Open Access Article
Open Access ArticleSynthesis of 2,5-furandicarboxylic acid by a TEMPO/laccase system coupled with Pseudomonas putida KT2440†
Lihua Zou‡
a,
Zhaojuan Zheng‡abc,
Huanghong Tana,
Qianqian Xua and
Jia Ouyang *abc
*abc
aJiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, People's Republic of China. E-mail: hgouyj@njfu.edu.cn
bKey Laboratory of Forestry Genetics & Biotechnology (Nanjing Forestry University), Ministry of Education, Nanjing 210037, People's Republic of China
cJiangsu Province Key Laboratory of Green Biomass-based Fuels and Chemicals, Nanjing 210037, People's Republic of China
First published on 8th June 2020
Abstract
As a useful and renewable chemical building block from biomass, 2,5-furandicarboxylic acid (FDCA) has become an increasingly desirable platform chemical as a terephthalic acid replacement for polymerization. In this work, an efficient and highly selective biocatalytic approach for the synthesis of FDCA from 5-hydroxymethylfurfural (HMF) was successfully developed using a TEMPO/laccase system coupled with Pseudomonas putida KT2440. TEMPO/laccase afforded the selective oxidation of the hydroxymethyl group of HMF to form 5-formyl-2-furancarboxylic acid as a major product, which was subsequently oxidized to FDCA by P. putida KT2440. Manipulating the reaction conditions resulted in a good conversion of HMF (100%) and an excellent selectivity of FDCA (100%) at substrate concentrations up to 150 mM within 50 h. The cascade catalytic process established in this work offers a promising approach for the green production of FDCA.
Introduction
The massive depletion of nonrenewable fossil resources and the emission of greenhouse gases have produced the widest range of changes in the world.1 There has been growing interest in developing renewable and sustainable fuels and platform chemicals.2–5 Lignocellulosic biomass is an important renewable carbon resource, and its derived carbohydrates can be converted into various biobased platform compounds for the production of high-value products.6,7 Among these compounds, 5-hydroxymethylfurfural (HMF) is such a compound that has a furan ring, a hydroxymethyl group and an aldehyde group, which makes it an appealing starting material for catalytic upgrades. It can be selectively oxidized to versatile building blocks, including 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), 2,5-diformylfuran (DFF), 5-formyl-2-furancarboxylic acid (FFCA), and 2,5-furandicarboxylic acid (FDCA).8 Among them, FDCA, obtained through the full oxidation of the hydroxymethyl and aldehyde moieties in HMF, is only listed as one of the 12 key value-added chemicals from biomass by the U.S. Department of Energy.9 It has been considered to have a huge market potential because it can be used as a ‘green’ substitute of terephthalic acid for the production of poly(ethylene terephthalate).10In the past few years, intensive research efforts have been conducted to synthesize FDCA by the oxidation of HMF through chemical pathways,11 such as the use of traditional stoichiometric oxidants,12 homogeneous catalysts,13 and heterogeneous catalysts (various noble metal catalysts, such as supported Au, Pt, and Pd catalysts).14 However, these chemocatalytic approaches are characterized by problems associated with harsh reaction conditions, such as high temperature, high pressure, and the presence of metal salts and organic solvents, which render the process expensive and polluting. In addition, the selectivity of some catalysts is poor in specific reactions, resulting in the formation of byproducts.15,16
Biocatalysis is emerging as a valuable tool to address these problems in the context of green chemistry since it is typically performed under mild conditions, usually requires fewer and less toxic reagents and solvent than chemocatalysis, and exhibits excellent selectivity.17 Currently, biocatalytic production of FDCA mainly involves microbial and enzymatic conversion approaches. Since the FDCA concentration produced by wild-type strains is too low,18 gene modification or metabolic engineering becomes an indispensable step for the microbial conversion process. Two notable examples of microbial bioconversion are the recombinant strains Pseudomonas putida S12 (ref. 19) and Raoultella ornithinolytica BF60.20 Although both of them give relatively high FDCA titers, they still suffer from several disadvantages: complex molecular operation and low productivity or yield of FDCA. Enzymatic bioconversion is another promising alternative approach, but there are limited examples in the literature. An HMF oxidase was identified with a remarkable capability of oxidizing HMF to FDCA, which was conducted at very low concentrations (2–4 mM).21,22 The full oxidation of HMF more often needs a combination of two enzymes, as the production of FDCA entails three consecutive oxidation steps. For example, aryl-alcohol oxidase coupled with an unspecific peroxygenase,23 galactose oxidase coupled with lipase,24 and galactose oxidase variant M3–5 coupled with aldehyde oxidase25 have been reported in the past five years. Compared with the corresponding bioconversion by microbes, the bioconversion of HMF to FDCA proceeded by enzymes needs extra expression and purification steps. Sometimes, the reaction conditions of two enzymes do not match well, which further complicates the whole process.
The shortcomings of the existing methods and the potential importance of FDCA production motivate us to further explore efficient and robust oxidation method. In this study, we designed a method of coupling reactions to produce FDCA from HMF. The key feature of this approach is the oxidation of the hydroxymethyl group via a TEMPO/laccase system and the oxidation of the aldehyde moiety by P. putida KT2440 cells. Combining the advantages of two catalysts, a satisfactory HMF conversion and FDCA selectivity were realized even at high HMF concentrations (150–200 mM). This study provides a highly green and efficient biocatalytic alternative for the production of FDCA from HMF.
Experimental section
Chemical materials
DFF (>98%), FFCA (>98%) and FDCA (>98%) were purchased from TCI (Japan), while the laccase from Trametes versicolor, HMF (>99%), TEMPO (>98%) and 2,2′-azinobis(3-ethylbenzylthiozoline-6-sulfonate) (ABTS) were purchased from Sigma-Aldrich (USA).Preparation of whole-cell biocatalysts
For preparation of seed cultures, P. putida KT2440 cells were precultivated at 30 °C and 200 rpm for 12 h in Luria Broth (LB) medium containing 0.5% yeast extract, 1% tryptone, and 1% NaCl. Then, 2% seed culture was incubated in 1 L shake flasks containing 200 mL fresh LB medium. After incubation for 12 h under the same conditions, the cells were harvested by centrifugation (6000g, 8 min, 4 °C) and washed twice with phosphate buffer (0.2 M, pH 6.0), followed by dispersing in corresponding buffer for subsequent experiments. The biomass concentrations were measured by determining the optical density at 600 nm and converted to the dry cell weight (dcw) using the following equation: dcw (g L−1) = 0.348 × optical density at 600 nm [OD600] − 0.76.Laccase activity assay
The laccase activity was determined spectrophotometrically in a sodium acetate buffer (0.1 M, pH 5.0) containing 0.5 mM ABTS as the substrate at room temperature and a suitable amount of enzyme. Oxidation of ABTS was followed by absorbance increase at 420 nm (ε420 = 3.6 × 104 M−1 cm−1) in 3 min.24 One unit of laccase activity was defined as follow: the amount of laccase that can oxidized 1 μmol of ABTS per minute. The concentrations of proteins were determined by the Bradford assay using bovine serum albumin as the standard. The specific activities of laccases from T. versicolor was 5.8 U mg−1.Oxidation of the hydroxymethyl group of HMF by TEMPO/laccase
In a typical experiment, 4 mL buffer or deionized water containing 30 mM HMF and TEMPO/laccase were incubated in a 50 mL centrifuge tube capped with a septum at 150 rpm with air bubbling for 3 min each 12 h. Parameters affecting the above typical bio-oxidation experiment were investigated, including temperature, buffer pH, and laccase concentration. The parameters varied as follows. Temperature ranged from 20 to 30 °C, and pH was varied from 4.5 to 7.0 using different buffers (50 mM) (CH3COOH–CH3COONa for pH 4.5–6.0 and NaH2PO4–Na2HPO4 for pH 6.0–7.0, and distilled water as the control). The enzyme dosage was set from 0 to 7.5 mg mL−1. Aliquots were withdrawn from the reaction mixtures at specified times and diluted with the corresponding mobile phase prior to HPLC analysis. All experiments were conducted in triplicate, and all the data are expressed as the mean ± standard deviation.Representative procedure for the synthesis of FDCA
Experiments for the biotransformation of HMF to FDCA by the TEMPO/laccase system coupled with P. putida KT2440 were performed as follows. Typically, HMF (30–200 mM), a certain proportion of TEMPO (molar TEMPO/HMF) and laccase were added to acetate buffer (50–200 mM, pH 6.0), and the reaction was incubated at 25 °C, 150 rpm in a shaking incubator capped with a septum. When the first step oxidation was completed by the TEMPO/laccase system, the pH was carefully monitored and adjusted to 6.0 with NaOH, which was followed by an addition of P. putida KT2440 cells and calcium carbonate (at same molarity as the substrate). The reaction progressed for a few hours in the incubator at 30 °C, 200 rpm. Aliquots were withdrawn at specified time intervals from the reaction mixture. The samples were treated at 100 °C for 5 min to denature the cells and then diluted prior to HPLC analysis.Analytical methods
The reaction mixtures were analyzed using an Agilent 1260 HPLC system using an external standard calibration curve method. The sample was separated by an Aminex HPX-87H column (Bio-Rad, USA) at 55 °C, with elution performed by the use of 5 mM H2SO4 at 0.6 mL min−1 and detection performed with a UV detector at a fixed wavelength of 268 nm. The conversion (%) of HMF and the selectivity (%) of oxidation products were calculated as follows:
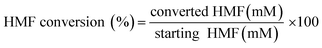 | (1) |
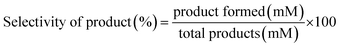 | (2) |
Statistical analysis
Statistical analyses were performed using SPSS statistics 16.0. The differences of the corresponding values between exposed groups were tested by one-way analysis of variance (ANOVA). p < 0.05 was considered to be a significant difference.Results and discussion
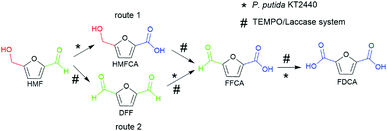
Design and selection of routes for FDCA production
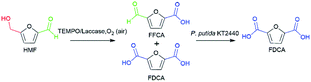
The synthesis of FDCA from HMF requires a catalyst to act on both hydroxymethyl and aldehyde groups. Interestingly, a previous study in our group demonstrated that wild-type P. putida KT2440 could effectively convert HMF into HMFCA, and no DFF, FFCA or FDCA formation was observed,26 which shows perfect selective oxidation toward the aldehyde group of HMF. For the oxidation of the hydroxymethyl group, TEMPO/laccase system has been employed in the literature toward HMF.24,27 Herein, we attempted to combine the abilities of the TEMPO/laccase system and P. putida KT2440 to oxidize the hydroxymethyl group and the aldehyde group, respectively, hoping that they match perfectly and efficiently achieve FDCA production under mild reaction conditions.Based on the above assumptions, there are two possible pathways for the oxidation of HMF to FDCA (Scheme 1). In the first pathway, the aldehyde group of HMF is first oxidized by P. putida KT2440 to yield HMFCA. Then, TEMPO/laccase is added to the reaction mixtures to further oxidize the hydroxymethyl group of HMFCA and thus complete full oxidation of HMF. In this pathway, it is uncertain whether TEMPO/laccase can oxidize the hydroxymethyl of HMFCA. In the second pathway, the TEMPO/laccase catalysis operates prior to P. putida KT2440. In this way, the conversion of the hydroxymethyl group of HMF is first catalyzed by the TEMPO/laccase system to generate furan mixtures, which are then converted to FDCA by P. putida KT2440. Regarding this pathway, we have not detailed and comprehensively investigated the biocatalytic ability of P. putida KT2440 toward other furanics except HMF.
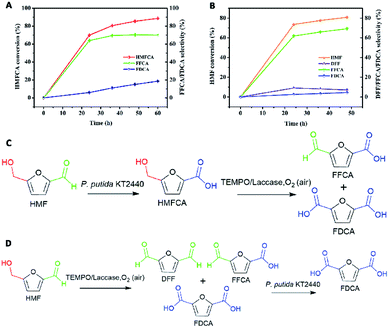
To verify the feasibility of the first route, considering the oxidation of HMF to HMFCA by P. putida KT2440 had been proved, we set up a test reaction using HMFCA with the TEMPO/laccase system to identify whether the hydroxymethyl group of HMFCA could be oxidized. The reaction was started with addition of the commercially available fungal laccase. When HMFCA was incubated with the TEMPO/laccase system in acetate buffer, almost 64% FFCA and only 6% FDCA were detected after 24 h of incubation, and these percentages changed slightly during the subsequent incubation (approximately 19% FDCA after 60 h) (Fig. 1A). The TEMPO/laccase oxidation of HMFCA resulted in mixtures in which FFCA was dominant. Qin and coworkers ever reported that TEMPO/laccase could convert HMF into DFF as initial dominate intermediate and further transform into FFCA, but its oxidation efficiency of FFCA into FDCA was poor.24 Our results further confirmed the low activity of the TEMPO/laccase system on FFCA. In general, the hydrated form (gem-diol) of aldehydes act as the key intermediates play a central role in the aldehyde oxidation catalyzed by TEMPO/laccase system. Whereas, only a small fraction of FFCA may exist in hydrated form.22 Thus, route 1 will not be sufficiently active to produce FDCA (Fig. 1C).
Next, the second route was evaluated. HMF was mixed with TEMPO/laccase to test the ability of this system to oxidize it. Most of the HMF (80% conversion) was converted into FFCA in 48 h, and very little DFF and FDCA were formed. The proportion of FFCA slowly decreased over time because of the formation of low amounts of FDCA (4.5% selectivity after 48 h) (Fig. 1B). Then, the ability of P. putida KT2440 to oxidize the intermediates of DFF and FFCA was tested (Fig. S1†). The results revealed that, given enough time, P. putida KT2440 can fully oxidize the aldehyde groups of both DFF and FFCA to yield FDCA. This means that the oxidation of HMF to FDCA via route 2 is indeed feasible (Fig. 1D). For this route, a critical task is determining how to improve the conversion of HMF to 100%, as the surplus HMF in the TEMPO/laccase system would be completely converted to HMFCA by P. putida KT2440, increasing the difficulty and cost of the separation and purification of FDCA.
Effects of key reaction conditions on HMF oxidation
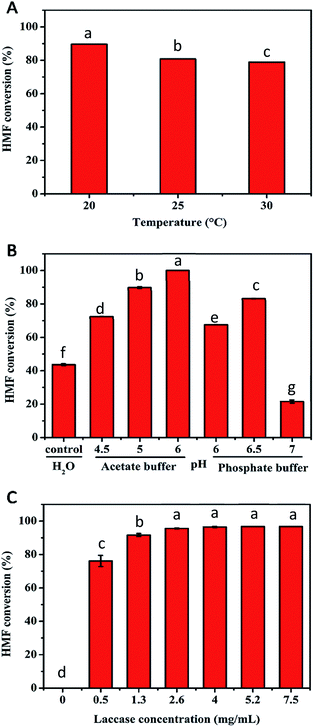
To obtain higher FDCA selectivity and concomitantly eliminate HMFCA generation in the second step, a series of experiments were performed to improve the conversion of HMF. The influence of temperature on the oxidation of HMF in the TEMPO/laccase system was tested in the range of 20–30 °C for 48 h (Fig. 2A). The highest conversion of HMF (nearly 90%) was obtained at 20 °C. But considering that 25 °C is closer to room temperature, we selected 25 °C for the next experiment. It has been reported that the type of buffer and pH used can influence the conversion of substrates in reaction systems.28 For this reason, the effects of buffer and pH on the oxidation of HMF were explored at 25 °C for 24 h. It was found that buffer type and pH exerted a significant effect on the catalytic performance. Compared to unbuffered aqueous solutions, all the buffered solutions tested were capable of considerably enhancing HMF conversion apart from phosphate buffer at pH 7.0. The use of sodium acetate buffer at pH 6.0 led to an almost perfect HMF conversion (100%). At the same pH of 6.0, the conversion of HMF in phosphate buffer decreased to 67.6%. For phosphate buffer, the best results were observed at pH 6.5 (HMF conversion of 83.1%) (Fig. 2B). In general, the results suggested that a buffered solution was necessary for the conversion of HMF because of the production of acidic intermediates during the reaction course. Thus, we selected sodium acetate buffer of pH 6.0 for subsequent work. During the aerobic oxidation of laccase/TEMPO, laccase can easily oxidise the stable oxyl-radical form of TEMPO to the oxoammonium ion. The oxoammonium ion of TEMPO as the actual oxidant could selectively oxidize the hydroxymethyl group of HMF.27 Therefore, the dose of laccase was also crucial to HMF conversion (Fig. 2C). A control experiment confirmed that no substrate transformation occurred in the absence of laccase under the investigated conditions, which was in good agreement with previous reported results in the literature.29 A maximum conversion of 95.6% was achieved at 2.6 mg mL−1 laccase. When the amount of enzyme was more than 2.6 mg mL−1, there was no significant improvement in the conversion of HMF. Therefore, considering the cost of enzyme, a laccase dose of 2.6 mg mL−1 was used for the subsequent studies.The oxidation of HMF in the TEMPO/laccase system at high substrate concentrations
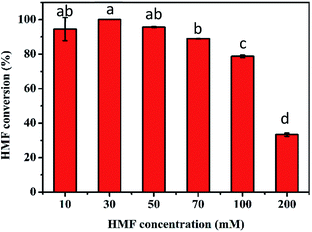
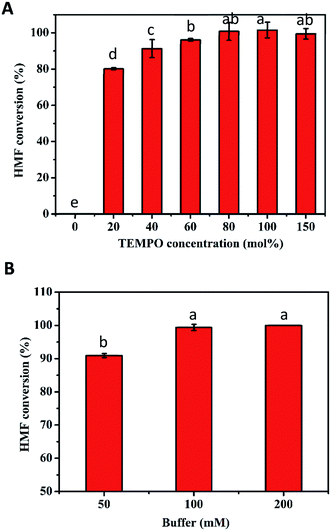
The excellent conversion at high concentrations of HMF obtained in the first step is conducive to the production of FDCA in the second step. Thus, the transformation of 10–200 mM HMF by the TEMPO/laccase system was firstly assessed under the optimum conditions obtained above. As presented in Fig. 3, after 24 h of reaction, 100% conversion was achieved when the HMF concentration was ≤30 mM. For 50 mM and 70 mM HMF, the conversion was slightly decreased to 96% and 89%, respectively. However, the conversion was reduced markedly to 79% and 34% at HMF concentrations of 100 and 200 mM. The major reason for the unsatisfactory conversion at high concentrations might be the inappropriate amount of catalyst and poor buffer capacity in the reaction system because of TEMPO decomposition30 and pH drop with time. Based on the above speculation, we next investigated the influence of these two factors on HMF conversion. First, the influence of TEMPO concentration on the oxidation of HMF by the TEMPO/laccase system was evaluated. As shown in Fig. 4A, when the laccase amount was constant, the conversion of HMF was promptly increased with increasing TEMPO concentration. When a TEMPO concentration of 80 mol% was used a high HMF conversion of nearly 100% was obtained after 12 h in the presence of 70 mM HMF, and further increasing the TEMPO concentration resulted in no significant changes in the conversion of HMF. This result means that a suitable TEMPO amount is crucial for the efficient conversion of HMF. A higher mediator/laccase ratio could greatly promote the conversion of HMF at high concentrations. Therefore, 80 mol% TEMPO was used for further research.Upon examining the second possibility for unsatisfactory conversion, we found that the continuous accumulation of intermediates (mainly FFCA) resulted in an appreciably decreased pH in the reaction system, which affected the progress of the reaction. For this reason, we investigated the effect of buffer concentrations (50, 100, 200 mM) on the catalytic reaction in the case of a higher HMF concentration. After a reaction period of 36 h, 100 and 200 mM sodium acetate buffer gave the best performance, and HMF conversion was close to 100% (Fig. 4B).
Given these facts, we further increased the HMF concentration to 150 mM and 200 mM, and this TEMPO/laccase system was still capable of oxidizing HMF with conversion of 100% and 98.2%, respectively (Table 1). In the literature, Qin et al. reported a conversion of 79% after 48 h starting with 30 mM HMF.24 Apparently, the results reported in this work were better than the previous results. To our knowledge, this is the best HMF conversion at such a high substrate concentration in the TEMPO/laccase system ever reported.
| Entry | HMF (mM) | Buffer (mM) | TEMPO (mol%) | HMF conversion (%) | ta (h) | FDCA selectivity (%) | Cell dosage (g L−1) | tb (h) |
|---|---|---|---|---|---|---|---|---|
| a The reaction time of TEMPO/laccase oxidation.b The reaction time of P. putida KT2440 biotransformation. | ||||||||
| 1 | 30 | 50 | 20 | 100 | 24 | 100 | 6 | 1 |
| 2 | 70 | 50 | 80 | 100 | 12 | 100 | 10 | 1 |
| 3 | 100 | 100 | 80 | 100 | 36 | 100 | 10 | 1 |
| 4 | 150 | 200 | 80 | 100 | 48 | 100 | 16 | 2 |
| 5 | 200 | 200 | 80 | 98.2 | 60 | 82.4 | 24 | 5 |
Synthesis of FDCA via the TEMPO/laccase system and P. putida KT2440 in tandem
With improved HMF conversion, we turned our attention to the second step. Considering that the intermediate FFCA and the acetate buffer in the first step might affect the biocatalytic performance of P. putida KT2440 in the last step, the effects of these factors on P. putida KT2440 were tested by employing the commercially available FFCA as a substrate. As shown in Fig. S2,† when the pH values varied from 4.5 to 6.0, P. putida KT2440 cells exhibited good catalytic performances, and a conversion of 100% was achieved after the short reaction time of 0.5 h. In addition, all FFCA was oxidized to FDCA with an excellent selectivity of 100%. Better yet, P. putida KT2440 maintained its outstanding capability under all tested FFCA and acetate buffer concentrations (Table S1†), which further demonstrated its great potential for oxidizing various furan aldehydes.Based on the above experimental results, the TEMPO/laccase system and P. putida KT2440 cells were coupled to test the conversion of HMF to FDCA in a sequential manner at different concentrations of HMF. When HMF was completely converted by the TEMPO/laccase system, the pH of the reaction mixtures was adjusted to 6.0 with NaOH, and P. putida KT2440 cells were added for further oxidation. The results of the two-step cascade oxidation of HMF to FDCA are summarized in Table 1. In most cases, almost 100% conversion of HMF was acquired in the TEMPO/laccase system. Increasing the initial concentration of TEMPO and buffer was beneficial for HMF conversion at high concentrations. The complete oxidation of HMF by TEMPO/laccase resulted in the predominant formation of FFCA and minor amounts of FDCA. HMF to FDCA conversion was faltered at the aldehyde acid stage (FFCA) because of the low activity of the TEMPO/laccase system on FFCA. FFCA was not efficiently oxidized by TEMPO/laccase system, which correlates with the low degree of hydration. After P. putida KT2440 cells, which can directly oxidize the aldehyde group of FFCA, were added, FDCA was synthesized quickly, and FFCA decreased sharply in a short reaction time. A full conversion of intermediates from the previous step was realized even at HMF concentrations up to 150 mM. Moreover, excellent selectivity (100%) was retained during the reaction. Yang and coworkers exploited Comamonas testosteroni SC1588 cells for synthesis of FDCA from FFCA in TEMPO/laccase system.31 However, it took about 15 h in the last step and achieved around 90% of conversion. Because a relatively high concentration of TEMPO in the catalytic system significantly inhibited the activity of the cells. However, P. putida KT2440 in this study can completely convert FFCA to FDCA within few hours in the second step. These results suggested that P. putida KT2440 is an excellent biocatalyst for the oxidation of FFCA. Besides, P. putida KT2440 has good compatibility with TEMPO/laccase system, which would likely be a potential and promising workhorse in biological conversion. Meanwhile, it was observed that improvement of the biocatalyst concentration was pivotal to obtaining a full conversion of FFCA with good product selectivity. When the concentration of starting HMF was increased to 200 mM, only 82.4% selectivity of FDCA was observed. Unsatisfactory FDCA production may be attributed to insufficient amount of biocatalyst at higher substrate loadings.
The results obtained in this work were compared with those of previous studies. As shown in Table 2, the electrochemical oxidation of HMF to FDCA was achieved using a catalytic anode under strongly acidic conditions.32 Although the method appeared to be clean, the initial HMF concentration and FDCA yield reported in the literature were relatively low and accompanied by the formation of byproducts. Homogeneous catalysts have some problems in the synthesis of FDCA, such as high FDCA yield usually being obtained at the expense of harsh reaction conditions.13 Compared with homogeneous catalysts, heterogeneous noble metal catalysts for the conversion of HMF to FDCA have been extensively studied due to their recoverability and reusability.14 However, noble metal materials are expensive, and slashing reaction conditions was required. The biosynthesis of FDCA from HMF is of considerable interest because it offers many advantages: mild conditions, excellent selectivity and relatively little environmental pollution. Although some biocatalytic oxidation methods have been reported for the synthesis of FDCA, they still suffer from some weaknesses, such as long reaction time, low HMF loading, and unsatisfactory FDCA yield. For example, the sequential oxidation of HMF to FDCA by fungal aryl alcohol oxidase (AAO) and unspecific peroxygenase (UPO) has been described,23 but its transformation efficacies were very poor (a reaction time of 120 h was required to achieve maximum yield). The TEMPO/laccase system coupled with P. putida KT2440 proved to be a good biocatalytic approach for the oxidation of HMF to FDCA because of its good conversion and excellent selectivity even at HMF concentrations up to 150 mM and 200 mM.
| Catalysts | HMF (mM) | Reaction conditions | t [h] | HMF Ca [%] | FDCA S/Yb [%] | Ref. |
|---|---|---|---|---|---|---|
| a C: conversion.b Y: yield, S: selectivity.c n.a.: not available. | ||||||
| Manganese oxide (MnOx) anode | 20 | pH 1H2SO4 solution, 60 °C, 1400 rpm, E = 2.0 V vs. RHE, 250C charge passed | n.a.c | >99.9 | 53.8 (Y) | 32 |
| Co/Mn/Br | ∼377 | Co/Mn/Br = 1/0.015/0.5, T = 180 °C, P = 30 bar (molar CO2/O2 = 1), H2O/HOAc = 7/93 (v/v), n = 1200 rpm | 0.5 | n.a. | 90 (Y) | 13 |
| Pt/C | 150 | 690 kPa O2, 2 equiv. of NaOH, 22 °C | 6 | 100 | 79 (S) | 14 |
| Recombinant Raoultella ornithinolytica BF60 | 100 | 45 g per L cells, 30 °C, 50 mM phosphate buffer (pH 8) | 170 | n.a. | 89 (Y) | 20 |
| HMF oxidase (HMFO) | 4 | 20 μM HMFO, 20 μM FAD, 25 °C, potassium phosphate buffer of pH 7 | 24 | 100 | 95 (Y) | 22 |
| AAO + UPO successively | 3 | AAO (5 μM), UPO (0.65 μM), sodium phosphate (pH 7) | 120 | 100 | 91 (Y) | 23 |
| TEMPO/laccase + C. testosterone SC1588 | 100 | 20 mol% TEMPO, 8.1 mg mL−1 laccase, 140 g per L cells, phosphate buffer (200 mM, pH 7) | 36 | 100 | 87 (Y) | 31 |
| TEMPO/laccase + P. putida KT2440 in tandem | 150 | 80 mol% TEMPO, 2.6 mg mL−1 laccase, 16 g per L cells, acetate buffer of pH 6 | 50 | 100 | 100 (S) | This work |
Conclusions
In this study, we developed a promising approach for the production of FDCA from HMF by a TEMPO/laccase system coupled with P. putida KT2440. This method exhibits 100% conversion of HMF and 100% selectivity of FDCA under mild conditions at substrate concentrations up to 150 mM and does not require complex gene modification, enzyme purification or expensive cofactor addition. Currently, the preparation of HMF from inexpensive and highly abundant lignocellulosic biomass has been well documented in the literature. The synthesis of FDCA from biomass resources can be attained readily by the combination approach established in this work. Additionally, the success of the coupling system will provide a similar alternative approach for further biomanufacturing of other value-added chemicals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Key Research and Development Program of China (2018YFA0902200), and the National Natural Science Foundation of China (51776099).Notes and references
- W.-C. Chen, Y.-C. Lin, I. M. Chu, L.-F. Wang, S.-L. Tsai and Y.-H. Wei, Biochem. Eng. J., 2020, 154, 107440 CrossRef.
- J. J. Bozell, Science, 2010, 329, 522–523 CrossRef CAS PubMed.
- C. Chatterjee, F. Pong and A. Sen, Green Chem., 2015, 17, 40–71 RSC.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- H. Li, A. Riisager, S. Saravanamurugan, A. Pandey, R. S. Sangwan, S. Yang and R. Luque, ACS Catal., 2018, 8, 148–187 CrossRef CAS.
- R. J. van Putten, J. C. Van Der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- T. Werpy and G. Petersen, Top Value Added Chemicals From Biomass Volume 1 - Results of Screening for Potential Candidates From Sugars and Synthesis Gas, National Renewable Energy Lab., Golden, CO (US), 2004 Search PubMed.
- A. J. J. E. Eerhart, A. P. C. Faaij and M. K. Patel, Energy Environ. Sci., 2012, 5, 6407–6422 RSC.
- Z. Zhang and K. Deng, ACS Catal., 2015, 5, 6529–6544 CrossRef CAS.
- T. Miura, H. Kakinuma, T. Kawano and H. Matsuhisa, US Pat. 7,411,078, 2008.
- X. Zuo, P. Venkitasubramanian, D. H. Busch and B. Subramaniam, ACS Sustainable Chem. Eng., 2016, 4, 3659–3668 CrossRef CAS.
- S. E. Davis, L. R. Houk, E. C. Tamargo, A. K. Datye and R. J. Davis, Catal. Today, 2011, 160, 55–60 CrossRef CAS.
- L. Hu, A. He, X. Liu, J. Xia, J. Xu, S. Zhou and J. Xu, ACS Sustainable Chem. Eng., 2018, 6, 15915–15935 CrossRef CAS.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- S. M. Thomas, R. DiCosimo and V. Nagarajan, Trends Biotechnol., 2002, 20, 238–242 CrossRef CAS PubMed.
- R. O. Rajesh, T. K. Godan, A. K. Rai, D. Sahoo, A. Pandey and P. Binod, Bioresour. Technol., 2019, 284, 155–160 CrossRef CAS PubMed.
- F. Koopman, N. Wierckx, J. H. de Winde and H. J. Ruijssenaars, Bioresour. Technol., 2010, 101, 6291–6296 CrossRef CAS PubMed.
- G. S. Hossain, H. Yuan, J. Li, H. D. Shin, M. Wang, G. Du, J. Chen and L. Liu, Appl. Environ. Microbiol., 2017, 83, e02312–16 CrossRef CAS PubMed.
- W. P. Dijkman and M. W. Fraaije, Appl. Environ. Microbiol., 2014, 80, 1082–1090 CrossRef PubMed.
- W. P. Dijkman, D. E. Groothuis and M. W. Fraaije, Angew. Chem., Int. Ed., 2014, 53, 6515–6518 CrossRef CAS PubMed.
- J. Carro, P. Ferreira, L. Rodriguez, A. Prieto, A. Serrano, B. Balcells, A. Arda, J. Jimenez-Barbero, A. Gutierrez, R. Ullrich, M. Hofrichter and A. T. Martinez, FEBS J., 2015, 282, 3218–3229 CrossRef CAS PubMed.
- Y.-Z. Qin, Y.-M. Li, M.-H. Zong, H. Wu and N. Li, Green Chem., 2015, 17, 3718–3722 RSC.
- S. M. McKenna, S. Leimkühler, S. Herter, N. J. Turner and A. J. Carnell, Green Chem., 2015, 17, 3271–3275 RSC.
- Q. Xu, Z. Zheng, L. Zou, C. Zhang, F. Yang, K. Zhou and J. Ouyang, Bioprocess Biosyst. Eng., 2020, 43, 67–73 CrossRef CAS PubMed.
- K. F. Wang, C. L. Liu, K. Y. Sui, C. Guo and C. Z. Liu, Chembiochem, 2018, 19, 654–659 CrossRef CAS PubMed.
- J. Gross, K. Tauber, M. Fuchs, N. G. Schmidt, A. Rajagopalan, K. Faber, W. M. F. Fabian, J. Pfeffer, T. Haas and W. Kroutil, Green Chem., 2014, 16, 2117–2121 RSC.
- C. Zhang, X. Chang, L. Zhu, Q. Xing, S. You, W. Qi, R. Su and Z. He, Int. J. Biol. Macromol., 2019, 128, 132–139 CrossRef CAS PubMed.
- I. W. C. E. Arends, Y. X. Li, R. Ausan and R. A. Sheldon, Tetrahedron, 2006, 62, 6659–6665 CrossRef CAS.
- Z.-Y. Yang, M. Wen, M.-H. Zong and N. Li, Catal. Commun., 2020, 139, 105979 CrossRef CAS.
- S. R. Kubota and K. S. Choi, ChemSusChem, 2018, 11, 2138–2145 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03089a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |