Open Access Article
Open Access ArticleA chitosan-based edible film with clove essential oil and nisin for improving the quality and shelf life of pork patties in cold storage
Karthikeyan Venkatachalam and
Somwang Lekjing
and
Somwang Lekjing *
*
Department of Food Technology, Faculty of Science and Industrial Technology, Prince of Songkla University, Surat Thani Campus, Makhamtia, Muang, Surat Thani 84000, Thailand. E-mail: somwang.s@psu.ac.th
First published on 7th May 2020
Abstract
This study assessed chitosan (CS)-based edible films with clove essential oil (CO) and nisin (NI) singly or in combination, for improving quality and shelf life of pork patties stored in cold conditions. The treatments were control (without chitosan film coating), CS, CS-CO, CS-NI, and CS-CO-NI, and these were tested for physicochemical, microbiological and sensory qualities for 15 days (3 days per interval) on samples in cold storage (4 ± 2 °C). Overall, the results showed that the lightness (L* value) (53.47 to 67.58), yellowness (b* value) (1.32 to 2.88), pH (5.31 to 7.98), metmyoglobin (MetMb) content (54.10 to 63.36%), free fatty acid (FFA) (0.67 to 3.17%), peroxide value (PV) (0.80 to 3.67 milliequivalent/100 g), thiobarbituric acid reactive substances (TBARS) (0.69 to 3.27 mg MDA per kg), total viable count (TVC) (2.97 to 7.63 log CFU g−1), psychotrophic bacteria count (psychrotrophs) (2.94 to 6.59 log CFU g−1), Enterobacteriaceae (2.59 to 6.57 log CFU g−1), lactic acid bacteria (LAB) (2.53 to 6.81 log CFU g−1) and sensory scores (red non-discolored part (1 to 4.70), discoloration (1 to 4.40) and off-odor (1 to 5.00)) were gradually increased during storage and whereas redness (a* value) (16.43 to 8.62) and redness index (12.54 to 3.01) were decreased. However, the quality changes were minimal in the pork patties treated with CS-CO-NI. Based on sensory and microbiological evaluations, the shelf life of treated pork patties was 6 days for control, 9 days for CS and CS-NI, and 12 days for CS-CO and CS-CO-NI.
Introduction
Fresh pork meat is a perishable food raw material high in nutrients and moisture, and in lipid and protein. It has a short shelf life of a few days in the refrigerator, mainly limited by microorganism growth and lipid oxidation.1–3 The growth of microorganisms can lead to off flavors, off odors and slime production.4 On the other hand, color, odor and flavor changes from lipid oxidation can limit the shelf life of a food product.5Biodegradable and/or edible films and coatings are receiving increased attention because of environmental concerns, especially the need to reduce the amount of disposable packaging. There is also potential for reducing food spoilage and lipid oxidation, and to prolong the shelf lives of meat and meat products.6 The bio-based edible films and coatings are based on various materials, including proteins, polysaccharides and lipids. Among these, CS-based films or coatings are well-known and widely applied in meat and meat products, due to high antioxidant activity, antimicrobial activity, and barrier properties.7–9 CS is a cationic polymer and derived from the deacetylation process of chitin. It is comprised of copolymers of glucosamine and N-actyl-glucosamine. CS is biodegradable, low toxic, and ability to biocompatible with variety of substances. CS posses excellent water permeability and film forming ability. Due to its abundant oxygen functional groups, it tightly bonds with the substances and avoid relative displacements and deformations and consequently, provide a strong film.10 Several studies have reported that the CS incorporated with some bioactive compounds, such as thyme oil,11,12 cinnamon oil,13,14 sunflower oil,15 oregano oil,16 Zataria multiflora essential oil,17 green tea extract18 and rosemary oil19 could enhance the antioxidant and/or antimicrobial properties of the film and thus, extended the shelf life of meat or meat products.
CO (Syzygium aromaticum, Lin), has eugenol as its dominant active compound, and is a natural essential oil that possesses excellent antioxidant and antibacterial properties.20,21 As CO has been listed as a “Generally Recognized as Safe (GRAS)” substance by the United States Food and Drug Administration, many studies have tested CO, in buffalo meat,22 fresh mutton,23 ground sheep meat,24 pork sausage25 and ground pork meat26 to prolong the shelf life of these products, since it has strong antioxidant and antimicrobial activities. NI, a bacteriocin produced by Lactococcus lactis subsp. lactis, is the first commercial bacteriocin with GRAS status for use in food products in many countries.27,28 It is very active in inhibiting Gram-positive bacteria including Listeria monocytogenes, but only rarely active against Gram-negative bacteria.29 Wang, Yang et al.28 suggested that combinations of NI, potassium sorbate and Salmonella bacteriophage were effective in microbial inhibition, and could extend the shelf life of fresh pork in chilled storage more than NI alone. Theivendran et al.27 also revealed that NI combined with grape seed extract or green tea extract showed higher potential to inhibit L. monocytogenes in turkey frankfurters than NI alone.
Previous studies have investigated the combination effect of CS and CO in cooked pork sausage,25 combination of CS/NI/gallic acid in pork loin,30 combination of NI/tea polyphenols/CS in chilled pork,29 and combination of NI and CO in beef31 and their results found that the combination technique had effectively preserved the qualities and extend shelf life. However, the preservation and shelf life extension of pork patties by a combination of CS, CO and NI would have yet to be demonstrated. Therefore, the objective of this study was to evaluate the effects of CS film with CO and NI, singly or in combination, on physical, chemical, microbiological, and sensory quality of pork patties during refrigerated storage.
Results and discussion
Surface color
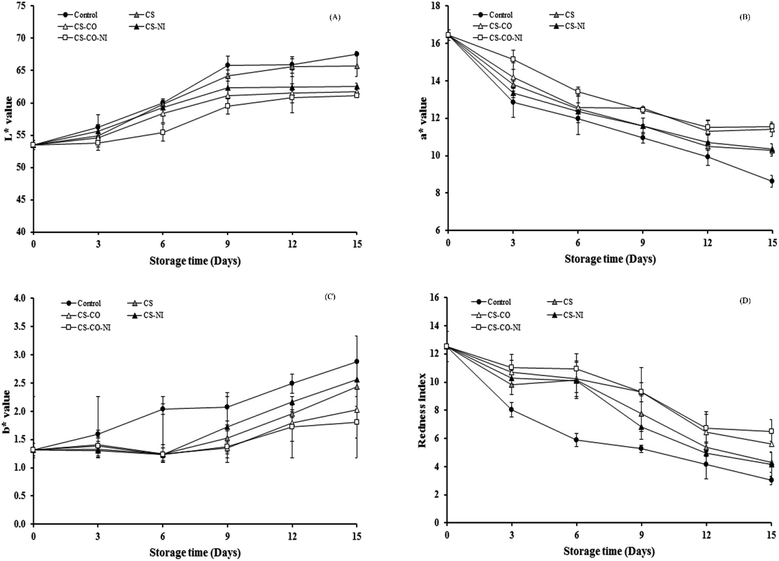
Fig. 1 represents the overview of the coated pork patties and its appearance during storage. The storage period had gradually influenced the appearance of the pork parties in all conditions. However, the treated patties were showed better performance on retaining the appearance as compared to the control. Particularly, CS-CO-NI treated patties retained better appearance compared to the other treatments. Fig. 2 shows changes in the color coordinates (lightness (L*), redness (a*) and yellowness (b*)) and redness index of pork patties with alternative treatments during chilled storage. It was found that L* and b* tended to increase, while a* and redness index decreased with storage time for up to 15 days, in all cases (P < 0.05). This was probably due to lipid oxidation and microbial spoilage, and is similar to the report by Siripatrawan and Noipha18 regarding pork sausages in chilled storage (4 °C) for up to 20 days. The surface color of control treatments rapidly changed more than with CS, CS-NI, CS-CO and CS-CO-NI treatments, and this persisted throughout the storage time. This may be because CS, CO and NI act as preventive antioxidants and antimicrobials. Regarding L* and b*, the L* with all treatments rapidly increased until day-9 (P < 0.05) and then slightly increased (P ≥ 0.05) during further storage; and similarly, b* gradually increased with storage time, indicating that the meat color became paler and brownish. The rates of increase of L* and b* were the lowest with the CS-CO-NI treatment throughout the storage time (Fig. 2A and C). So, the CS-CO-NI treatment slowed down the lightness increase of pork samples. Other studies have reported that CS with NI and gallic acid,30 CS mixed with green tea extract,18 and CS loaded with cinnamon essential oil13 also could slow the increase of L* during chilled storage of pork. Giatrakou et al.11,12 also reported that CS and thyme oil could slow down the increase in L* of a ready-to eat chicken product during chilled storage. The a* and redness index during chilled storage are shown in Fig. 2B and D across the various treatments. Decreasing a* and redness index with storage time were found in all cases. This was probably due to the oxidation of deoxymyoglobin or oxymyoglobin into MetMb.32 In addition, Siripatrawan and Noipha18 indicated that the decrease of redness in meat may be caused by accumulation of hydrogen peroxide from the growth of LAB, which can react with nitric oxide, myoglobin or hemochromogen and produce oxidized porphyrin. Comparing the control with the four other treatments, it was found that the changes in a* and redness index were more rapid in control samples than in samples wrapped with combination of CS, CO or NI throughout the storage time. Moreover, CS-CO and CS-CO-NI samples had only slowly decreasing a*. This suggests that incorporating CO and/or NI in the CS coating can delay the redness changes in pork during storage, probably via effective antioxidant and antimicrobial activities. Based on previous studies, CS acts as an antioxidant18,33 and effectively inhibits the growth of various pathogenic bacteria, yeasts and molds,25,34 as well as LAB.35 CO has been reported to effectively inhibit the growth of pathogenic bacteria and microbial spoilage, and could retard oxidation in meat products.20,25 NI is a commercially available bacteriocin and is characterized as a preservative in various products.30,36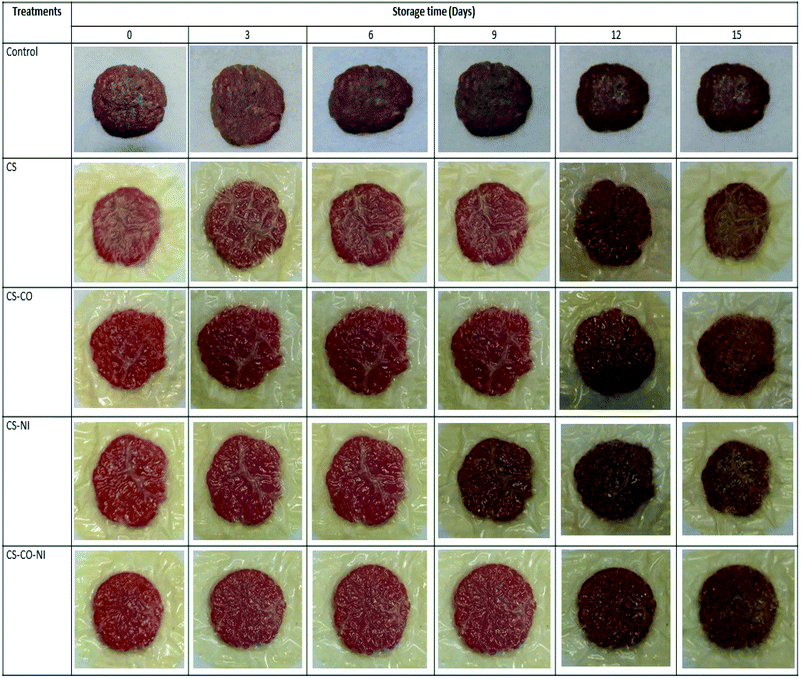 | ||
| Fig. 1 Effect of various treatments on the appearance of pork patties during refrigerated storage for up to 15 days. | ||
 | ||
| Fig. 2 Effect of various treatments on the surface color coordinates L* (A), a* (B) and b* (C) and on redness index (D) of pork patties during refrigerated storage for up to 15 days. | ||
pH and metmyoglobin (MetMb)
The pH and MetMb content of pork patties with alternative treatments during chilled during are shown in Fig. 3. The initial pH of fresh pork was 5.31 and it increased to 7.98, 6.13, 6.00, 6.12 and 6.03 for control, CS, CS-CO, CS-NI and CS-CO-NI treatments, respectively, during 15 days of chilled storage (Fig. 3A). Cao et al.30 also reported that the pH of pork loin coated with CS including gallic acid or NI gradually increased from 5.54 to 5.79 during 20 days of cold storage (2 ± 1 °C). Similar trend was reported by Wang, Xia et al.37 with the pH of lean pork slices coated with CS film containing cinnamon and ginger increasing from 6.01 to 6.75 during refrigerated storage (4 °C) for 9 days. These results agree with those reported by Cao et al.7 for stewed pork, and by Wang, Yang et al.28 for fresh chilled pork. The pH increase with storage time may due to accumulation of the volatile bases ammonia and trimethylamine, produced by either endogenous or microbial enzymes.8 During storage, the pH of pork patties covered with films having CO or NI had slower rate of increased than with control treatment, throughout the storage time. This was probably due to CS, CO, and NI having antimicrobial activity toward various spoilage bacteria, including LAB and volatile basic nitrogen producing microorganisms, thus inhibiting the normal increase of acidity.26,30,38 The initial MetMb content in fresh pork was about 54% and gradually increased during storage with all the treatments (Fig. 3B), as the meat color changed from reddish to brown. This suggests that oxymyoglobin and deoxymyoglobin were oxidized to MetMb, leading to brown discoloration of meat.32 There are several exogenous and endogenous factors affecting this meat color stability, namely prooxidants, antioxidants, lipid oxidation, muscle source, meat species, pH, mitochondrial activity, microbial population, packaging system and storage temperature.32,39 Chan et al.40 mentioned that lipid oxidation products significantly increase the rate of myoglobin oxidation. Hence, myoglobin discoloration might be controlled by retarding the lipid oxidation rate with antioxidants, such as CS and CO. In the present study, the MetMbs increased at slower rate with combination of CS and CO (CS-CO and CS-CO-NI) than with CS or CS-NI treatments. This indicates CO as the most effective antioxidant in the currently studied system. Aliakbarlu and Khalili Sadaghiani24 have also reported that CO could inhibit MetMb formation in ground sheep meat during refrigerated storage for 9 days. Similarly, Kumudavally et al.23 reported that clove extract could reduce the rate of discoloration in fresh mutton during storage at ambient temperature (25 ± 2 °C) for 5 days.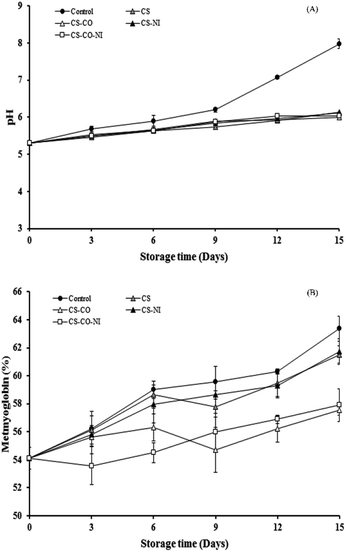 | ||
| Fig. 3 Effect of the various treatments on pH (A) and metmyoglobin (B) in pork patties during refrigerated storage for up to 15 days. | ||
Free fatty acid (FFA), peroxide value (PV) and thiobarbituric acid reactive substances value (TBARS)
The FFA, PV and TBARS of pork patties with different treatments during chilled storage are presented in Fig. 4. The initial FFA content in fresh pork was 0.67% and gradually increased with storage time for up to 15 days in all cases (Fig. 4A), probably due to the lipolytic enzyme activities. Alasnier et al.41 reported on FFA content in rabbit muscles during refrigerated storage, suggesting that the formation of FFA was due to the breakdown of triglycerides and phospholipids. In the present study, the amount of FFA in control treatment changed at a dramatically higher rate than when CS film was used with NI and/or CO, throughout the storage (P < 0.05). This may be related to bacterial load increase. Psychrotrophs, mainly Pseudomonas species, are reported to produce lipases and phospholipases increasing FFA.42,43 The results show that the combination CO/NI/CS could delay FFA formation during storage, by inhibiting the growth of Pseudomonas. On the other hand, the samples treated with CO had major inhibition of microbial growth in this experiment. In a similar report Kumudavally et al.23 have studied fresh mutton. Hydroperoxides are the primary products of lipid oxidation by oxygen attacking polyunsaturated fatty acids. Therefore, measuring peroxide concentration in meat samples seems a reasonable way to assess the extent of oxidation.44 In the present study, the initial PV of the control sample was 0.80 milliequivalent/100 g and gradually increased during 15 days of storage, significantly more than with the other actual treatments (P < 0.05) (Fig. 4B). CO in the CS film (CS-CO and CS-CO-NI) gave stronger resistance to oxidation as indicated by PV than CS or CS-NI at longer storage times. This was probably because CO has a high antioxidant activity, while NI was not effective against lipid oxidation in this study. Zhao et al.29 also reported that treatment with NI could not inhibit lipid oxidation, whereas combination of NI, tea polyphenols and CS could effectively inhibit the lipid oxidation of fresh pork during refrigerated storage. Both CS and CO as preservatives could delay the oxidative rancidity of meat. CS acts as a chelator of transition metal ions from the hemoprotein.33 Eugenol is the major ingredient in CO and could prevent lipid peroxidation in meat samples by acting as a free radical scavenger.45 However, combination of clove oil and CS may have a synergistic effect in improving the oxidative stability of meat samples, as suggested by the present study. Lekjing25 also suggested that the combination of CS and CO was more effective in retarding lipid oxidation in pork sausages than the antioxidant or CS individually. TBARS quantifies secondary lipid oxidation products, such as aldehydes and ketones, that are responsible for undesirable rancid off-odors. The effects of different treatments on TBARS of pork patties over 15 days of chilled storage are shown in Fig. 4C. The initial TBARS of pork patties was 0.69 mg MDA per kg and gradually increased with storage time up to 15 days in all cases. This increase was the most pronounced in the control samples at 15 days of storage, as TBARS reached its higher value (3.27 MDA per kg) than the actual treatments (1.33–2.10 MDA per kg). Treatments with CO (CS-CO and CS-CO-NI) significantly (P < 0.05) lowered TBARS relative to the other actual treatments (CS and CS-NI) throughout the storage time, maybe because CO acted as a very high efficacy antioxidant in these meat samples. Previous studies have been reported that clove oil exhibited the highest effective antioxidant activity in raw and cooked minced chicken meat,46 raw pork,3 and sheep meat.24 In the present study, the combination of CS and CO appeared to have synergy in improving the oxidative stability of pork patties during storage for 15 days. Lekjing25 also found a synergistic antioxidant effect between clove oil and CS in pork sausages. On the other hand, Naveena et al.22 indicated that clove extract and lactic acid were similarly synergistic in buffalo meat. In contrast, there was no difference (P ≥ 0.05) between the TBARS of CS and CS-NI, or between CS-CO and CS-CO-NI (Fig. 4C), which suggests that NI in the CS coating did not affect the TBARS of pork samples under chilled storage. This is similar to the report by Cao et al.,30 on investigating the addition of NI into CS coating for preservation of pork loin under high oxygen modified atmosphere packaging in cold storage for 20 days.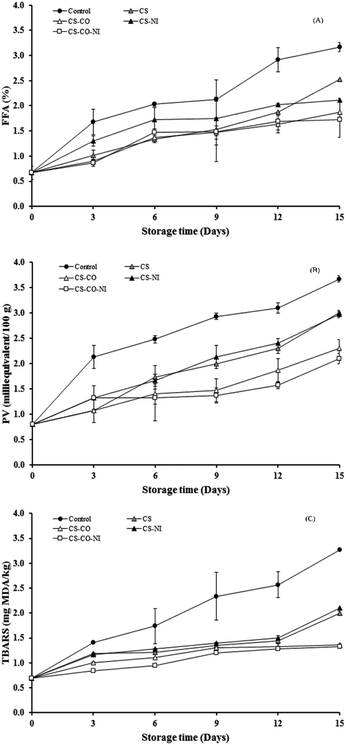 | ||
| Fig. 4 Effect of the various treatments on FFA (A), PV (B), TBARS (C) of pork patties during refrigerated storage for up to 15 days. | ||
Microbiological quality
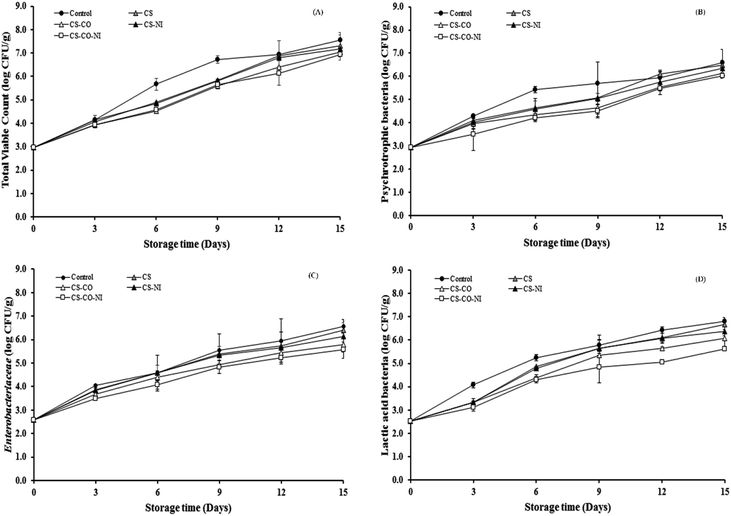
Results of TVC, psychrotrophs, Enterobacteriaceae and LAB in pork patties of various treatment groups during 15 day storage at 4 ± 2 °C are presented in Fig. 5. The initial TVC of fresh pork samples was 2.97 log CFU g−1, which is less than 3.82 and 4.76 log CFU g−1 reported by Pogorzelska et al.47 and Zhao et al.,29 respectively, indicating comparatively low bacterial loads in the pork samples of this current study. The TVC significantly (P < 0.05) increased to 7.63, 7.30, 7.03, 7.18 and 6.95 log CFU g−1 for control, CS, CS-CO, CS-NI and CS-CO-NI, respectively, by day 15 (Fig. 5A). The Department of Medical Sciences of Thailand48 proposed that the TVC limit of 5 × 106 CFU g−1 or of 6.70 log CFU g−1 could be considered the upper acceptable limit for fresh pork meat. In the present study, this limit was exceeded on day 6 in the control sample, on day 9 with CS and CS-NI treatments, and on day 12 with CS-CO and CS-CO-NI treatments. The actual treatments had significantly (P < 0.05) lower TVC than the control samples during the storage period, suggesting that CS coating alone or in combination with CO and/or NI effectively inhibited TVC growth in pork samples. Thus, a 3 to 6 days extension of the microbiological shelf life was achieved with the actual treatments (CS, CS-NI, CS-CO and CS-CO-NI). The psychrotrophs, mainly Pseudomonas spp. as a common foodborne spoilage bacterium, were found in the meat during low temperature storage. Fig. 5B shows time profiles of psychrotrophs in pork patties with different treatments, during chilled storage for up to 15 days. The initial 2.94 log CFU g−1 of psychrotrophs in fresh pork samples gradually increased (P < 0.05) to 6.59, 6.48, 6.13, 6.34 and 6.03 log CFU g−1 for control, CS, CS-CO, CS-NI and CS-CO-NI, respectively, at 15 days. The results indicate that CS-CO-NI combination treatment gave excellent inhibitory effect on the growth of Pseudomonas spp. in chilled pork patties during storage. The Enterobacteriaceae growth counts were also inhibited by the addition of CS, CO and NI, are shown in Fig. 5C. It was found that the initial amount of Enterobacteriaceae in pork patties was 2.59 log CFU g−1 and gradually increased (P < 0.05) to 6.57, 6.40, 5.80, 6.14 and 5.57 log CFU g−1 for control, CS, CS-CO, CS-NI and CS-CO-NI, respectively, at 15 days. During the whole storage, the Enterobacteriaceae counts with CS-CO-NI coating showed a lower value than the other treatments (P < 0.05). Thus, the addition of CS, CO and NI reduced Enterobacteriaceae growth in meat effectively. The amount of LAB in pork patties with the various treatments during cold storage are shown in Fig. 5D. In the present study, the initial population of LAB in fresh pork was 2.53 log CFU g−1, and reached 6.81, 6.68, 6.08, 6.38 and 5.62 log CFU g−1 in control, CS, CS-CO, CS-NI and CS-CO-NI samples, respectively, on day 15 of storage. Furthermore, the CS-CO-NI treatment had the lowest LAB counts throughout the storage time, maybe because of synergistic bactericidal effects in the combination of CS, CO and NI. Of the treatments examined in the present study, CS-CO-NI was the most effective in controlling the microbial growth throughout the tested storage time, probably due to synergistic bactericidal effects of these 3 agents against the microorganisms in pork meat. Many studies have reported that CS has effective antimicrobial activity in meat samples.49,50 It inhibits spoilage microorganisms and pathogens by changing the permeability of the cytoplasmatic membrane, leading to the leakage of intracellular electrolytes and proteinaceous constituents, and finally to cell death.49 Naveena et al.22 and Shan et al.3 have suggested that clove oil acts as an antimicrobial agent, inhibiting the production of amylase and proteases in the cell, inducing cell wall deterioration and a high degree of cell lysis, and preventing enzyme action by binding to proteins. NI has been reported antibacterial against Gram-positive bacteria, such as Listeria spp. and LAB.28,29 The inhibition is achieved by pore formation in bacterial lipid membranes and inhibiting cell wall synthesis through mislocalisation or binding to lipid II that is an essential bacterial cell wall precursor.51Sensory assessment
The results of sensory evaluations of red color, discoloration, off-odor and clove odor in pork patties with various treatments during cold storage are shown in Table 1. All these attributes showed similarly decreasing acceptance (P < 0.05) with storage time, suggesting that microbial growth and lipid oxidation are the major causes of off-odor, off-flavor and discoloration, and can reduce the shelf life of food products.4,5 In the present study, the redness of pork patties in all the treatments faded during storage, probably due to the formation of MetMb by oxidation of oxymyoglobin. The results show that control, CS and CS-NI treatment had more color change by day 6 than the other treatments (day 9 for CS-CO, and day 12 for CS-CO-NI). Considering the score 3 as the threshold for acceptability, the shelf life was 6 days for the control sample, whereas CS, CS-CO, CS-NI and CS-CO-NI samples remained acceptable for up to 12 days of storage. In case of discoloration attribute, the pork patties in all the treatments turned more discolored (became brownish) with storage time, maybe caused by microbial growth and lipid oxidation which are associated with microbial counts and oxidation indicators. It was found that the discoloration score of control treatment changed significantly (P < 0.05) faster (day 6) than other treatments (day 9 for CS, CS-NI, CS-CO and CS-CO-NI). For consumer acceptance (scores < 3), the discoloration scores of control sample were accepted for up to 6 days of storage whereas CS, CS-CO, CS-NI and CS-CO-NI samples were accepted for up to 12 days of storage. Regarding the off-odor attribute, the pork patties in all the treatments got stronger off-odor with storage time, caused by the growth of microorganisms and lipid oxidation (related to colony counts and oxidation indicators). It was found that the off-odor scores of control treatment significantly changed (P < 0.05) faster (day 3) than other actual treatments (day 9 for CS, CS-NI, CS-CO and CS-CO-NI). Based on sensory evaluation, control sample, and those with actual treatments (CS, CS-CO, CS-NI and CS-CO-NI) reached unacceptable sensory scores (score < 3) by days 9 and 15, respectively, suggesting that CS inhibited the growth of microorganisms, and that its antimicrobial properties could be enhanced by CO and/or NI incorporated into the film. Regarding the clove odor attribute, the control, CS and CS-NI did not give detectable clove odor throughout the storage time as they lacked CO. On the other hand, CS-CO and CS-CO-NI treatments had slight clove odor according to the panelists, with no significant changes throughout the storage time (P ≥ 0.05). It can be concluded that the clove odor scores were acceptable throughout the storage in all cases. Naveena et al.22 and Lekjing25 have reported that the addition of 0.1% v/v or 1.5% v/v clove oil in meat samples had a remarkable effect on the color and odor attributes of meat samples. In the present study, clove odor did not strongly affect acceptability of pork patties because it was not directly added into meat, and the concentration of CO added in the film was very low but had antioxidant and antimicrobial activities. Since the meat was considered acceptable when all sensory scores remained better than 3.0, the pork patties could be kept for 6 days with control treatment and for 12 days with the actual treatments (CS, CS-CO, CS-NI and CS-CO-NI). The results suggest that incorporating CO and/or NI into CS film enhanced the antioxidant and antimicrobial properties of the film, helping maintain the quality and prolong the shelf life of pork patties. According to Muzolf-Panek et al.26 and Shan et al.,3 adding clove oil to pork meat could delay lipid oxidation and extend the shelf life. Clove oil was also applied in ground sheep meat to prolong its shelf life at 4 °C.24 Kumudavally et al.23 have reported that adding clove extract was effective in preserving fresh mutton at 25 ± 2 °C for up to 4 days. A combination of lactic acid, clove oil and vitamin C also extended buffalo meat's shelf life by 9 days at 4 ± 1 °C.22 Shelf lives have been extended by a combination of NI, tea polyphenols and CS to fresh chilled pork, for up to 11 days in cold (4 °C) storage.29| Storage time (days)a | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | ||
| a The results are shown in mean ± standard deviation. Different lowercase superscripts indicate significant differences within the same column, and different uppercase superscripts show significant different within the rows. | |||||||
| Red color | Control | 1.00 ± 0.00aA | 1.30 ± 0.48aA | 2.40 ± 0.52bB | 3.20 ± 0.42cC | 4.10 ± 0.88cD | 4.70 ± 0.48bE |
| CS | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.50 ± 0.53aB | 2.00 ± 0.47bC | 2.70 ± 0.48bD | 3.70 ± 0.67aE | |
| CS-CO | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.20 ± 0.42aAB | 1.50 ± 0.53aB | 2.00 ± 0.47aC | 3.30 ± 0.48aD | |
| CS-NI | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.40 ± 0.52aB | 2.10 ± 0.57bC | 2.60 ± 0.52bD | 3.80 ± 0.42aE | |
| CS-CO-NI | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.20 ± 0.42aA | 1.40 ± 0.52aA | 1.90 ± 0.57aB | 3.40 ± 0.70aC | |
| Discoloration | Control | 1.00 ± 0.00aA | 1.10 ± 0.32aA | 1.30 ± 0.48bB | 3.00 ± 0.00dC | 4.20 ± 0.79cD | 4.40 ± 0.52bD |
| CS | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.30 ± 0.48aA | 1.80 ± 0.42bcB | 2.50 ± 0.53bC | 3.80 ± 0.79aD | |
| CS-CO | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.10 ± 0.32aA | 1.50 ± 0.53abBC | 1.80 ± 0.63aC | 3.50 ± 0.71aD | |
| CS-NI | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.40 ± 0.52aA | 1.90 ± 0.32cB | 2.60 ± 0.52bC | 3.70 ± 0.67aD | |
| CS-CO-NI | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.10 ± 0.32aA | 1.40 ± 0.52aBC | 1.60 ± 0.52aC | 3.40 ± 0.70aD | |
| Off odor | Control | 1.00 ± 0.00aA | 1.60 ± 0.52bB | 2.40 ± 0.52bC | 3.10 ± 0.32cD | 4.60 ± 0.52cE | 5.00 ± 0.00dF |
| CS | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.30 ± 0.48aA | 1.80 ± 0.42abB | 2.70 ± 0.48bC | 3.80 ± 0.63cD | |
| CS-CO | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.10 ± 0.32aA | 1.50 ± 0.53abB | 2.00 ± 0.47aC | 3.30 ± 0.48abD | |
| CS-NI | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.30 ± 0.48aA | 1.90 ± 0.32bB | 2.60 ± 0.52bC | 3.70 ± 0.67bcD | |
| CS-CO-NI | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.10 ± 0.32aAB | 1.40 ± 0.52aB | 1.80 ± 0.42aC | 3.20 ± 0.42aD | |
| Clove odor | Control | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA |
| CS | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | |
| CS-CO | 1.60 ± 0.52bA | 1.50 ± 0.53bA | 1.50 ± 0.53bA | 1.30 ± 0.48bA | 1.30 ± 0.48bA | 1.20 ± 0.42abA | |
| CS-NI | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | 1.00 ± 0.00aA | |
| CS-CO-NI | 1.60 ± 0.52bA | 1.60 ± 0.52bA | 1.40 ± 0.52bA | 1.50 ± 0.53bA | 1.50 ± 0.53bA | 1.40 ± 0.52bA | |
Experimental
Chemicals and media
The food grade CS powder with 200 mesh particle size had molecular weight of 8.97 × 105 DA and 80% degree of deacetylation. It was purchased from Sinudom Agriculture Products Co., Ltd. (Surat Thani, Thailand). NI was provided by Shandong Freda Biotechnology Co., Ltd. (Shandong, China). CO (Syzygium aromaticum, Lin), with eugenol content in 70–80% range (manufacturer's data) was purchased from Thai China Flavors and Fragrances Industry Co., Ltd. (Bangkok, Thailand). The chemical agents used were analytical grade: chloroform, dipotassium hydrogen phosphate, phenolphthalein, methanol, potassium iodide, trichloroacetic acid, hydrochloric acid, 2-propanal, sodium hydroxide, sodium thiosulfate, thiobarbituric acid, potassium dihydrogen phosphate, anhydrous sodium sulfate, (Merck, Darmstadt, Germany), acetic acid (Lab-Scan, Bangkok, Thailand), glycerol (Vidhyasom, Thailand), Tween 80 (Labchem, Australia), palmitic acid, and 1,1,3,3-tetramethoxypropane (Sigma-Aldrich, St. Louis., MO, USA). The media for microbiological analyses were analytical grade: plate count agar; peptone; deMan, Rogosa, and Sharpe (MRS) agar; and violet red bile glucose agar (VRBG) (Merck, Darmstadt, Germany).Preparation of film
CS-based edible film was prepared according to the method of Lekjing.25 A CS solution (2% w/v) was prepared by dispersing 2 g of CS powder in 100 ml of glacial acetic acid (1% w/v) and stirring overnight at room temperature. To prepare CS solutions with CO and NI, 0.5 ml of glycerol per g CS and 0.1% w/v of Tween 80 were added to the CS solution. Following the addition of plasticizer, stirring was continued for a further 30 min. Then, CO and/or NI were added to the CS solution with stirring until fully dissolved. The concentrations of CO and NI were 6400 μg ml−1 and 204![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 IU ml−1, respectively, based on preliminary experiments including sensory analysis, film properties analysis and minimum inhibitory concentration (MIC) determination. The MICs of CO against Staphylococcus aureus, Salmonella typhimurium, Escherichia coli and Listeria monocytogenes were 800, 3,200, 3200 and 1600 μg ml−1, respectively. The MICs of NI against S. aureus, S. typhimurium, E. coli and L. monocytogenes were 25
800 IU ml−1, respectively, based on preliminary experiments including sensory analysis, film properties analysis and minimum inhibitory concentration (MIC) determination. The MICs of CO against Staphylococcus aureus, Salmonella typhimurium, Escherichia coli and Listeria monocytogenes were 800, 3,200, 3200 and 1600 μg ml−1, respectively. The MICs of NI against S. aureus, S. typhimurium, E. coli and L. monocytogenes were 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, 25
600, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, 102
600, 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and 51
400 and 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 IU ml−1, respectively. The following 4 solutions were prepared: (i) CS, CS film without antibacterial agents; (ii) CS-CO, CS film with CO; (iii) CS-NI, CS film with NI; (iv) CS-CO-NI, CS film with CO and NI. Then, fifteen mL of a film forming solution was cast on a 10 cm diameter Petri dish and dried in an oven at 40 °C for 5 h. The dry films were then peeled off and stored in low density polyethylene (LDPE) Ziplock bags in desiccators at 25 ± 2 °C and 50% relative humidity until further experiments.
200 IU ml−1, respectively. The following 4 solutions were prepared: (i) CS, CS film without antibacterial agents; (ii) CS-CO, CS film with CO; (iii) CS-NI, CS film with NI; (iv) CS-CO-NI, CS film with CO and NI. Then, fifteen mL of a film forming solution was cast on a 10 cm diameter Petri dish and dried in an oven at 40 °C for 5 h. The dry films were then peeled off and stored in low density polyethylene (LDPE) Ziplock bags in desiccators at 25 ± 2 °C and 50% relative humidity until further experiments.
Preparation of pork patties
Fresh pork meat (Longissimus thoracis and/or Longissimus lumborum as described by Kauffman et al.52) was purchased from a local processor and after that the meat was trimmed to remove visible connective tissue as well as subcutaneous and intramuscular fat. Then, the meat was ground through 4 mm plates. After mincing the samples were mixed with 2% sodium chloride. Fifteen-gram pork patties were shaped by hand to approximately 5 cm diameter and 1 cm thickness. The patties were randomly assigned to five alternative treatments: control group, CS group, CS-CO group, CS-NI group and CS-CO-NI group. All the samples were placed in plastic boxes and stored at 4 ± 2 °C for up to 15 days. Samples were taken for physical, chemical, microbiological and sensory quality analyses every 3 days. The above experiment was carried out in duplicate on the same day of preparation.Physical quality analysis
Surface color measurement. Pork patties from each treatment group were measured for the L*, a* and b* CIElab color coordinates, using a HunterLab colorimeter (MiniScan EZ, USA) that had been calibrated with a standard black and white plate. The illuminant used was *C (D65), the standard observer angle was 10°, and the aperture was 2.5 cm. The redness index was calculated as the ratio a*/b*, as described by Chen et al.53Chemical quality analyses
| MetMb (%) = {1.395 − [(A572 − A700)/(A525 − A700)]} × 100 |
Microbiological quality analyses
TVC and psychrotrophs were determined according to the method of BAM.61 The TVC and psychrotrophs were performed by the pour plate method, using plate count agar incubated at 35 °C for 48 h, or at 7 °C for 10 days. The counts are expressed in log CFU g−1.The food spoilage microorganisms Enterobacteriaceae and LAB were determined according to the method of Radha Krishnan et al.,62 performed by the spread on an agar plate. The plate counting used VRBG agar incubated at 37 °C after 24 h for Enterobacteriaceae and MRS agar incubated at 30 °C after 72 h for LAB. The microbial colonies were counted, and results are expressed in log CFU g−1.
Sensory evaluation
Sensory evaluation by a 10-member panel (5 males and 5 females with ages ranging from 20 to 40 years) was performed to estimate the shelf life of pork patties using a 5-point descriptive scale. The attributes were modified from Camo et al.55 The attribute ‘red color’ was scored in the red non-discolored part of the pork, using a 5-point scale for intensity, with 1 for extremely brilliant fresh meat red and 5 for extremely faded red. Scores for ‘discoloration’ were based on area fraction of discolored surface: 1 = none, 2 = 0–10%, 3 = 11–20%, 4 = 21–60%, and 5 = 61–100%. Scores for off-odor referred to the intensity of odors associated with meat oxidation: 1 = none; 2 = slight; 3 = small; 4 = moderate; and 5 = extreme. The clove odor attribute referred to the intensity of clove odor perception after pack opening: 1 = none; 2 = slight; 3 = small; 4 = moderate; and 5 = extreme. A score of 3 or higher in any of the attributes was chosen to make the sample unacceptable to consumers.Statistical analysis
In this study, the data were analyzed statistically by the Statistical Package for Social Science (SPSS) software (v16.0 for windows) using the factorial arrangements (5 treatments × 6 storage times) and focusing on the interaction within the treatments and storage time. Analyses such as color were tested of six replications, and the other parameters such as pH, MetMb, FFA, PV, TBARS, TVC, psychrotrophs, Enterobacteriaceae and LAB were tested of three replications. A completely randomized design was applied for physicochemical and microbial qualities, and whereas sensory analysis (test day and panelists) was analyzed (10 replications) using the randomized complete block design. The data are shown in mean ± standard deviation (SD). Analysis of variance (ANOVA) and Duncan's multiple range test was used to evaluate the significances of differences between mean values and the statistical significance was set to 5% level (P < 0.05).Conclusions
The present study observed that the application of CS and combination of CS, CO, NI had efficiently prevented the quality loss of pork patties. The CO was stronger in antioxidant and antimicrobial activities than CS or NI, when tested in treatments of pork patties. However, a CS-based film containing clove oil and NI (CS-CO-NI) had shown possible synergistic effects on the antioxidative and antimicrobial activities in preserving pork patties. It can be evidenced by the lower changes in MetMb, FFA, PV, TBARS in CS-CO-NI treated patties. Furthermore, based on the sensory and microbiological evaluations, the combination of CS-CO-NI could prolong the shelf life about twofold over the control treatment (6 days for control and 12 days for CS-CO-NI film coating).Conflicts of interest
This work has no conflict of interest to declare.Acknowledgements
The authors gratefully acknowledge the Prince of Songkla University, Grant SIT601297S, and the Prince of Songkla University, Surat Thani campus, 2016 for financial support. Furthermore, the authors would like to sincerely thank Food Innovation and Product Development Laboratory for the experiments and for equipment support.References
- A. B. Falowo, P. O. Fayemi and V. Muchenje, Food Res. Int., 2014, 64, 171–181 CrossRef CAS PubMed.
- V. Pothakos, F. Devlieghere, F. Villani, J. Björkroth and D. Ercolini, Meat Sci., 2015, 109, 66–74 CrossRef CAS PubMed.
- B. Shan, Y. Z. Cai, J. D. Brooks and H. Corke, J. Sci. Food Agric., 2009, 89(11), 1879–1885 CrossRef CAS.
- L. Gram, L. Ravn, M. Rasch, J. B. Bruhn, A. B. Christensen and M. Givskov, Int. J. Food Microbiol., 2002, 78(1–2), 79–97 CrossRef PubMed.
- C. Faustman and R. G. Cassens, J. Muscle Foods, 1990, 1(3), 217–243 CrossRef.
- G. H. Zhou, X. L. Xu and Y. Liu, Meat Sci., 2010, 86(1), 119–128 CrossRef CAS PubMed.
- Y. Cao, W. Gu, J. Zhange, Y. Chu, X. Ye, Y. Hu and J. Chen, Food Chem., 2013, 141, 1655–1660 CrossRef CAS PubMed.
- W. Fan, J. Sun, Y. Chen, J. Qiu, Y. Zhang and Y. Chi, Food Chem., 2009, 115(1), 66–70 CrossRef CAS.
- M. T. Yen, J. H. Yang and J. L. Mau, Carbohydr. Polym., 2009, 75(1), 15–21 CrossRef CAS.
- J. Shi, H. Kang, N. Li, K. Teng, W. Sun, Z. Xu, X. Qian and Q. Liu, Appl. Surf. Sci., 2019, 478, 38–48 CrossRef CAS.
- V. Giatrakou, A. Ntzimani and I. N. Savvaidis, Food Microbiol., 2010, 27(1), 132–136 CrossRef CAS PubMed.
- V. Giatrakou, A. Ntzimani, M. Zwietering and I. N. Savvaidis, J. Food Prot., 2010, 73, 663–669 CrossRef CAS PubMed.
- J. Hu, X. Wang, Z. Xiao and W. Bi, LWT - Food Sci. Technol., 2015, 63(1), 519–526 CrossRef CAS.
- S. M. Ojagh, M. Rezaei, S. H. Razavi and S. M. H. Hosseini, Food Chem., 2010, 120(1), 193–198 CrossRef CAS.
- M. Vargas, A. Albors and A. Chiralt, Procedia Food Sci., 2011, 1, 39–43 CrossRef CAS.
- S. Petrou, M. Tsiraki, V. Giatrakou and I. N. Savvaidis, Int. J. Food Microbiol., 2012, 156(3), 264–271 CrossRef CAS PubMed.
- B. Bazargani-Gilani, J. Aliakbarlu and H. Tajik, Innovative Food Sci. Emerging Technol., 2015, 29, 280–287 CrossRef CAS.
- U. Siripatrawan and S. Noipha, Food Hydrocolloids, 2012, 27(1), 102–108 CrossRef CAS.
- G. C. Vasilatos and I. N. Savvaidis, Int. J. Food Microbiol., 2013, 166(1), 54–58 CrossRef CAS PubMed.
- S. Burt, Int. J. Food Microbiol., 2004, 94(3), 223–253 CrossRef CAS PubMed.
- I. Gülçin, M. Elmastaş and H. Y. Aboul-Enein, Arabian J. Chem., 2012, 5(4), 489–499 CrossRef.
- B. M. Naveena, M. Muthukumar, A. R. Sen, Y. Babji and T. R. K. Murthy, Meat Sci., 2006, 74(2), 409–415 CrossRef CAS PubMed.
- K. V. Kumudavally, A. Tabassum, K. Radhakrishna and A. S. Bawa, J. Food Sci. Technol., 2011, 48(4), 466–471 CrossRef CAS PubMed.
- J. Aliakbarlu and S. Khalili Sadaghiani, J. Food Qual., 2015, 38(4), 240–247 CrossRef CAS.
- S. Lekjing, Meat Sci., 2016, 111, 192–197 CrossRef CAS PubMed.
- M. Muzolf-Panek, A. Kaczmarek, J. Tomaszewska-Gras, R. Cegielska-Radziejewska and M. Majcher, Int. J. Food Prop., 2019, 22(1), 111–129 CrossRef CAS.
- S. Theivendran, N. S. Hettiarachchy and M. G. Johnson, J. Food Sci., 2006, 71(2), M39–M44 CrossRef CAS.
- C. Wang, J. Yang, X. Zhu, Y. Lu, Y. Xue and Z. Lu, Food Contr., 2017, 73, 869–877 CrossRef CAS.
- S. Zhao, N. Li, Z. Li, H. He, Y. Zhao, M. Zhu, Z. Wang, Z. Kang and H. Ma, Int. J. Food Prop., 2019, 22(1), 1047–1063 CrossRef CAS.
- Y. Cao, R. D. Warner and Z. Fang, Food Contr., 2019, 101, 9–16 CrossRef CAS.
- Z. Koplay and C. Sezer, Ataturk Univ. Vet. Bilim. Derg., 2013, 8(1), 9–19 Search PubMed.
- R. A. Mancini and M. C. Hunt, Meat Sci., 2005, 71(1), 100–121 CrossRef CAS PubMed.
- M. T. Yen, J. H. Yang and J. L. Mau, Carbohydr. Polym., 2008, 74(4), 840–844 CrossRef CAS.
- S. R. Kanatt, R. Chander and A. Sharma, Food Chem., 2008, 107, 845–852 CrossRef CAS.
- V. Coma, A. Martial-Giros, S. Garreau, A. Copinet, F. Salin and A. Deschamps, J. Food Sci., 2002, 67, 1162–1168 CrossRef CAS.
- L. He, L. Zou, Q. Yang, J. Xia, K. Zhou, Y. Zhu, X. Han, B. Pu, B. Hu, W. Deng and S. Liu, J. Food Sci., 2016, 81(6), M1466–M1471 CrossRef CAS PubMed.
- Y. Wang, Y. Xia, P. Zhang, L. Ye, L. Wu and S. He, Food Bioprocess Technol., 2017, 10, 503–511 CrossRef CAS.
- A. Ruiz, S. K. Williams, N. Djeri, A. Hinton Jr and G. E. Rodrick, Poult. Sci., 2010, 89(2), 353–358 CrossRef CAS PubMed.
- S. P. Suman and P. Joseph, Annu. Rev. Food Sci. Technol., 2013, 4, 79–99 CrossRef CAS PubMed.
- W. K. M. Chan, C. Faustman and E. A. Decker, J. Food Sci., 1997, 62, 709–712 CrossRef CAS.
- C. Alasnier, E. Devid-Briand and G. Gandemer, Meat Sci., 2000, 54, 127–134 CrossRef CAS.
- Y. J. Chung Wang, M. E. Bailey and R. T. Marshall, J. Appl. Microbiol., 1997, 82, 317–324 CrossRef CAS PubMed.
- R. Koka and B. C. Weimer, J. Dairy Res., 2001, 68, 109–116 CrossRef CAS PubMed.
- J. Choe, H. Kim and C. Kim, Korean Journal for Food Science of Animal Resources, 2017, 37(2), 254–263 CrossRef PubMed.
- K. G. Lee and T. Shibamoto, Food Chem., 2001, 74(4), 443–448 CrossRef CAS.
- S. S. L. A. El-Alim, A. Lugasi, J. Hóvári and E. Dworschák, J. Sci. Food Agric., 1999, 79(2), 277–285 CrossRef.
- E. Pogorzelska, J. Godziszewska, M. Brodowska and A. Wierzbicka, Meat Sci., 2018, 135, 54–61 CrossRef CAS PubMed.
- Department of Medical Sciences, The criteria of food microbiological quality and food containers, Department of Medical Sciences, Ministry of Public Health, Bangkok, Thailand, 2017 Search PubMed.
- K. V. Harish Prashanth and R. N. Tharanathan, Trends Food Sci. Technol., 2007, 18(3), 117–131 CrossRef.
- E. Sayas-Barberá, J. Quesada, E. Sánchez-Zapata, M. Viuda-Martos, F. Fernández-López, J. A. Pérez-Alvarez and E. Sendra, Meat Sci., 2011, 88(4), 740–749 CrossRef PubMed.
- H. Zhou, J. Fang, Y. Tian and X. Y. Lu, Ann. Microbiol., 2014, 64(2), 413–420 CrossRef CAS.
- R. Kauffman, R. E. Habel, F. M. Smulders, P. L. Bergstrom and W. Hartman, Meat Sci., 1990, 28(3), 259–265 CrossRef.
- H. H. Chen, E. M. Chiu and J. R. Huang, J. Food Sci., 1997, 62(5), 985–991 CrossRef CAS.
- S. Songsaeng, P. Sophanodora, J. Kaewsrithong and T. Ohshima, Food Chem., 2010, 123(2), 286–290 CrossRef CAS.
- J. Camo, A. Lorés, D. Djenane, J. A. Beltrán and P. Roncalés, Meat Sci., 2011, 88(1), 174–178 CrossRef CAS PubMed.
- K. Krzywicki, Meat Sci., 1982, 7, 29–35 CrossRef CAS.
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37(8), 911–917 CrossRef CAS PubMed.
- AOCS, The official methods and recommended practices of the American Oil Chemists' Society, The American Oil Chemists' Society, Champaign, I.L., 1994 Search PubMed.
- L. K. Low and C. S. Ng, Determination of peroxide value, Marine Fisheries Research Department, Southeast Asian Fisheries Development Center, Singapore, 1978, pp. C7.1–C7.3 Search PubMed.
- J. A. Buege and S. D. Aust, Methods Enzymol., 1978, 52, 302–310 CAS.
- BAM, http://www.cfsan.fda.gov/%7Eebam/bam-3.htm, 2001.
- K. Radha Krishnan, S. Babuskin, P. Azhagu Saravana Babu, M. Sasikala, K. Sabina, G. Archana, M. Sivarajan and M. Sukumar, Int. J. Food Microbiol., 2014, 171, 32–40 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |