an alumina column (AR, Tianjin Fuchen Chemical Plant, Tianjin, China).Reaction of alkaline earth metals ions with Kaem and Api
All UV-vis absorption spectra were measured on a Cary60 spectrophotometer (Varian, Inc., Palo Alto, CA, USA) using 1.0 cm quartz cells in a thermostated room at 25 °C. The absorption spectra were obtained by two methods: (i) adding increasing amounts of AEM(II) ions to 20 μM Kaem or Api, (ii) mixing solutions of Kaem or Api and AEM(II) ions with invariable total molar concentrations but varying molar ratios from 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 for Mg(II) or Ca(II), and from 9
19 for Mg(II) or Ca(II), and from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 for Sr(II) or Ba(II) ions.
9 for Sr(II) or Ba(II) ions.
Infrared (IR) spectra and mass spectra (MS)
IR spectra were obtained on a Bruker Tensor 27 FT-IR spectrometer (Karlsruhe, Germany). Solid samples of AEM(II)–kaempferol/apigenin complexes for IR tableting were prepared from a solution of Kaem/Api and AEM(II) salts in ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 by evaporation of ethanol under a nitrogen gas flow.
10 by evaporation of ethanol under a nitrogen gas flow.
MS were obtained on a Thermo Scientific™ Q Exactive™ HF (Waltham, MA, USA) spectrometer operated in positive ion mode. Samples of AEM(II)–kaempferol complexes were prepared by filtering solutions of Kaem and AEM(II) salts after mixing them through a nylon membrane with 220 nm sieve pores. The samples were analyzed by direct infusion electrospray ionization (ESI) by means of a syringe pump (Thermo UltiMate 3000, Waltham, MA, USA) at a flow rate of 5 μL min−1 at a capillary temperature of 320 °C and spray voltage of 3.50 kV.
Determination of oxidation potentials
Cyclic voltammetry (CV) was performed on a three-electrode CHI 760D electrochemical analyzer (ChenHua Instruments Inc., Shanghai, China) both in ethanol and in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform = 7
chloroform = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The working electrode was a glassy carbon piece (diameter = 4 mm), the reference electrode was a silver wire, and the auxiliary electrode was a platinum wire. 0.10 M NaClO4 was used as a supporting electrolyte. 50 μM ferrocene was used as an internal standard, and CVs were obtained in the potential range of −0.5 to 0.9 V at a scan rate of 0.05 V s−1.
3. The working electrode was a glassy carbon piece (diameter = 4 mm), the reference electrode was a silver wire, and the auxiliary electrode was a platinum wire. 0.10 M NaClO4 was used as a supporting electrolyte. 50 μM ferrocene was used as an internal standard, and CVs were obtained in the potential range of −0.5 to 0.9 V at a scan rate of 0.05 V s−1.
β-Carotene radical cation and DPPH˙ scavenging
The laser flash photolysis apparatus for the study of the kinetics of β-Car˙+ scavenging has been previously described in detail.30,31 The laser pulses at 532 nm (4 mJ per pulse, 7 ns, and 10 Hz) for the bleaching assay were supplied by a Nd3+:YAG laser (Quanta-Ray PRO-230, Spectra Physics Lasers, Inc., Mountain View, CA, USA). 940 and 510 nm probe lights were provided by a laser-driven white light source (LDLS-EQ-99 LAMP MODULE, Energetiq Technology, Inc., Woburn, MA). The absorbance was detected using a photodiode (model S3071, Hamamatsu Photonics, Hamamatsu, Japan). The optical path length of the cuvette was 1 cm and all measurements were carried out at 25 °C. Ethanol/chloroform (7/3, v/v) was used as the solvent for the equilibrated solution of β-Car and AEM(II) salts and Kaem.The kinetics of DPPH˙ scavenging by AEM(II)–kaempferol complexes were investigated using a stopped-flow technique with the same method as previously performed on an RX2000 Rapid-Mixing Stopped-Flow Unit (Applied Photophysics Ltd, Surrey, United Kingdom).5,6 For one syringe solution, DPPH˙ was dissolved in ethanol to obtain an absorbance of 0.92 ± 0.02 (extinction coefficient ε = 9660 L mol−1 cm−1) at 516 nm,32 and the final concentration of the DPPH˙ was calculated to be 100 μM. The other syringe solution was an equilibrated sample to be measured.
Quantum chemical calculations
Structural optimization of Kaem or Api and the AEM(II) were performed using the Gaussian 09 package and self-consistent reaction field (SCRF) model with the Becke3 and Lee Yang Parr (B3LYP) hybrid functional method along with Def2-SVP basis set.33The proton dissociation enthalpy (PDE), ionization potential (IP) and bond dissociation enthalpy (BDE) of both Kaem and Api and their AEM(II) complexes were calculated as the gas phase enthalpy difference:
| Ar-OH → Ar-O˙ + H˙ |
| Ar-OH → Ar-O− + H+ |
| Ar-OH → Ar-OH˙+ + e |
| Ar-O− →Ar-O˙ + e |
Dihedral angles (α) between the two planes of the two Kaem ligands in complexes were calculated according to eqn (1):
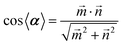 | (1) |
![[m with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006d_20d1.gif) and
and ![[n with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006e_20d1.gif) represent two normal vectors of two Kaem planes, respectively.
represent two normal vectors of two Kaem planes, respectively. ![[m with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006d_20d1.gif) (A1, B1, C1) and
(A1, B1, C1) and ![[n with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006e_20d1.gif) (A2, B2, C2) were obtained from the following two equations of two planes including metal ions and the two oxygen atoms within the same plane,
(A2, B2, C2) were obtained from the following two equations of two planes including metal ions and the two oxygen atoms within the same plane,| A1x + B1y + C1z + D1 = 0 | (2) |
| A2x + B2y + C2z + D2 = 0 | (3) |
![[m with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006d_20d1.gif) and
and ![[n with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006e_20d1.gif) , and x, y, z represent the coordinates of optimized metal ions and the four oxygen atoms in two Kaem planes coordinated to metal ions, and D1 and D2, are constants, respectively.
, and x, y, z represent the coordinates of optimized metal ions and the four oxygen atoms in two Kaem planes coordinated to metal ions, and D1 and D2, are constants, respectively.
Results
Reactions of AEM(II) ions with Kaem or Api and the formation of complexes
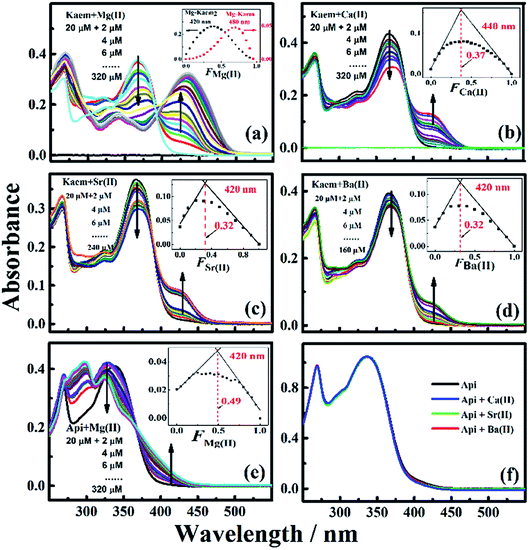
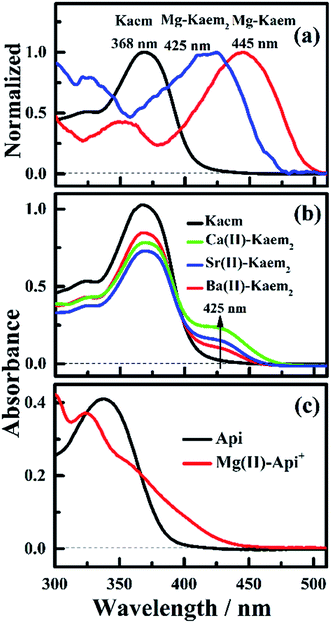
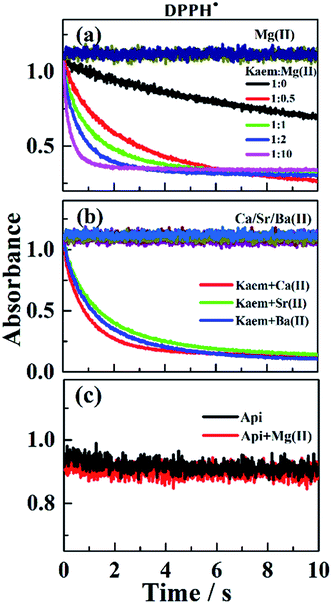
The UV-vis absorption spectra of 20 μM of Kaem with a maximum absorption at 368 nm in ethanol clearly shifted to the long wavelength region by addition of Mg/Ca/Sr/Ba(II) ions, as seen in Fig. 1a–d. Addition of 2 to 20 μM Mg(II) ions to Kaem showed a similar spectral shift to around 425 nm upon the addition of the heavier AEM(II) ions Ca/Sr/Ba(II). Further addition of up to 30 μM of Mg(II) ions to Kaem resulted in a further red shift and the formation of a new species with a maximum peak at 445 nm, while no further red shift occurred upon the addition of even excessive concentrations of Ca/Sr/Ba(II) ions to Kaem solution. Both Mg(II)–kaempferol complexes were found to have very similar characteristics in terms of their absorption spectra and Jobs plot (Fig. 1a, inset), as also observed for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn(II)–Kaem and 1
1 Zn(II)–Kaem and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn(II)–Kaem2 binding at the 3-hydroxyl and 4-carbonyl groups in our recent study.5
2 Zn(II)–Kaem2 binding at the 3-hydroxyl and 4-carbonyl groups in our recent study.5
The same method of deconvolution and calculations of the equilibrium distribution between the two species as used for the Zn(II)–Kaem complexes in ref. 5, gave 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cationic Mg(II)–(Kaem–H)+ and 1
1 cationic Mg(II)–(Kaem–H)+ and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
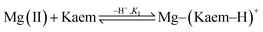
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 neutral Mg(II)–(Kaem–H)2 complexes (Scheme 1c and d) with maximum absorption peaks at 445 and 425 nm (Fig. 2a), formed from the binding of Mg(II) to Kaem at the 3-hydroxyl and 4-carbonyl groups, resulting in the deprotonation of the 3-hydroxyl group, as shown in eqn (4) and (5). The 1
2 neutral Mg(II)–(Kaem–H)2 complexes (Scheme 1c and d) with maximum absorption peaks at 445 and 425 nm (Fig. 2a), formed from the binding of Mg(II) to Kaem at the 3-hydroxyl and 4-carbonyl groups, resulting in the deprotonation of the 3-hydroxyl group, as shown in eqn (4) and (5). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–kaempferol complex was found to transform into a 1
1 Mg(II)–kaempferol complex was found to transform into a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–kaempferol complex upon the addition of acetic acid (Fig. S1†), which indicates that the most stable 1
2 Mg(II)–kaempferol complex upon the addition of acetic acid (Fig. S1†), which indicates that the most stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–kaempferol complex is neutral, Mg(II)–(Kaem–2H) (Scheme 1e), formed from the spontaneous deprotonation of cationic Mg(II)–(Kaem–H)+ due to the increased acidity of phenol upon Mg(II) coordination. This result is also supported by the calculated proton deprotonation enthalpy (PDE) values and will be further explained in the Structural and thermodynamic analyses section. The formation of the 1
1 Mg(II)–kaempferol complex is neutral, Mg(II)–(Kaem–2H) (Scheme 1e), formed from the spontaneous deprotonation of cationic Mg(II)–(Kaem–H)+ due to the increased acidity of phenol upon Mg(II) coordination. This result is also supported by the calculated proton deprotonation enthalpy (PDE) values and will be further explained in the Structural and thermodynamic analyses section. The formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes and the transformation of the two complexes are shown in eqn (4)–(6):
2 complexes and the transformation of the two complexes are shown in eqn (4)–(6):
 | (4) |
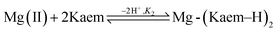 | (5) |
| 2Mg(II)–(Kaem–2H) + 2H+ ⇌ Mg(II)–(Kaem–H)2 + Mg(II) | (6) |
For convenience, the formulae Mg(II)–Kaem+ and Mg(II)–Kaem are used to represent the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cationic Mg(II)–(Kaem–H)+ and the neutral Mg(II)–(Kaem–2H) complexes, respectively. The 1
1 cationic Mg(II)–(Kaem–H)+ and the neutral Mg(II)–(Kaem–2H) complexes, respectively. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem complex is neutral, formed by the loss of a proton from each of the 3-hydroxyl groups of the two Kaem ligands, and is written as Mg(II)–Kaem2. The stability constants were calculated to be 8.8 × 104 L mol−1 for Mg(II)–Kaem and 1.9 × 1010 L2 mol−2 for Mg(II)–Kaem2 in ethanol. A similar binding pattern was also observed in ethanol
2 Mg(II)–Kaem complex is neutral, formed by the loss of a proton from each of the 3-hydroxyl groups of the two Kaem ligands, and is written as Mg(II)–Kaem2. The stability constants were calculated to be 8.8 × 104 L mol−1 for Mg(II)–Kaem and 1.9 × 1010 L2 mol−2 for Mg(II)–Kaem2 in ethanol. A similar binding pattern was also observed in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform (7
chloroform (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), and stability constants were 1.9 × 104 L mol−1 for Mg(II)–Kaem and 3.2 × 1010 L2 mol−2 for Mg(II)–Kaem2.
3, v/v), and stability constants were 1.9 × 104 L mol−1 for Mg(II)–Kaem and 3.2 × 1010 L2 mol−2 for Mg(II)–Kaem2.
Absorption spectra (Fig. 1b–d) and the derived Job plots (Fig. 1b–d insets) resulting from addition of the heavier AEM(II) ions Ca/Sr/Ba(II) to Kaem indicate only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 M(II)–Kaem2 (M = Ca, Sr, Ba) complexes formed, with a shoulder peak at 425 nm, as seen in eqn (7) and Fig. 2b. The obtained stability constants for Ca/Sr/Ba(II)–Kaem2, 4.9 × 109/2.4 × 1010/1.2 × 1010 L2 mol−2 in ethanol, and 6.4 × 109/6.9 × 109/5.6 × 109 L2 mol−2 in ethanol
2 M(II)–Kaem2 (M = Ca, Sr, Ba) complexes formed, with a shoulder peak at 425 nm, as seen in eqn (7) and Fig. 2b. The obtained stability constants for Ca/Sr/Ba(II)–Kaem2, 4.9 × 109/2.4 × 1010/1.2 × 1010 L2 mol−2 in ethanol, and 6.4 × 109/6.9 × 109/5.6 × 109 L2 mol−2 in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform (7
chloroform (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), respectively, are listed in Table 1 together with the values for the 1
3, v/v), respectively, are listed in Table 1 together with the values for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–kaempferol complexes.
2 Mg(II)–kaempferol complexes.
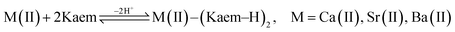 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform (eth
chloroform (eth![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chl, 7/3), and absolute second order rate constants kCar˙+(L mol−1 s−1) for the scavenging of β-Car˙+ and kDPPH˙(L mol−1 s−1) for the scavenging of DPPH˙ by metal–flavonoid complexes. The parameters of Zn(II)–kaempferol complexes are shown for comparison
chl, 7/3), and absolute second order rate constants kCar˙+(L mol−1 s−1) for the scavenging of β-Car˙+ and kDPPH˙(L mol−1 s−1) for the scavenging of DPPH˙ by metal–flavonoid complexes. The parameters of Zn(II)–kaempferol complexes are shown for comparison
| Flav | Metal ions | ra (pm) | Polarizationb | M(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Flav Flav |
Binding site | K1/Keth2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L mol−1)/1 1 (L mol−1)/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (L2 mol−2) 2 (L2 mol−2) |
K1/Keth:chl2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L mol−1)/1 1 (L mol−1)/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (L2 mol−2) 2 (L2 mol−2) |
Eeth (V) | Eeth:chl (V) | kCar˙+ (L mol−1 s−1) | kDPPH˙ (L mol−1 s−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
a From ref. 35.b From ref. 36.c Formation constants, oxidation potentials (solvent is methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform = 7 chloroform = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and rate constants are from ref. 5. 3) and rate constants are from ref. 5. |
|||||||||||
| Kaem | Zn(II)c | 74 | 5.41 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3,4 | 4.8 × 105 | 1.0 × 105 | −0.097 | (1.88 ± 0.05) × 108 | (2.5 ± 0.03) × 104 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3,4 | 1.2 × 1011 | 2.0 × 1010 | 0.170 | (1.3 ± 0.07) × 104 | ||||||
| Ca(II) | 99 | 4.04 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3,4 | 4.9 × 109 | 6.4 × 109 | −0.093 | −0.081 | (5.44 ± 0.02) × 108 | (2.9 ± 0.02) × 105 | |
| Sr(II) | 113 | 3.54 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3,4 | 2.4 × 1010 | 6.9 × 109 | −0.096 | −0.084 | (3.38 ± 0.02) × 108 | (8.1 ± 0.01) × 104 | |
| Ba(II) | 135 | 2.96 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3,4 | 1.2 × 1010 | 5.6 × 109 | −0.122 | −0.098 | (3.17 ± 0.02) × 108 | (1.3 ± 0.03) × 105 | |
| Api | Mg(II) | 65 | 6.15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4,5 | 2.4 × 105 | 0.409 | ||||
In contrast, only Mg(II) among the AEM(II) ions binds to apigenin (Api) (Fig. 1e and 2c). A shift to a lower wavenumber for the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond in the IR spectra (Table S1†) and the Job plot (Fig. 1e inset) characteristics indicate that the Mg(II) ions bind Api at the 4-carbonyl and 5-hydroxyl groups in a 1
O double bond in the IR spectra (Table S1†) and the Job plot (Fig. 1e inset) characteristics indicate that the Mg(II) ions bind Api at the 4-carbonyl and 5-hydroxyl groups in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio, forming a cationic Mg(II)–Api+ complex, with the structure shown in Scheme 1f. Nevertheless, no reaction occurred between the Ca/Sr/Ba(II) ions and Api, as evidenced by lack of spectral changes both in the UV-vis and IR absorption spectra following the addition of Ca/Sr/Ba(II) ions to Api solution (Fig. 1f and Table S1†). These observations support Ca/Sr/Ba(II) ion bonding with Kaem at the 3-hydroxyl and 4-carbonyl groups of the C-ring, as in the Mg(II)–Kaem complexes. The structures of the AEM(II)–kaempferol and Mg(II)–apigenin complexes are shown in Scheme 1c–f. Mass spectrometry (Tables S2†) further confirmed the composition obtained from the UV-visible spectra of the four 1
1 stoichiometric ratio, forming a cationic Mg(II)–Api+ complex, with the structure shown in Scheme 1f. Nevertheless, no reaction occurred between the Ca/Sr/Ba(II) ions and Api, as evidenced by lack of spectral changes both in the UV-vis and IR absorption spectra following the addition of Ca/Sr/Ba(II) ions to Api solution (Fig. 1f and Table S1†). These observations support Ca/Sr/Ba(II) ion bonding with Kaem at the 3-hydroxyl and 4-carbonyl groups of the C-ring, as in the Mg(II)–Kaem complexes. The structures of the AEM(II)–kaempferol and Mg(II)–apigenin complexes are shown in Scheme 1c–f. Mass spectrometry (Tables S2†) further confirmed the composition obtained from the UV-visible spectra of the four 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM(II)–Kaem2 complexes and the 1
2 AEM(II)–Kaem2 complexes and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem and Mg(II)–Api+ complex.
1 Mg(II)–Kaem and Mg(II)–Api+ complex.
Determinations of oxidation potentials
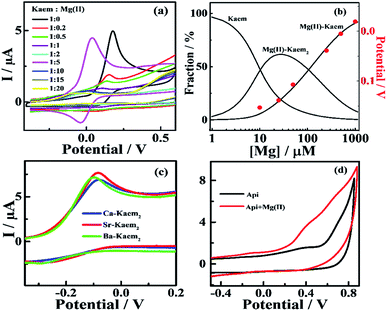
A significant decrease in the oxidation potential of 50 μM of Kaem from 0.166 to −0.044 V versus ferrocene from the CV scan was observed following the addition of Mg(II) to Kaem with a Kaem![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :Mg
:Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (II) ratio ranging from 1
(II) ratio ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 to 1
0.2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 in ethanol/chloroform (7/3, v/v), as shown in Fig. 3a. Oxidation potentials obtained from the initial oxidation peak corresponded to the oxidation of phenolic groups.37,38 The potential for the 1
20 in ethanol/chloroform (7/3, v/v), as shown in Fig. 3a. Oxidation potentials obtained from the initial oxidation peak corresponded to the oxidation of phenolic groups.37,38 The potential for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex was estimated to be at −0.044 V, as obtained from a sample of Kaem and Mg(II) in ratio of 1
1 Mg(II)–Kaem complex was estimated to be at −0.044 V, as obtained from a sample of Kaem and Mg(II) in ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 with 62% of the 1
20 with 62% of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex dominating in equilibrium with 3% Kaem and 35% Mg(II)–Kaem2, as shown in Table S3.† The decrease in oxidation potential for the solutions with increasing addition of Mg(II) was found to have a similar tendency as the increase in the fraction of 1
1 Mg(II)–Kaem complex dominating in equilibrium with 3% Kaem and 35% Mg(II)–Kaem2, as shown in Table S3.† The decrease in oxidation potential for the solutions with increasing addition of Mg(II) was found to have a similar tendency as the increase in the fraction of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem, as shown in Fig. 3b, excluding the contribution of 1
1 Mg(II)–Kaem, as shown in Fig. 3b, excluding the contribution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 to the decrease in the oxidation potential. The oxidation potential of the 1
2 Mg(II)–Kaem2 to the decrease in the oxidation potential. The oxidation potential of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 complex was estimated as 0.153 V for the combination of Kaem
2 Mg(II)–Kaem2 complex was estimated as 0.153 V for the combination of Kaem![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg(II) = 1
Mg(II) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 with 34% of Mg(II)–Kaem2 present in equilibrium with 64% Kaem and 2% 1
0.2 with 34% of Mg(II)–Kaem2 present in equilibrium with 64% Kaem and 2% 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem, which is close to values for the parent Kaem and 1
1 Mg(II)–Kaem, which is close to values for the parent Kaem and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn(II)–Kaem2.
2 Zn(II)–Kaem2.
In addition, significant decreases in oxidation potentials were observed for combinations of Ca/Sr/Ba(II) ions and Kaem in ethanol/chloroform (7/3, v/v). The oxidation potentials were found to be at approximately −0.081 V for Ca(II)–Kaem2, −0.084 V for Sr(II)–Kaem2 and −0.098 V for Ba(II)–Kaem2 as obtained from Kaem and Ca/Sr/Ba(II) ions in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with >94% of 1
10 with >94% of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 as the predominate components, see Fig. 3c and Table 1. The oxidation peak of 50 μM of Api is weak and has the approximate value of 0.409 V. No change in the oxidation potentials was observed for Api upon the addition of excess Mg(II), as shown in Fig. 3d.
2 Ca/Sr/Ba(II)–Kaem2 as the predominate components, see Fig. 3c and Table 1. The oxidation peak of 50 μM of Api is weak and has the approximate value of 0.409 V. No change in the oxidation potentials was observed for Api upon the addition of excess Mg(II), as shown in Fig. 3d.
Similar changes were also observed for AEM(II)–Kaem complexes in ethanol and the corresponding oxidation potentials were determined and are listed in Table 1.
Radical scavenging kinetics
Antioxidant activities of Kaem/Api, and of the complexes of Mg/Ca/Sr/Ba(II) with Kaem and of Mg(II) with Api were evaluated using the two radical scavenging methods as previously reported in the literature:5 (i) scavenging of the photo-induced carotenoid radical cations β-Car˙+ in ethanol/chloroform (7/3, v/v), which is now a well-established method for determining the radical scavenging of polyphenols and of Zn(II)–Kaem complexes via electron transfer (ET); and (ii) the scavenging of the semi-stable radical DPPH˙ in ethanol, which has now has been accepted to occur via hydrogen atom transfer (HAT) from polyphenolic antioxidants.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg(II) at varying ratios from 1
Mg(II) at varying ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 to 1
0.2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were used to investigate the β-Car˙+ scavenging reactivity of the 1
5 were used to investigate the β-Car˙+ scavenging reactivity of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem and 1
1 Mg(II)–Kaem and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 complexes. Solutions of Kaem with an excess of AEM(II) ions were used for investigating the combinations of Kaem and Ca/Sr/Ba(II), with the 1
2 Mg(II)–Kaem2 complexes. Solutions of Kaem with an excess of AEM(II) ions were used for investigating the combinations of Kaem and Ca/Sr/Ba(II), with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 complexes dominating. Notably, the decay of β-Car˙+ by Kaem became notably faster upon the addition of AEM(II) ions compared to previously reported5 results in which the same amount of Zn(II) was added, while the absorbance amplitudes for the bleaching recovery of the β-Car ground state were smaller. These results together imply that other reactions than ET to β-Car˙+ occur for the AEM(II)–Kaem complexes as the reaction products do not completely return to the ground state of β-Car. Stronger absorbances were observed at 940 nm for the solutions of Kaem with Ca/Sr/Ba(II) ions than for the solutions of only Kaem or of each of the AEM(II) ions, especially for the combinations of Kaem and Sr/Ba(II) (Fig. 4c). The fast decay of β-Car˙+ without full recovery of the β-Car ground state molecule was also observed for tea polyphenols and m-hydroxybenzoic acid in our previous studies and has been ascribed to the formation of radical adducts.40,41 The scavenging of β-Car˙+ in the present study seems accordingly to include both β-Car˙+ reduction to β-Car upon accepting an electron from AEM(II)–Kaem complexes, and addition of AEM(II)–Kaem complexes to β-Car˙+, as shown in eqn (8) and (9):
2 Ca/Sr/Ba(II)–Kaem2 complexes dominating. Notably, the decay of β-Car˙+ by Kaem became notably faster upon the addition of AEM(II) ions compared to previously reported5 results in which the same amount of Zn(II) was added, while the absorbance amplitudes for the bleaching recovery of the β-Car ground state were smaller. These results together imply that other reactions than ET to β-Car˙+ occur for the AEM(II)–Kaem complexes as the reaction products do not completely return to the ground state of β-Car. Stronger absorbances were observed at 940 nm for the solutions of Kaem with Ca/Sr/Ba(II) ions than for the solutions of only Kaem or of each of the AEM(II) ions, especially for the combinations of Kaem and Sr/Ba(II) (Fig. 4c). The fast decay of β-Car˙+ without full recovery of the β-Car ground state molecule was also observed for tea polyphenols and m-hydroxybenzoic acid in our previous studies and has been ascribed to the formation of radical adducts.40,41 The scavenging of β-Car˙+ in the present study seems accordingly to include both β-Car˙+ reduction to β-Car upon accepting an electron from AEM(II)–Kaem complexes, and addition of AEM(II)–Kaem complexes to β-Car˙+, as shown in eqn (8) and (9):
 | (8) |
 | (9) |
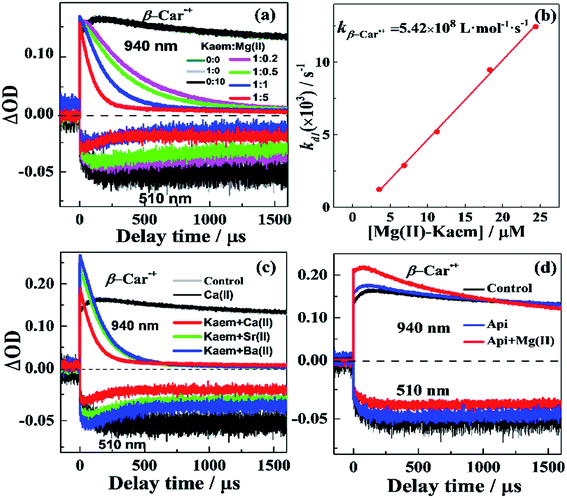 | ||
Fig. 4 Decay of β-Car˙+ as monitored at 940 nm and the bleaching recovery of the β-Car ground state at 510 nm (a) in the absence and presence of 50 μM of Kaem and of 50 μM of Kaem with 10, 25, 50, and 250 μM of Mg(CH3COO)2, (c) in the presence of 50 μM of Kaem with 500 μM of Ca(CH3COO)2, Sr(CH3COO)2, Ba(CH3COO)2, and (d) in the absence and presence of 50 μM of Api and of 50 μM of Api with 750 μM of Mg(CH3COO)2. Kinetics with only β-Car and with only β-Car and AEM acetate salts were also investigated and are shown for comparison. (b) Observed rate constants obtained from exponential fitting of the fast decay of β-Car˙+ (kd1, Table 2) at 940 nm in (a) against the concentration of Mg(CH3COO)2. The second-order rate constant for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem scavenging of β-Car˙+ was calculated to have the value kβ-Car˙+ = (5.42 ± 0.04) × 108 L mol−1 s−1 by linear regression. The solvent used was ethanol/chloroform (7/3) and measurements were made at 25 °C. 1 Mg(II)–Kaem scavenging of β-Car˙+ was calculated to have the value kβ-Car˙+ = (5.42 ± 0.04) × 108 L mol−1 s−1 by linear regression. The solvent used was ethanol/chloroform (7/3) and measurements were made at 25 °C. | ||
The radical adduct observed at 940 nm was a reaction intermediate, which further dissociated into a final product, as evidenced by the absence of absorption in the near-infrared region (870 to 950 nm) after 532 nm laser radiation for 5 min of the solutions of β-Car and the AEM(II)–Kaem complexes (Fig. S2†). The kinetics observed for the absorption at 940 nm for all samples could be accommodated by a tri-exponential function Aie−t/τi + Ad1(1 − e−t/τd1) + Ad2(1 − e−t/τd2), including a fast increase (τi, ki), a relatively fast and dominating decay (τd1, kd1), and a slower minor decay (τd2, kd2), as shown in Table 2. For the combination of Kaem and Mg(II), kd1 accounting for >90% amplitude of the decay was found to be linearly dependent on the concentration of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex present, as calculated from the formation constants (Fig. 4b). The 1
1 Mg(II)–Kaem complex present, as calculated from the formation constants (Fig. 4b). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex was accordingly confirmed to be the only β-Car˙+ radical scavenger and the 1
1 Mg(II)–Kaem complex was accordingly confirmed to be the only β-Car˙+ radical scavenger and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem complex was non-reactive in β-Car˙+ scavenging. The second order rate constant was found to have the value kCar˙+ = (5.42 ± 0.04) × 108 L mol−1 s−1, as obtained by the linear fitting of the observed pseudo first-order rate constant kd1 against the concentration of the 1
2 Mg(II)–Kaem complex was non-reactive in β-Car˙+ scavenging. The second order rate constant was found to have the value kCar˙+ = (5.42 ± 0.04) × 108 L mol−1 s−1, as obtained by the linear fitting of the observed pseudo first-order rate constant kd1 against the concentration of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem (Fig. 4b and Table 1). For combinations of Kaem with Ca(II), Sr(II) or Ba(II), for which the results are shown in Fig. 4c, the second order rate constants kCar˙+ were obtained from the observed pseudo first-order rate constant kd1 by division with the concentration of the 1
1 Mg(II)–Kaem (Fig. 4b and Table 1). For combinations of Kaem with Ca(II), Sr(II) or Ba(II), for which the results are shown in Fig. 4c, the second order rate constants kCar˙+ were obtained from the observed pseudo first-order rate constant kd1 by division with the concentration of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 complex as the only complex formed and accordingly as the only β-Car˙+ radical scavenger present. The second order rate constant for the decay of β-Car˙+ by the AEM complexes was the largest for 1
2 Ca/Sr/Ba(II)–Kaem2 complex as the only complex formed and accordingly as the only β-Car˙+ radical scavenger present. The second order rate constant for the decay of β-Car˙+ by the AEM complexes was the largest for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2, with the value (5.44 ± 0.02) × 108 L mol−1 s−1, as compared with the 1
2 Ca(II)–Kaem2, with the value (5.44 ± 0.02) × 108 L mol−1 s−1, as compared with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex with the value (5.24 ± 0.04) × 108 and with (3.38 ± 0.02) × 108 L mol−1 s−1 for Sr(II)–Kaem2 and (3.17 ± 0.02) × 108 L mol−1 s−1 for Ba(II)–Kaem2, where a quantitative comparison is shown in Fig. 5a.
1 Mg(II)–Kaem complex with the value (5.24 ± 0.04) × 108 and with (3.38 ± 0.02) × 108 L mol−1 s−1 for Sr(II)–Kaem2 and (3.17 ± 0.02) × 108 L mol−1 s−1 for Ba(II)–Kaem2, where a quantitative comparison is shown in Fig. 5a.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v)
3, v/v)
| λ/nm | Fitting parameters | Mg(II) + Kaem | Ca(II)–Kaem2 | Sr(II)–Kaem2 | Ba(II)–Kaem2 | |||
|---|---|---|---|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
||
| 940 nm | τi/μs | 40.0 | 41.1 | 37.4 | 25.4 | 26.9 | 35.0 | 38.2 |
| ki(×104)/s−1 | 2.5 | 2.4 | 2.7 | 3.9 | 0.037 | 0.029 | 0.026 | |
| Ai | −0.032 | −0.031 | −0.032 | −0.026 | −0.026 | −0.050 | −0.054 | |
| τd1/μs | 402.3 | 320.3 | 175.3 | 86.8 | 96.7 | 150.3 | 149.9 | |
| kd1(×103)/s−1 | 2.5 | 3.1 | 5.7 | 12 | 1000 | 6.7 | 6.7 | |
| Ad1 | 0.17 | 0.17 | 0.18 | 0.17 | 0.20 | 0.27 | 0.30 | |
| τd2/μs | 4285 | 4613 | 4263 | 3286 | 2705 | 1801 | 1303 | |
| kd2(×102)/s−1 | 2.3 | 2.2 | 2.3 | 3.0 | 3.7 | 5.6 | 7.7 | |
| Ad2 | 0.017 | 0.014 | 0.015 | 0.014 | 0.012 | 0.011 | 0.012 | |
| 510 nm | τf/μs | 16.0 | 17.6 | 8.8 | 34.7 | 4.4 | 79.1 | 73.0 |
| kf(×104)/s−1 | 6.3 | 5.7 | 11 | 2.9 | 23 | 1.3 | 1.4 | |
| Af | −0.030 | −0.027 | −0.023 | −0.019 | −0.024 | −0.031 | −0.040 | |
| τr/μs | 1868 | 860 | 338 | 76 | 135.6 | 170.4 | 153.4 | |
| kr(×103)/s−1 | 0.54 | 1.2 | 3.0 | 13 | 7.4 | 509 | 6.5 | |
| Ar | −0.013 | −0.015 | −0.014 | −0.025 | −0.014 | −0.040 | −0.049 | |
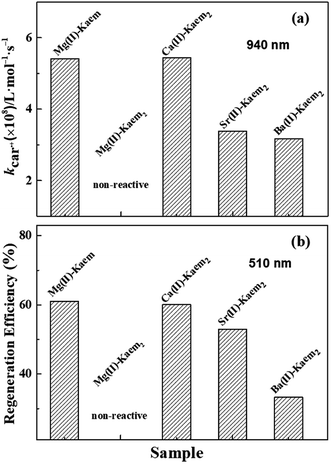 | ||
Fig. 5 (a) Second-order rate constants of β-Car˙+ scavenging by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem and by 1 1 Mg(II)–Kaem and by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 complexes obtained from the linear fitting of pseudo first-order rate constants at 940 nm, as shown in Fig. 4a and c. (b) Regeneration efficiency of β-Car˙+ to yield β-Car by the 1 2 Ca/Sr/Ba(II)–Kaem2 complexes obtained from the linear fitting of pseudo first-order rate constants at 940 nm, as shown in Fig. 4a and c. (b) Regeneration efficiency of β-Car˙+ to yield β-Car by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem and 1 1 Mg(II)–Kaem and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 complexes, as obtained from amplitude ratios of the bleaching kinetics at 510 nm in the presence and absence of the complexes in Fig. 4a and c. 2 Ca/Sr/Ba(II)–Kaem2 complexes, as obtained from amplitude ratios of the bleaching kinetics at 510 nm in the presence and absence of the complexes in Fig. 4a and c. | ||
The time constants and rate constants of the formation and recovery of bleaching at 510 nm for all AEM(II)–Kaem complexes could be accommodated by a di-exponential function Afe−t/τf + Ar(1 − e−t/τr), including a fast formation (τf, kf) without recovery and a subsequently slow recovery (τr, kr), as shown in Table 2. The recovery at 510 nm for all complexes was found to be slower than the decay at 940 nm, which implies that the decay of β-Car˙+ at 940 nm only partially regenerates to the β-Car ground state, further supporting the presence of an additional reaction that occurs for the decay of β-Car˙+ formed by laser flash photolysis. The fraction of β-Car˙+ regenerated by electron transfer was quantified using the ratios of the absorbance of β-Car bleaching at 510 nm at a longer delay time between the samples in the presence and absence of the complexes, as shown in Fig. 5b. The results indicate that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem and 1
1 Mg(II)–Kaem and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2 complexes are the most efficient radical scavengers that regenerate β-Car from β-Car˙+ compared to 1
2 Ca(II)–Kaem2 complexes are the most efficient radical scavengers that regenerate β-Car from β-Car˙+ compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Sr(II)–Kaem2 and Ba(II)–Kaem2 (Fig. 5a and b). β-Car regeneration efficiency was 61% for 1
2 Sr(II)–Kaem2 and Ba(II)–Kaem2 (Fig. 5a and b). β-Car regeneration efficiency was 61% for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem, 60% for 1
1 Mg(II)–Kaem, 60% for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2, 53% for Sr(II)–Kaem2 and 33% for Ba(II)–Kaem2, all of which are lower than the 87% for 1
2 Ca(II)–Kaem2, 53% for Sr(II)–Kaem2 and 33% for Ba(II)–Kaem2, all of which are lower than the 87% for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn(II)–Kaem.
1 Zn(II)–Kaem.
In contrast, Api alone had no effect on the decay of β-Car˙+and the combination of Mg(II) and Api only slightly accelerated the decay of β-Car˙+, as evidenced by the higher absorbance at 940 nm in the presence of complexes than for only β-Car, with no recovery of the bleaching at 510 nm, as shown in Fig. 4d.
For the combination of Kaem and Mg(II) in DPPH˙ scavenging, the reaction rate depends on three parallel reactions of Kaem in equilibrium with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem complexes. Individual second order rate constants, kKaem2 = (1.59 ± 0.01) × 103 L mol−1 s−1, kMg(II)Kaem2 = (3.5 ± 0.03) × 104 L mol−1 s−1 and
2 Mg(II)–Kaem complexes. Individual second order rate constants, kKaem2 = (1.59 ± 0.01) × 103 L mol−1 s−1, kMg(II)Kaem2 = (3.5 ± 0.03) × 104 L mol−1 s−1 and 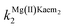 = (3.4 ± 0.03) × 103 L mol−1 s−1 were calculated from the distributions of Kaem, 1
= (3.4 ± 0.03) × 103 L mol−1 s−1 were calculated from the distributions of Kaem, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem and 1
1 Mg(II)–Kaem and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 using the same method as for Zn(II)–kaempferol complexes to analyze the observed rate constant kobs upon varying the Mg(II)
2 Mg(II)–Kaem2 using the same method as for Zn(II)–kaempferol complexes to analyze the observed rate constant kobs upon varying the Mg(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kaem ratios (Table S3†). The asymmetric 1
Kaem ratios (Table S3†). The asymmetric 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II) Kaem was found to be 20 times and 10 times as efficient as the parent Kaem and symmetric 1
1 Mg(II) Kaem was found to be 20 times and 10 times as efficient as the parent Kaem and symmetric 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 complex in DPPH˙ scavenging, respectively.
2 Mg(II)–Kaem2 complex in DPPH˙ scavenging, respectively.
For combinations of Kaem and the heavier AEM ions Ca/Sr/Ba(II), the reaction rates of DPPH˙ scavenging depend on two parallel reactions of Kaem in equilibrium with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 complexes. Individual second order rate constants were obtained as (2.9 ± 0.02) × 105, (8.1 ± 0.01) × 104, (1.3 ± 0.03) × 105 L mol−1 s−1 for the 1
2 Ca/Sr/Ba(II)–Kaem2 complexes. Individual second order rate constants were obtained as (2.9 ± 0.02) × 105, (8.1 ± 0.01) × 104, (1.3 ± 0.03) × 105 L mol−1 s−1 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2, Sr(II)–Kaem2 and Ba(II)–Kaem2 complexes, respectively, based on the distributions of the 1
2 Ca(II)–Kaem2, Sr(II)–Kaem2 and Ba(II)–Kaem2 complexes, respectively, based on the distributions of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 complexes in equilibrium with Kaem, which indicates that the 1
2 Ca/Sr/Ba(II)–Kaem2 complexes in equilibrium with Kaem, which indicates that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal–Kaem complexes of the heavier Ca/Sr/Ba(II) ions are far more efficient radical scavengers than the complexes of the lighter Mg(II) ion and the transition metal ion Zn(II) in reacting with DPPH˙ (Table 1). Ca(II)–Kaem2 has the highest scavenging rate constant, ∼3.6 times that of Sr(II)–Kaem2, 2.2 times that of Ba(II)–Kaem2, 8 times that of 1
2 metal–Kaem complexes of the heavier Ca/Sr/Ba(II) ions are far more efficient radical scavengers than the complexes of the lighter Mg(II) ion and the transition metal ion Zn(II) in reacting with DPPH˙ (Table 1). Ca(II)–Kaem2 has the highest scavenging rate constant, ∼3.6 times that of Sr(II)–Kaem2, 2.2 times that of Ba(II)–Kaem2, 8 times that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem, 85 times that of 1
1 Mg(II)–Kaem, 85 times that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2, and 182 times that of the parent Kaem. In contrast, no effect on the kinetics of DPPH˙ decay was observed upon the addition of Api or solutions of Api together with Mg(II), as can be seen in Fig. 6c.
2 Mg(II)–Kaem2, and 182 times that of the parent Kaem. In contrast, no effect on the kinetics of DPPH˙ decay was observed upon the addition of Api or solutions of Api together with Mg(II), as can be seen in Fig. 6c.
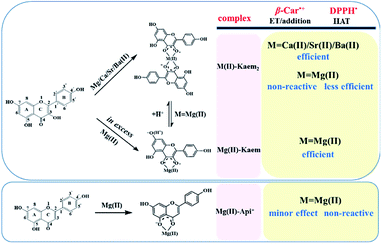
A proposed mechanism for Kaem and Api scavenging of DPPH˙ in the presence and absence of AEM(II) ions and the radical scavenging activities of the AEM(II)–Kaem and Mg(II)–Api complexes are outlined in Scheme 2.
Structural and thermodynamic analyses
Molecular structures of the AEM(II)–Kaem and Mg(II)–Api complexes were optimized using density functional theory (DFT) methods, and the structural parameters are listed in Table 3. The results indicate that the bond lengths of the metal ions and oxygen atoms in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 di-coordinated AEM(II)–Kaem2 complexes increase along with an increase in the radii of the AEM(II) ions (Table 3). Mono-coordinated Mg(II) with Kaem or Api as a ligand is planar (optimized structures not shown), while the two Kaem ligands in the 1
2 di-coordinated AEM(II)–Kaem2 complexes increase along with an increase in the radii of the AEM(II) ions (Table 3). Mono-coordinated Mg(II) with Kaem or Api as a ligand is planar (optimized structures not shown), while the two Kaem ligands in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 di-coordinated AEM(II)–Kaem2 complexes are arranged in staggered spatial structures different from the planar structure of the 1
2 di-coordinated AEM(II)–Kaem2 complexes are arranged in staggered spatial structures different from the planar structure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn(II)–Kaem2 complex, the structures of which are shown in Fig. S3.† The two Kaem planes in the 1
2 Zn(II)–Kaem2 complex, the structures of which are shown in Fig. S3.† The two Kaem planes in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2 complex have the highest dihedral angle of 89° (almost vertical) among all the AEM–kaempferol complexes, followed by 84° for 1
2 Ca(II)–Kaem2 complex have the highest dihedral angle of 89° (almost vertical) among all the AEM–kaempferol complexes, followed by 84° for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2, 77° for Sr(II)–Kaem2 and 75° for Ba(II)–Kaem2, as can be seen in Fig. S3† and Table 3.
2 Mg(II)–Kaem2, 77° for Sr(II)–Kaem2 and 75° for Ba(II)–Kaem2, as can be seen in Fig. S3† and Table 3.
| Compound | Group | Kaem | Mg–Kaem+ | Mg–Kaema | Mg–Kaem2 | Ca–Kaem2 | Sr–Kaem2 | Ba–Kaem2 | Api | Mg–Api+ |
|---|---|---|---|---|---|---|---|---|---|---|
| a The IP and BDE values were calculated for neutral Mg–Kaem with the 7-phenol deprotonated as the most acidic phenol in the cationic Mg–Kaem+ complex. | ||||||||||
| LO–M (Å) | 3-O | 1.95 | 1.98/1.98 | 2.32/2.32 | 2.52/2.52 | 2.79/2.79 | ||||
| 4-O | 2.04/2.04 | 2.42/2.42 | 2.60/2.60 | 2.89/2.90 | ||||||
| α (°) | 84 | 89 | 77 | 75 | ||||||
| PDE (kcal mol−1) | 5-OH | 301.46 | 296.30 | 300.40 | 303.52 | 303.62 | 305.07 | 305.44 | ||
| 7-OH | 295.27 | 292.26 | 294.81 | 296.51 | 296.93 | 297.59 | 296.09 | 294.37 | ||
| 3-OH | 297.81 | |||||||||
| 4′-OH | 297.06 | 294.89 | 297.72 | 299.57 | 299.87 | 300.62 | 295.35 | 295.71 | ||
| IP (kcal mol−1) | 126.55 | 178.66 | 107.56 | 114.71 | 114.51 | 111.55 | 107.01 | 133.86 | 185.82 | |
| BDE (kcal mol−1) | 5-OH | 89.99 | 84.93 | 84.72 | 84.91 | 83.36 | 80.94 | 80.53 | 94.33 | — |
| 7-OH | 87.11 | 82.91 | 81.10 | 77.94 | 76.36 | 74.47 | 88.96 | 90.93 | ||
| 3-OH | 76.69 | |||||||||
| 4′-OH | 81.54 | 79.72 | 74.82 | 77.90 | 74.84 | 73.46 | 71.66 | 84.79 | 86.10 | |
Thermodynamic parameters, including proton dissociation enthalpies (PDE), ionization potentials (IP), and bond dissociation energies (BDE) of the AEM(II)–Kaem and Mg(II)–Api complexes, as well as those for Kaem and Api for comparison were calculated by DFT and are listed in Table 3. The PDE values for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex were found to be 3 to 5 kcal mol−1 lower than for the parent Kaem, which further supports that the 1
1 Mg(II)–Kaem complex were found to be 3 to 5 kcal mol−1 lower than for the parent Kaem, which further supports that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cationic Mg(II)–Kaem+ easily loses a proton to form a more stable di-deprotonated neutral Mg(II)–Kaem complex, as observed for 1
1 cationic Mg(II)–Kaem+ easily loses a proton to form a more stable di-deprotonated neutral Mg(II)–Kaem complex, as observed for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn(II)–Kaem. In addition, the 1
1 Zn(II)–Kaem. In addition, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem complex also shows a lower IP and lower BDE and is more reducing and is a better radical scavenger than the parent Kaem.
1 Mg(II)–Kaem complex also shows a lower IP and lower BDE and is more reducing and is a better radical scavenger than the parent Kaem.
The PDE values in Table 3 of all four 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM(II)–kaempferol complexes are almost unaffected compared to those of the parent Kaem or Api, while both the IP and BDE values decreased remarkably, especially for the 4′-phenol group in the B-ring, generally accepted as the most active group in contributing towards the antioxidant properties of the complexes. The BDE and IP values for 1
2 AEM(II)–kaempferol complexes are almost unaffected compared to those of the parent Kaem or Api, while both the IP and BDE values decreased remarkably, especially for the 4′-phenol group in the B-ring, generally accepted as the most active group in contributing towards the antioxidant properties of the complexes. The BDE and IP values for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM(II)–Kaem2 decreased in the order of: Mg(II) > Ca(II) > Sr(II) > Ba(II). In contrast, all the thermodynamic parameters, including the PDE, BDE and IP values of Api were not affected by the coordination of Mg(II).
2 AEM(II)–Kaem2 decreased in the order of: Mg(II) > Ca(II) > Sr(II) > Ba(II). In contrast, all the thermodynamic parameters, including the PDE, BDE and IP values of Api were not affected by the coordination of Mg(II).
Discussion
AEM ion complexes of Kaem and Api
The AEM ions differ dramatically in terms of their binding of Kaem and Api, despite their minor structural differences. Mg(II) binds to Kaem at the 3,4-site to form equilibrium mixtures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem complexes and the parent Kaem, while Mg(II) binds with Api at the 4,5-site only forming a 1
2 Mg(II)–Kaem complexes and the parent Kaem, while Mg(II) binds with Api at the 4,5-site only forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Api complex. Different from Mg(II), the heavier AEM(II) ions Ca/Sr/Ba(II) only form 1
1 Mg(II)–Api complex. Different from Mg(II), the heavier AEM(II) ions Ca/Sr/Ba(II) only form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM–Kaem2 complexes at the 3,4-site, and no binding occurs between Ca/Sr/Ba(II) and Api. All four AEM(II) ions bind at the 3,4 site for Kaem, while Mg(II) only binds at the 4,5 site for Api. The absence of binding of Ca/Sr/Ba(II) with Api is in agreement with the fact that the 3-hydroxyl and 4-carbonyl groups are more preferential metal binding groups compared to the 4-carbonyl and 5-hydroxyl groups in flavonoids.42,43 A smaller radius, higher polarization and accordingly stronger attraction to Kaem and Api for Mg(II) compared to Ca/Sr/Ba(II), but similar to Zn(II) as seen in Table 1, may explain the same coordination modes and similar spectra of the Mg(II)– and Zn(II)–Kaem complexes. Only in the presence of higher concentrations of Mg(II) or Zn(II), the 1
2 AEM–Kaem2 complexes at the 3,4-site, and no binding occurs between Ca/Sr/Ba(II) and Api. All four AEM(II) ions bind at the 3,4 site for Kaem, while Mg(II) only binds at the 4,5 site for Api. The absence of binding of Ca/Sr/Ba(II) with Api is in agreement with the fact that the 3-hydroxyl and 4-carbonyl groups are more preferential metal binding groups compared to the 4-carbonyl and 5-hydroxyl groups in flavonoids.42,43 A smaller radius, higher polarization and accordingly stronger attraction to Kaem and Api for Mg(II) compared to Ca/Sr/Ba(II), but similar to Zn(II) as seen in Table 1, may explain the same coordination modes and similar spectra of the Mg(II)– and Zn(II)–Kaem complexes. Only in the presence of higher concentrations of Mg(II) or Zn(II), the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 and Zn(II)–Kaem2 complexes transform into the 1
2 Mg(II)–Kaem2 and Zn(II)–Kaem2 complexes transform into the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem and Zn(II)–Kaem complexes via metal–oxygen cleavage. Due to the lower polarization of the heavier AEM(II) ions Ca/Sr/Ba(II) compared to Mg(II), no 1
1 Mg(II)–Kaem and Zn(II)–Kaem complexes via metal–oxygen cleavage. Due to the lower polarization of the heavier AEM(II) ions Ca/Sr/Ba(II) compared to Mg(II), no 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ca/Sr/Ba(II)–Kaem complexes were formed, even at high Ca/Sr/Ba(II) concentrations in Kaem solution, in agreement with that Ca/Sr/Ba(II) do not bind to Api in contrast to Mg(II) and Zn(II), which both bind with Api at the 4,5-site.
1 Ca/Sr/Ba(II)–Kaem complexes were formed, even at high Ca/Sr/Ba(II) concentrations in Kaem solution, in agreement with that Ca/Sr/Ba(II) do not bind to Api in contrast to Mg(II) and Zn(II), which both bind with Api at the 4,5-site.
The absorption spectra of the four 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM(II)–Kaem2 complexes, obtained by deconvolution, all have new absorption bands at around 425 nm. The most intense absorption of the new spectral line is observed for Mg(II), followed by Ca(II), Sr(II), and Ba(II), corresponding to a decrease in polarization along with an increase in the metal ions radius. Among the AEM(II) ions, Mg(II) has the smallest radius, and accordingly the strongest polarization of the AEM(II)–Kaem2 complexes, affecting the 3-hydroxyl and 4-carbonyl groups, resulting in a significant increase in the intensity of the new peak at 425 to 445 nm (Fig. 2a and b).
2 AEM(II)–Kaem2 complexes, obtained by deconvolution, all have new absorption bands at around 425 nm. The most intense absorption of the new spectral line is observed for Mg(II), followed by Ca(II), Sr(II), and Ba(II), corresponding to a decrease in polarization along with an increase in the metal ions radius. Among the AEM(II) ions, Mg(II) has the smallest radius, and accordingly the strongest polarization of the AEM(II)–Kaem2 complexes, affecting the 3-hydroxyl and 4-carbonyl groups, resulting in a significant increase in the intensity of the new peak at 425 to 445 nm (Fig. 2a and b).
Radical scavenging of Kaem coordinated to AEM ions
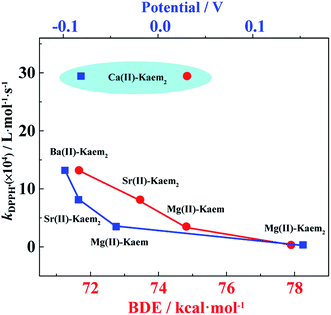
Both the β-Car˙+ and DPPH˙ radical scavenging efficiencies of Kaem were significantly increased by the presence of any one of the four acetates of AEM(II), as evidenced by the scavenging rate both for the ultra-fast reactions determined using sub-microsecond laser flash photolysis, and for the slower scavenging rates determined by stopped-flow techniques. Compared with the Zn(II)–Kaem complex, a faster formation of new species at the initial reaction time as seen in the near-infrared spectral region (940 nm) and a faster decay of β-Car˙+ with less recovery of β-Car following bleaching (510 nm) for solutions of both AEM(II) and Kaem confirmed a parallel addition reaction in the ET reaction of the AEM(II)–Kaem complexes with β-Car˙+. Based on the present results alone, it is, however, not possible to distinguish whether ET, hydrogen atom transfer (HAT) or an additional mechanism are the most important in the scavenging of DPPH˙ by the AEM(II)–Kaem complexes. This was possible for β-Car˙+ scavenging but not for the scavenging of DPPH˙ by the AEM(II)–Kaem complexes due to the spectral overlaps with the final products. Absolute second reaction rate constants of DPPH˙ radical scavenging indicate, with the notable exception of Ca(II)–Kaem2, that Ba(II)–Kaem2 is the most efficient radical scavenger, followed by Sr(II)–Kaem2, Mg(II)–Kaem, Mg(II)–Kaem2, which strictly agrees with the hierarchy of their oxidation potentials, the BDE and the IP values in the order of: Ba(II)–Kaem2 < Sr(II)–Kaem2 < Mg(II)–Kaem < Mg(II)–Kaem2 (Fig. 7). These data are consistent with the widely accepted HAT mechanism for DPPH˙ scavenging by antioxidants.371![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 is non-reactive in β-Car˙+ scavenging, as is the parent Kaem and the 1
2 Mg(II)–Kaem2 is non-reactive in β-Car˙+ scavenging, as is the parent Kaem and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn(II)–Kaem2 complex, and is also the poorest DPPH˙ radical scavenger among the AEM(II)–Kaem complexes studied. In contrast, its DPPH˙ scavenging is fast compared to the parent Kaem, while the 1
2 Zn(II)–Kaem2 complex, and is also the poorest DPPH˙ radical scavenger among the AEM(II)–Kaem complexes studied. In contrast, its DPPH˙ scavenging is fast compared to the parent Kaem, while the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca/Sr/Ba(II)–Kaem2 complexes have increased scavenging rates for both radicals studied. These findings are consistent with the most positive oxidation potential determined electrochemically, and the calculated largest IP and BDE values for 1
2 Ca/Sr/Ba(II)–Kaem2 complexes have increased scavenging rates for both radicals studied. These findings are consistent with the most positive oxidation potential determined electrochemically, and the calculated largest IP and BDE values for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2 (Fig. 7 and Table 3). The remarkable increase in radical scavenging efficiencies seen for the Ca/Sr/Ba(II)–Kaem2 complexes may be understood by the increasing electron withdrawing capability of the (n − 1)d orbital in the heavier AEM Ca(II), Sr(II) and Ba(II) ions, which accordingly makes them behave almost like transition metal ions, reducing the BDE and IP values of the ligand Kaem.22 Such effects are not noted for Kaem coordinated to the lighter Mg(II) ion in 1
2 Mg(II)–Kaem2 (Fig. 7 and Table 3). The remarkable increase in radical scavenging efficiencies seen for the Ca/Sr/Ba(II)–Kaem2 complexes may be understood by the increasing electron withdrawing capability of the (n − 1)d orbital in the heavier AEM Ca(II), Sr(II) and Ba(II) ions, which accordingly makes them behave almost like transition metal ions, reducing the BDE and IP values of the ligand Kaem.22 Such effects are not noted for Kaem coordinated to the lighter Mg(II) ion in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Mg(II)–Kaem2. For both transition metal ions, Zn(II) and Cu(II), the 1
2 Mg(II)–Kaem2. For both transition metal ions, Zn(II) and Cu(II), the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal–flavonoid complex was previously found to have a higher radical scavenging ability as the result of asymmetric electron withdrawal from the phenolic group by the metal ions. In contrast, the 1
1 metal–flavonoid complex was previously found to have a higher radical scavenging ability as the result of asymmetric electron withdrawal from the phenolic group by the metal ions. In contrast, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes, in which bi-coordinated flavonoid ligands symmetrically balance the electron withdrawal effect of the metal ion, were noted to show decreased radical scavenging ability.5,6
2 complexes, in which bi-coordinated flavonoid ligands symmetrically balance the electron withdrawal effect of the metal ion, were noted to show decreased radical scavenging ability.5,6
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2 complex has a higher β-Car˙+ and DPPH˙ radical scavenging capacity than both the Sr(II)–Kaem2 and Ba(II)–Kaem2 complexes, although the thermodynamic parameters of 1
2 Ca(II)–Kaem2 complex has a higher β-Car˙+ and DPPH˙ radical scavenging capacity than both the Sr(II)–Kaem2 and Ba(II)–Kaem2 complexes, although the thermodynamic parameters of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2, including experimentally determined oxidation potentials and calculated BDE and IP values, are less favorable than Sr(II)–Kaem2 and Ba(II)–Kaem2 (Fig. 7 and Table 3). Other factors must accordingly be important for radical scavenging. The dihedral angle of the two flavone backbones in Ca(II)–Kaem2 is 89°, obviously larger than the 77° in Sr(II)–Kaem2 and 75° in Ba(II)–Kaem2. The lower steric hindrance in Ca(II)–Kaem2 compared to other complexes may kinetically facilitate the formation and stability of a radical adduct both in the scavenging of β-Car˙+ and DPPH˙, and Ca(II)–Kaem2 thus making it the most efficient radical scavenger. Ba(II)–Kaem2 and Sr(II)–Kaem2 are marginally less efficient in β-Car˙+ radical scavenging reactivity than Ca(II)–Kaem2, although they are thermodynamically more favorable.
2 Ca(II)–Kaem2, including experimentally determined oxidation potentials and calculated BDE and IP values, are less favorable than Sr(II)–Kaem2 and Ba(II)–Kaem2 (Fig. 7 and Table 3). Other factors must accordingly be important for radical scavenging. The dihedral angle of the two flavone backbones in Ca(II)–Kaem2 is 89°, obviously larger than the 77° in Sr(II)–Kaem2 and 75° in Ba(II)–Kaem2. The lower steric hindrance in Ca(II)–Kaem2 compared to other complexes may kinetically facilitate the formation and stability of a radical adduct both in the scavenging of β-Car˙+ and DPPH˙, and Ca(II)–Kaem2 thus making it the most efficient radical scavenger. Ba(II)–Kaem2 and Sr(II)–Kaem2 are marginally less efficient in β-Car˙+ radical scavenging reactivity than Ca(II)–Kaem2, although they are thermodynamically more favorable.
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mono-coordinated Mg(II)–Kaem complex has similar β-Car˙+ scavenging reactivity as the 1
1 mono-coordinated Mg(II)–Kaem complex has similar β-Car˙+ scavenging reactivity as the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2 complex, but higher DPPH˙ scavenging efficiency. This pattern could arise from the strong electron attraction by the polar Mg(II), increasing the radical scavenging efficiency of Kaem as a ligand, as clearly demonstrated for 1
2 Ca(II)–Kaem2 complex, but higher DPPH˙ scavenging efficiency. This pattern could arise from the strong electron attraction by the polar Mg(II), increasing the radical scavenging efficiency of Kaem as a ligand, as clearly demonstrated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn(II) and Cu(II) transition metal–flavonoid complexes.5,6 However, 1
1 Zn(II) and Cu(II) transition metal–flavonoid complexes.5,6 However, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Kaem is only formed in the presence of excessive concentrations of Mg(II) ions, and accordingly is of less significance in biological systems than the 1
1 Mg(II)–Kaem is only formed in the presence of excessive concentrations of Mg(II) ions, and accordingly is of less significance in biological systems than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ca(II)–Kaem2 complex.
2 Ca(II)–Kaem2 complex.
For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Mg(II)–Api complex, its formation shows an insignificant increase in β-Car˙+ scavenging efficiency, and the complex formation has no effect on DPPH˙ scavenging. A comparison of the Mg(II)–Kaem and Mg(II)–Api complexes indicates that Mg(II) binding at the 3-hydroxyl and 4-carbonyl groups is an important structural factor for promoting the radical scavenging efficiency of Kaem. 4-Carbonyl and 5-hydroxyl binding to the Mg(II) ions for Api and binding to the Cu(II) for genistein both have insignificant effects on the radical scavenging efficiencies of flavonoids.18
1 Mg(II)–Api complex, its formation shows an insignificant increase in β-Car˙+ scavenging efficiency, and the complex formation has no effect on DPPH˙ scavenging. A comparison of the Mg(II)–Kaem and Mg(II)–Api complexes indicates that Mg(II) binding at the 3-hydroxyl and 4-carbonyl groups is an important structural factor for promoting the radical scavenging efficiency of Kaem. 4-Carbonyl and 5-hydroxyl binding to the Mg(II) ions for Api and binding to the Cu(II) for genistein both have insignificant effects on the radical scavenging efficiencies of flavonoids.18
Conclusions
Coordination of kaempferol in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 AEM(II)–Kaem2 complexes by the heavier AEM(II) ions Ca(II), Sr(II) and Ba(II) increases the radical scavenging efficiency in contrast to binding to the lighter Mg(II) ion and to the transition metal ion Zn(II). This difference is suggested to be the result of the stronger electron withdrawing capability of the (n − 1)d orbital in the heavier Ca(II), Sr(II) and Ba(II) ions compared to the lighter Mg(II) ion and the transitional metal ion Zn(II). Symmetry differences between the 1
2 AEM(II)–Kaem2 complexes by the heavier AEM(II) ions Ca(II), Sr(II) and Ba(II) increases the radical scavenging efficiency in contrast to binding to the lighter Mg(II) ion and to the transition metal ion Zn(II). This difference is suggested to be the result of the stronger electron withdrawing capability of the (n − 1)d orbital in the heavier Ca(II), Sr(II) and Ba(II) ions compared to the lighter Mg(II) ion and the transitional metal ion Zn(II). Symmetry differences between the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes also increase the radical scavenging rate in favor of the 1
2 complexes also increase the radical scavenging rate in favor of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, as observed for Zn(II). The Ca(II)–Kaem2 complex is a far more efficient radical scavenger than the other AEM(II)– and Zn(II)–kaempferol complexes, and may accordingly find use as a food additive and an antioxidant, especially in dairy products with high calcium content. Based on the surprising findings for Kaem, calcium complexes of flavonoids should also be investigated as new metal-based drug candidates.
1 complexes, as observed for Zn(II). The Ca(II)–Kaem2 complex is a far more efficient radical scavenger than the other AEM(II)– and Zn(II)–kaempferol complexes, and may accordingly find use as a food additive and an antioxidant, especially in dairy products with high calcium content. Based on the surprising findings for Kaem, calcium complexes of flavonoids should also be investigated as new metal-based drug candidates.
Abbreviations
| AEM | Alkaline earth metal |
| Kaem | Kaempferol |
| Api | Apigenin |
| β-Car | β-Carotene |
| β-Car˙+ | β-Carotene radical cation |
| CV | Cyclic voltammetry |
| DPPH˙ | 2,2-Diphenyl-1-picrylhydrazyl radical |
| ET | Electron transfer |
| DFT | Density functional theory |
| PDE | Proton dissociation energy |
| IP | Ionization potential |
| BDE | Bond dissociation energy |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (Nos. 21573284 and 21673288).References
- P. G. Pietta, J. Nat. Prod., 2000, 63, 1035–1042 CrossRef CAS PubMed.
- W. Ngwa, R. Kumar, D. Thompson and W. Lyerly, Molecules, 2020, 25, 2707 CrossRef CAS PubMed.
- S. Eghbaliferiz and M. Iranshahi, Phytother. Res., 2016, 30, 1379–1391 CrossRef CAS PubMed.
- D. Procházková, I. Boušová and N. Wilhelmová, Fitoterapia, 2011, 82, 513–523 CrossRef PubMed.
- Y. Xu, L.-L. Qian, J. Yang, R.-M. Han, J.-P. Zhang and L. H. Skibsted, J. Phys. Chem. B, 2018, 122, 10108–10117 CrossRef CAS PubMed.
- Y. Xu, J. Yang, Y. Lu, L.-L. Qian, Z.-Y. Yang, R.-M. Han, J.-P. Zhang and L. H. Skibsted, J. Phys. Chem. B, 2020, 124, 380–388 CrossRef CAS PubMed.
- M. M. Kasprzak, A. Erxleben and J. Ochocki, RSC Adv., 2015, 5, 45853–45877 RSC.
- Y. Qi, M. Jiang, Y.-L. Cui, L. Zhao and S. Liu, J. Hazard. Mater., 2015, 285, 336–345 CrossRef CAS PubMed.
- M. Samsonowicz, E. Regulska and M. Kalinowska, Chem.-Biol. Interact., 2017, 273, 245–256 CrossRef CAS PubMed.
- S. Selvaraj, S. Krishnaswamy, V. Devashya, S. Sethuraman and U. M. Krishnan, Med. Res. Rev., 2014, 34, 677–702 CrossRef CAS PubMed.
- A. Raza, S. Bano, X. Xu, R. X. Zhang, H. Khalid, F. M. Iqbal, C. Xia, J. Tang and Z. Ouyang, Biol. Trace Elem. Res., 2017, 178, 160–169 CrossRef CAS PubMed.
- N. Ghosh, T. Chakraborty, S. Mallick, S. Mana, D. Singha, B. Ghosh and S. Roy, Spectrochim. Acta, Part A, 2015, 151, 807–813 CrossRef CAS PubMed.
- Q. K. Panhwar and S. Memon, J. Coord. Chem., 2011, 64, 2117–2129 CrossRef CAS.
- A. Raza, X. Xu, L. Xia, C. Xia, J. Tang and Z. Ouyang, J. Fluoresc., 2016, 26, 2023–2031 CrossRef CAS PubMed.
- S. Roy, S. Mallick, T. Chakraborty, N. Ghosh, A. K. Singh, S. Manna and S. Majumdar, Food Chem., 2015, 173, 1172–1178 CrossRef CAS PubMed.
- Q. K. Panhwar, S. Memon and M. I. Bhanger, J. Mol. Struct., 2010, 967, 47–53 CrossRef CAS.
- M. Samsonowicz and E. Regulska, Spectrochim. Acta, Part A, 2017, 173, 757–771 CrossRef CAS PubMed.
- J. Yang, Y. Xu, H.-Y. Liu, R.-M. Han, J.-P. Zhang and L. H. Skibsted, Molecules, 2017, 22, 1757–1771 CrossRef PubMed.
- Z.-Y. Yang, L.-L. Qian, Y. Xu, M.-T. Song, C. Liu, R.-M. Han, J.-P. Zhang and L. H. Skibsted, Molecules, 2020, 25, 1975–1987 CrossRef CAS PubMed.
- G. Dehghan and Z. Khoshkam, Food Chem., 2012, 131, 422–426 CrossRef CAS.
- Q. K. Panhwar and S. Memon, Inorg. Chim. Acta, 2013, 407, 252–260 CrossRef CAS.
- N. Sugihara, T. Arakawa, M. Ohnishi and K. Furuno, Free Radical Biol. Med., 1999, 27, 1313–1323 CrossRef CAS PubMed.
- K. Mai, R. Divyashree, G. Francesca and M. O. Helen, Future Med. Chem., 2019, 11, 2845–2867 CrossRef PubMed.
- X. Wu, L. Zhao, J. Jin, S. Pan, W. Li, X. Jin, G. Wang, M. Zhou and F. Gernot, Science, 2018, 361, 912–916 CrossRef CAS PubMed.
- A. S. S. Wilson, M. S. Hill, M. F. Mahon, C. Dinoi and L. Maron, Science, 2017, 358, 1168–1171 CrossRef CAS PubMed.
- B. Maitland, M. Wiesinger, J. Langer, G. Ballmann, J. Pahl, H. Elsen, C. Farber and S. Harder, Angew. Chem., Int. Ed., 2017, 56, 11880–11884 CrossRef CAS PubMed.
- J. Yin, Y. Hu and J. Yoon, Chem. Soc. Rev., 2015, 44, 4619–4644 RSC.
- M. J. Berridge, M. D. Bootman and H. L. Roderick, Nat. Rev. Mol. Cell Biol., 2003, 4, 517–529 CrossRef CAS PubMed.
- P. J. Marie, P. Ammann, G. Boivin and C. Rey, Calcif. Tissue Int., 2001, 69, 121–129 CrossRef CAS PubMed.
- R.-M. Han, Y.-X. Tian, E. M. Becker, M. L. Andersen and L. H. Skibsted, J. Agric. Food Chem., 2007, 55, 2384–2391 CrossRef CAS PubMed.
- R.-M. Han, C.-H. Chen, Y.-X. Tian, J.-P. Zhang and L. H. Skibsted, J. Phys. Chem. A, 2010, 114, 126–132 CrossRef CAS PubMed.
- V. Butković, L. Klasinc and W. Bors, J. Agric. Food Chem., 2004, 52, 2816–2820 CrossRef PubMed.
- B. P. Pritchard, D. Altarawy, B. Didier, T. D. Gibson and T. L. Windus, J. Chem. Inf. Model., 2019, 59, 4814–4820 CrossRef CAS PubMed.
- J. E. Bartmess, J. Phys. Chem., 1994, 98, 6420–6424 CrossRef CAS.
- M. J. Stowell, Handbook of chemistry and Physics 50th edn, Phys. Bull., 1970, 21, 460 CrossRef.
- G.-X. Xu, Substance Structure, Higher Education Press, Beijing, 2nd edn, 1991 Search PubMed.
- R. A. Dar, G. A. Naikoo, I. U. Hassan and A. M. H. Shaikh, Anal. Chem. Res., 2016, 7, 1–8 CrossRef CAS.
- Y. Oztekin, Z. Yazicigil, A. Ramanaviciene and A. Ramanavicius, Sens. Actuators, B, 2011, 152, 37–48 CrossRef CAS.
- Y.-J. Shang, Y.-P. Qian, X.-D. Liu, F. Dai, X.-L. Shang, W.-Q. Jia, Q. Liu, J.-G. Fang and B. Zhou, J. Org. Chem., 2009, 74, 5025–5031 CrossRef CAS PubMed.
- L.-L. Song, R. Liang, D.-D. Li, Y.-D. Xing, R.-M. Han, J.-P. Zhang and L. H. Skibsted, J. Agric. Food Chem., 2011, 59, 12643–12651 CrossRef CAS PubMed.
- H. Cheng, R.-M. Han, J.-P. Zhang and L. H. Skibsted, J. Agric. Food Chem., 2014, 62, 942–949 CrossRef CAS PubMed.
- S. B. Bukhari, S. Memon, M. Mahroof-Tahir and M. I. Bhanger, Spectrochim. Acta, Part A, 2009, 71, 1901–1906 CrossRef PubMed.
- G. Dehghan and Z. Khoshkam, Food Chem., 2012, 131, 422–426 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03249b |
| This journal is © The Royal Society of Chemistry 2020 |