Open Access Article
Open Access ArticleFacet-, composition- and wavelength-dependent photocatalysis of Ag2MoO4†
Lucas Warmuth,
Christian Ritschel and
Claus Feldmann *
*
Institut für Anorganische Chemie, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, D-76131 Karlsruhe, Germany. E-mail: claus.feldmann@kit.edu; Tel: +49-721-60842855
First published on 14th May 2020
Abstract
Faceted β-Ag2MoO4 microcrystals are prepared by controlled nucleation and growth in diethylene glycol (DEG) or dimethylsulfoxide (DMSO). Both serve as solvents for the liquid-phase synthesis and surface-active agents for the formation of faceted microcrystals. Due to its reducing properties, truncated β-Ag2MoO4@Ag octahedra are obtained in DEG. The synthesis in DMSO allows avoiding the formation of elemental silver and results in β-Ag2MoO4 cubes and cuboctahedra. Due to its band gap of 3.2 eV, photocatalytic activation of β-Ag2MoO4 is only possible under UV-light. To enable β-Ag2MoO4 for absorption of visible light, silver-coated β-Ag2MoO4@Ag and Ag2(Mo0.95Cr0.05)O4 with partial substitution of [MoO4]2− by [CrO4]2− were prepared, too. The photocatalytic activity of all the faceted microcrystals (truncated octahedra, cubes, cuboctahedra) and compositions (β-Ag2MoO4, β-Ag2MoO4@Ag, β-Ag2(Mo0.95Cr0.05)O4) is compared with regard to the photocatalytic decomposition of rhodamine B and the influence of the respective faceting, composition and wavelength.
Introduction
Nanosized molybdenum and tungsten oxides have proven to be interesting with regard to a wide range of properties, including catalysis, applicability in high-power batteries and sensing, and luminescence.1 Beside the binary MoO3 and WO3,2 ternary molybdate and tungstate phases are intensely studied as well (e.g., CaWO4, CdMoO4, CoMoO4).3 With regard to their properties, photocatalysis can be considered as particularly important. Special attention has been recently paid to molybdates/tungstates with closed-shell and lone-pair containing metal cations like Pb(II), Sn(II) or Bi(III) due to their unique electronic and structural features mediated by the “non-bonding” electron pairs.3f,4 In this regard, especially, bismuth molybdate and bismuth tungstate have been intensely studied.5 For efficient photocatalysis, moreover, faceted microcrystals have turned out to be most promising.6Aiming at molybdates/tungstates with closed-shell cations, silver molybdate has been also classified to be an catalytically interesting material.7 The cubic β-Ag2MoO4 exhibits a direct band gap of 3.2 eV,8 which is too high in energy for visible-light activation, whereas a band gap of 1.26 eV was reported for the tetragonal α-Ag2MoO4 phase.9 However, α-Ag2MoO4 is metastable and was yet only described twice as a nanomaterial.9,10 The thermodynamically most stable β-Ag2MoO4 is already available with different morphologies, including spheres, rods, cylinders as well as octahedra and cube-like shapes.11 To this concern, microemulsions,11b hydrothermal and solvothermal synthesis were applied with water, methanol or ethanol heated in autoclaves up to temperatures of 180 °C.8,11a,c–e Shape control and formation of specific facets were predominately initiated by high-molecular-weight surface-active agents like as polyvinylpyrrolidone (PVP) or 2,3-bis(2-pyridyl)pyrazine (BPP).10,11a,c–e Despite of successful shape control, such high-molecular-weight stabilizers suffer from the necessity of their removal after the synthesis, since the photocatalytically active surface is otherwise blocked by the stabilizer. Motivated by the great interest in photocatalytic nanomaterials, we have already studied niobates, molybdates and tungstates such as Au@Nb@HxK1−xNbO3 nanopeapods,12 CuMoO4 nanoparticles,13 or faceted β-SnWO4.14 In this study, we report on a controlled liquid-phase synthesis of faceted β-Ag2MoO4 microcrystals, the chromate-driven red-shift of the light absorption as well as on the facet-, composition- and wavelength-depending photocatalysis.
Experimental
Material synthesis
| Morphology | Type of solvent | Temperature of injection/°C | Time of cooling/h | Composition |
|---|---|---|---|---|
| Sample color | ||||
| Spheres | DEG | 80 | 1.0 | β-Ag2MoO4@Ag |
| Greyish | ||||
| Truncated octahedra | DEG | 80 | 2.5 | β-Ag2MoO4@Ag |
| Greyish | ||||
| Spheres | DMSO/H2O | 0–80 | 1.0–2.5 | β-Ag2MoO4 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 to 80 80 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
Colorless | |||
| Cubes | DMSO/H2O | 0 | 2.5 | β-Ag2MoO4 |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
Colorless | |||
| Cuboctahedra | DMSO/H2O | 50 | 2.5 | β-Ag2MoO4 |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
Colorless | |||
| Cuboctahedra | DMSO/H2O | 50 | 2.5 | Ag2(Mo0.95Cr0.05)O4 |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
Yellow |
Analytical methods
Field emission scanning electron microscopy (FESEM, Zeiss Supra 40VP equipped with energy dispersive X-ray spectrometer, EDXS) was used to determine the morphology of the as-prepared faceted β-Ag2MoO4 microcrystals. The microcrystals were suspended in ethanol, dropped and dried on sliced pieces of a silicon wafer (5 × 5 mm).Crystallinity and chemical composition of the faceted β-Ag2MoO4 microcrystals were examined by XRD (Stoe STADI-MP diffractometer, equipped with Ge-monochromator and Cu-Kα1 radiation, 40 kV, 40 mA). About 10 mg of the microcrystals were deposited onto acetate foil and fixed with scotch tape.
To determine the zeta potential of microcrystal β-Ag2MoO4 suspensions, a Zetasizer Nano ZS from Malvern Instruments was used. About 1 mg of the microcrystals was suspended in water and ultrasonicated for 5 min prior to the analysis.
UV-Vis spectra of β-Ag2MoO4 microcrystals were recorded on a UV 2700 spectrometer (Shimadzu). 4–8 mg of the microcrystals were mixed with 100–120 mg of dried BaSO4 (spectroscopic grade) and measured against dried BaSO4 as a reference. The determination of the band gap was conducted based on the Kubelka–Munk formalism and Tauc plots.
Fourier-transform infrared (FT-IR) spectroscopy was performed with a Bruker Vertex 70 FT IR spectrometer. To this concern, the microcrystals were grounded with dried KBr (1–2 mg with 400 mg KBr) and pressed for 15 min under 50 kN.
Volumetric nitrogen sorption measurements (BEL BELSORP-max) were carried out at 77 K with nitrogen as analyte. According to the BET formalism (BET: Brunauer–Emmett–Teller) the specific surface area was deduced. Prior to the analysis, the microcrystalline samples were dried at 120 °C in vacuum.
Photocatalytic measurements
The photocatalytic dye degradation was performed with a Shimadzu Excalibur measuring head linked to the aforementioned UV-Vis spectrometer. For measurement, the quartz flask contained an aqueous suspension of the respective faceted β-Ag2MoO4 microcrystals (20 mg in 110 mL; 0.18 g L−1) and RhB (0.20 mg L−1). This suspension was continuously mixed with a magnetic stirrer. For photocatalytic degradation, a LOT Class ABA solar simulator (type LS0805) was applied that emits a spectrum of AM 1.5 G solar light (100 mW cm−2). Beside illumination with simulated sunlight, cut-off filters (Horiba, <350 nm and <400 nm) were applied to cut-off UV or UV and blue light. Prior to illumination, the suspensions were magnetically stirred in the dark for 1 h to ensure homogenous distribution of the photocatalyst and the dye. All experiments were carried out at room temperature and in air. The course of the RhB degradation was continuously monitored by comparing the intensity of the strongest dye absorption (λRhB = 554 nm) with the initial absorption at the beginning of the experiment. The obtained transmission-time plots were fitted by linear or allometric fits. For reasons of clarity, only every tenth raw data point is shown.Results and discussion
Morphology control
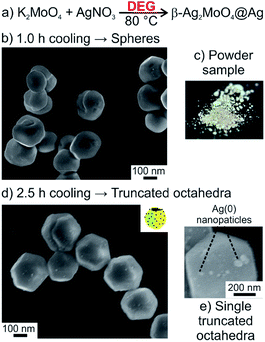
Based on our knowhow on the synthesis of molybdate and tungstate nanoparticles in high-boiling alcohols (so-called polyol synthesis)13,15 and the realization of faceted microcrystals of β-SnWO4,14 we have tried to prepare Ag2MoO4 nanoparticles via a polyol-mediated approach. For this purpose, diethylene glycol (DEG) was chosen (Fig. 1a). In general, the advantage of DEG as a water-comparable but high-boiling solvent relates to its moderately coordinating properties, so that additional high-molecular-weight surface-active agents are not required. K2MoO4 was selected as starting material containing the required tetrahedral [MoO4]2− anion. Here, it needs to be noticed that starting materials containing octahedral (MoO6) building units (e.g. (NH4)6Mo7O24) show inappropriate solubility and often result in incomplete reactions with several by-products.For the synthesis, a concentrated aqueous solution of AgNO3 was injected at a temperature high enough for the crystallization of β-Ag2MoO4 (≥80 °C) but low enough to avoid instantaneous DEG-driven reduction of Ag+ (<100 °C) (Table 1, compare Fig. 5a). Fast cooling (1.0 hour) indeed results in highly crystalline β-Ag2MoO4 but predominately yields non-faceted, spherical particles with diameters of 200–300 nm (Fig. 1b). Slow cooling (2.5 hours), in contrast, supports facet formation and results in truncated β-Ag2MoO4 octahedra with edge lengths of 100 nm (Fig. 1d). For both the spherical particles and the truncated octahedra, however, certain formation of silver could not be avoided. Its formation is qualitatively indicated by the greyish color of the samples (Fig. 1c) and can be quantified by UV-Vis spectra displaying the characteristic plasmon-resonance absorption of nanosized silver (Table 1, compare Fig. 5b).16 Moreover, silver nanoparticles, 32 ± 13 nm in diameter, are clearly visible on SEM images as bright spots on the surface of the β-Ag2MoO4 microcrystals (Fig. 1e). Since visible light could be absorbed via the plasmon-resonance absorption, these Ag(0) nanoparticle-coated β-Ag2MoO4 microcrystals (Ag2MoO4@Ag) nevertheless be relevant for photocatalysis.
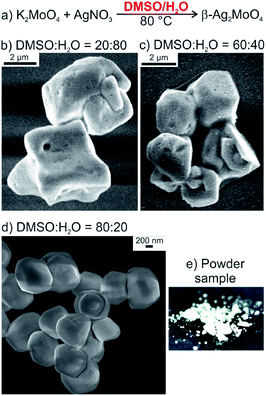
Aiming at pure, faceted β-Ag2MoO4 microcrystals without elemental silver, we have modified the synthesis. In order to maintain the polarity of the solvent in regard of the solubility of the starting materials but to avoid the reducing properties of DEG, we have selected a mixture of dimethylsulfoxide (DMSO) and water as alternative liquid phase (Fig. 2a). Highly crystalline β-Ag2MoO4 was indeed obtained upon injection of aqueous AgNO3 into a solution of KMoO4 in DMSO/water at a temperature of 0 to 50 °C (Table 1). The shape of the resulting Ag2MoO4 turned out to be highly dependent on the DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio. Thus, more-or-less spherical microcrystals were formed at high water contents (Table 1, Fig. 2b and c). Optimal conditions for the formation of well-faceted microcrystals including cuboctahedra and cubes were observed for a DMSO
water ratio. Thus, more-or-less spherical microcrystals were formed at high water contents (Table 1, Fig. 2b and c). Optimal conditions for the formation of well-faceted microcrystals including cuboctahedra and cubes were observed for a DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 85
H2O ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (Table 1 and Fig. 2d). In addition to the formation of specific crystal facets, the size of the microcrystals was significantly reduced from >5 μm to 1–2 μm upon decreasing the amount of water (Fig. 2d). The water content is limited to a minimum of 15% due to the poor solubility of K2MoO4. Finally, it should be noticed that the formation of elemental silver was prevented with these experimental conditions of the DMSO-mediated synthesis. Thus, the β-Ag2MoO4 microcrystals are colorless and do not show any plasmon-resonance absorption (Table 1 and Fig. 2e, compare Fig. 5b). They also do not show Ag(0)-related spots on electron-microscopy images (Fig. 2b–d). Small Ag(0)-related spots only appear on electron-microscopy images after certain period of exposition under the electron beam (compare Fig. 4).
15 (Table 1 and Fig. 2d). In addition to the formation of specific crystal facets, the size of the microcrystals was significantly reduced from >5 μm to 1–2 μm upon decreasing the amount of water (Fig. 2d). The water content is limited to a minimum of 15% due to the poor solubility of K2MoO4. Finally, it should be noticed that the formation of elemental silver was prevented with these experimental conditions of the DMSO-mediated synthesis. Thus, the β-Ag2MoO4 microcrystals are colorless and do not show any plasmon-resonance absorption (Table 1 and Fig. 2e, compare Fig. 5b). They also do not show Ag(0)-related spots on electron-microscopy images (Fig. 2b–d). Small Ag(0)-related spots only appear on electron-microscopy images after certain period of exposition under the electron beam (compare Fig. 4).
To obtain cuboctahedra and cubes – beside the aforementioned water content – the temperature for particle nucleation turned out to be relevant as well (Table 1 and Fig. 3a). Thus, injecting aqueous AgNO3 at 0 °C results in β-Ag2MoO4 cubes (Fig. 3b), whereas β-Ag2MoO4 cuboctahedra were formed after AgNO3 injection at 50 °C (Fig. 3c). Thereof, the cubes expose {100} crystal facets only, whereas the cuboctahedra show mixed {100} and {111} crystal facets. Again, the β-Ag2MoO4 microcrystals are colorless and do not show any plasmon-resonance absorption (Table 1 and Fig. 3d, compare Fig. 5b), and they also do not show Ag(0)-related spots on electron-microscopy images (Fig. 3b,c) if the exposition under the electron beam was not too long (Fig. 4).
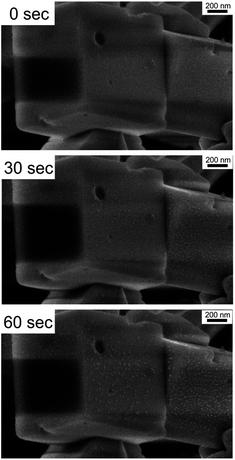 | ||
| Fig. 4 Evolution of Ag(0)-related spots, up to 10 ± 4 nm in diameter, due to electron-beam-driven reduction on the surface of β-Ag2MoO4 cubes after 60 seconds. | ||
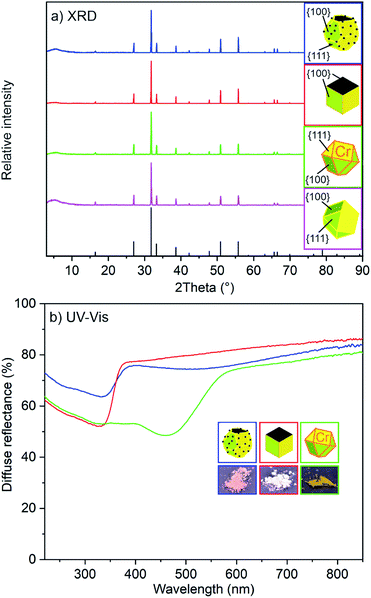
Crystallinity and optical properties
All faceted β-Ag2MoO4 microcrystals – independent of their respective synthesis in DEG or DMSO – are highly crystalline (Fig. 5a). X-ray powder diffraction (XRD) displays all characteristic Bragg peaks of β-Ag2MoO4.17 In fact, this finding is expected since faceted shapes need to be single crystalline for facet formation. The optical properties were characterized by UV-Vis spectroscopy. Accordingly, the β-Ag2MoO4 samples show a steep absorption edge between 330 and 370 nm (Fig. 5b), which can be related to the valence band-to-transition band excitation. Based on Tauc plots, the absorption of DMSO-made β-Ag2MoO4 can be ascribed to a direct band gap with an energy of 3.2 eV, which is well in agreement with literature data (3.14 eV).8 For the DEG-made β-Ag2MoO4, the band gap is slightly reduced to 3.0 eV. In contrast to β-Ag2MoO4 made in DMSO, moreover, those samples prepared in DEG show a weak and broad plasmon-resonance absorption at 420 to 750 nm due to the presence of Ag(0) nanoparticles (Fig. 5b). In regard of photocatalysis, these β-Ag2MoO4@Ag microcrystals can be promising in terms of visible-light absorption as already outlined by Li et al.11dDespite of a potential plasmon-resonance-driven visible-light absorption of β-Ag2MoO4@Ag, we intended to evaluate other options to enable β-Ag2MoO4 for visible-light absorption in absence of Ag(0) nanoparticles. To this concern, partial substitution of [MoO4]2− by [CrO4]2− seemed promising. On the one hand, [CrO4]2− is known for its bright yellow to orange color indicating light absorption in the blue spectral regime. Moreover, both complex anions are of similar size as indicated by solid solution series Ag2(Cr,Mo)O4 with complete substitution of [MoO4]2− by [CrO4]2− and vice versa.18 On the other hand, the blue-light absorption of [CrO4]2− relates to a ligand-to-metal charge-transfer (LMCT) of a finite complex anion, which – in the case of partial substitution – is not necessarily affecting the band gap of the infinite lattice of the molybdate. Pure Ag2CrO4, finally, was reported to have a reddish black color, which is less optimal for photocatalytic application.11b
As a result of the aforementioned considerations, we intended a substitution of 5 mol% of chromate in β-Ag2MoO4. Ag2(Mo0.95Cr0.05)O4 was prepared similar to β-Ag2MoO4 and, for instance, results in cuboctahedra with similar size and shape as observed for pure β-Ag2MoO4. In contrast to colorless β-Ag2MoO4, Ag2(Mo0.95Cr0.05)O4 indeed exhibits a bright yellow color (Fig. 5b). UV-Vis spectra show a steep absorption for Ag2(Mo0.95Cr0.05)O4, which is now red-shifted (470–570 nm) in comparison to the β-Ag2MoO4 cuboctahedra (Fig. 5b). The band gap of Ag2(Mo0.95Cr0.05)O4 was deduced to 2.1 eV, which lays in between of the values of β-Ag2MoO4 (3.14 eV)18 and Ag2CrO4 (1.75 eV).11b,19 This finding points to an active role of chromate for the band gap of Ag2(Mo0.95Cr0.05)O4.
Photocatalytic examination
For a comparative evaluation of the photocatalytic activity of the different β-Ag2MoO4 microcrystals in dependence of their respective surface faceting and chemical composition, the respective experiments were performed with comparable conditions. Thus, identical concentrations of the respective β-Ag2MoO4 microcrystals (0.18 g L−1) were suspended in water, and identical concentrations of zwitterionic rhodamine B (RhB, 2 mg L−1, 4.18 μmol L−1) were added. RhB was selected, on the one hand, since its absorption (500–600 nm) is different from the absorption of β-Ag2MoO4 (<500 nm). On the other hand, the adhesion of the zwitterionic RhB on the photocatalyst surface is independent from eventual charging of the respective surface of the photocatalyst. Additional criteria for the photocatalytic performance of the β-Ag2MoO4 microcrystals were examined as well (Table 2). First of all, the crystallinity and size of the β-Ag2MoO4 microcrystals (about 0.5 μm) can be considered as very comparable. Nitrogen sorption analysis also indicated the specific surface area to be similar (Table 2). Finally, the zeta potential was quantified to evaluate a potential influence of surface charging. As a result, all β-Ag2MoO4 microcrystals show negative charging ranging from −33 to −43 mV at pH = 7 (Table 2). Finally, any photoluminescence as potential competitive loss process to photoluminescence could be excluded (ESI: Fig. S1†). Taken together, several material parameters – including concentrations, size, crystallinity, surface area and surface charging – are similar, so that a direct correlation of the photocatalytic activity to the type of surface faceting and chemical composition is possible.| Composition (synthesis) | Morphology with exposed facets | Zeta potential/mV | Specific surface area/m2 g−1 | Band gap/eV | Photo-catalytic activity |
|---|---|---|---|---|---|
| Ag2MoO4@Ag (DEG) | Truncated octahedra {111} + {100} | −43 | 1 | 3.0 | Poor |
| Ag2MoO4 (DMSO) | Cubes {100} | −37 | 2 | 3.2 | Medium |
| Ag2MoO4 (DMSO) | Cuboctahedra {111} + {100} | −33 | 1 | 3.2 | High |
| Ag2(Mo,Cr)O4 (DMSO) | Cuboctahedra {111} + {100} | −35 | 1 | 2.1 | Medium |
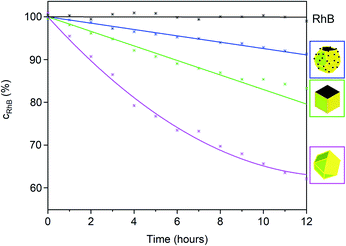
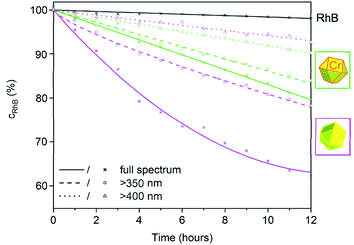
To examine the photocatalytic activity of the β-Ag2MoO4 microcrystals, the absorption of RhB was continuously monitored at λRhB = 554 nm under artificial sunlight (AM 1.5 G solar light, 100 mW cm−2) (Fig. 6 and 7). First of all, the autophotolysis of RhB (in absence of β-Ag2MoO4) was verified and showed no considerable effect within the significance of the experiment. Thereafter, the different β-Ag2MoO4 microcrystals were studied and evidence a significantly higher photocatalytic activity as compared to the autophotolysis of RhB. It should also be noticed that the size and shape of the faceted microcrystals was maintained subsequent to the photocatalytic reaction (ESI: Fig. S2†).
With simulated sunlight, the β-Ag2MoO4 cuboctahedra made in DMSO show the highest photocatalytic activity, which is about twice to thrice as high as for all other faceted microcrystals (Fig. 6). The β-Ag2MoO4@Ag truncated octahedra made in DEG show the lowest activity, followed by β-Ag2MoO4 cubes made in DMSO. In regard of the surface faceting, the photocatalytic activity of {111} crystal facets is obviously higher as compared to {100} crystal facets (Fig. 6). Such situation is well known for metal oxides such as Cu2O, γ-Al2O3 or Co3O4 and was related to the higher surface energy of the {111} crystal facets.20 Since cubes exhibit only {100} facets, they show the lowest photocatalytic activity. The β-Ag2MoO4 cuboctahedra, having the highest area of {111} crystal facets, consequently exhibit the highest photocatalytic activity.
Whereas the aforementioned β-Ag2MoO4 cubes and cuboctahedra only become photoactive upon absorption of UV photons (<390 nm due to a band gap at 3.2 eV), the truncated β-Ag2MoO4@Ag octahedra and Ag2(Mo0.95Cr0.05)O4 cuboctahedra are suitable for visible-light-driven photocatalysis, in principle. However, β-Ag2MoO4@Ag already under simulated sunlight with high-energy UV photons absorbed by β-Ag2MoO4@Ag shows low performance (Fig. 6). The low activity of β-Ag2MoO4@Ag that we observed is in contrast to former studies suggesting a considerable effect of Ag(0) particles on the photocatalytic activity of β-Ag2MoO4, which was claimed to be driven by plasmon-resonance absorption even at low wavelength (<420 nm)11d or with significantly larger Ag(0) nanoparticles (80–100 nm).21 Due to its low performance, β-Ag2MoO4@Ag was not examined further in our study.
A comparison of the β-Ag2MoO4 cuboctahedra as the most active photocatalyst under simulated sunlight (Fig. 6 and 7) and the Ag2(Mo0.95Cr0.05)O4 cuboctahedra (Fig. 7), finally, shows an increased relative photocatalytic activity of Ag2(Mo0.95Cr0.05)O4 the longer the wavelength of the irradiated light is. Thus, illumination only with visible light >400 nm results in a higher photocatalytic activity of the Ag2(Mo0.95Cr0.05)O4 cuboctahedra as compared to the β-Ag2MoO4 cuboctahedra (Fig. 7). Such effect of a chromate-shifted optical absorption of a molybdate-based photocatalyst is shown for the first time and offers an additional option for visible-light-driven photocatalysis of metal molybdates and tungstates.
Conclusions
Faceted β-Ag2MoO4 microcrystals are prepared by controlled nucleation and growth in diethylene glycol (DEG) and dimethylsulfoxide (DMSO). Both serve as solvents for the liquid-phase synthesis and surface-active agents for the formation of faceted microcrystals. Due to the reducing properties, truncated β-Ag2MoO4@Ag octahedra are obtained in DEG. The synthesis in DMSO allows avoiding the formation of elemental silver and results in β-Ag2MoO4 cubes and cuboctahedra. Whereas the colorless β-Ag2MoO4 (band gap: 3.2 eV) can be only activated by UV-light, β-Ag2MoO4@Ag (with Ag(0)-based plasmon-resonance absorption) as well as β-Ag2(Mo0.95Cr0.05)O4 (with partial substitution of [MoO4]2− by [CrO4]2−) are suitable for visible-light excitation.Based on the realized faceting and chemical composition – truncated octahedra, cubes, cuboctahedra, β-Ag2MoO4, β-Ag2MoO4@Ag, β-Ag2(Mo0.95Cr0.05)O4 – the photocatalytic properties were exemplarily monitored for the degradation of rhodamine B. As a result, the β-Ag2MoO4 cuboctahedra showed the highest photocatalytic activity under simulated sunlight, which could be related to their {111} crystal facets that turned out to be more active than {100} crystal facets. β-Ag2MoO4@Ag showed the lowest photocatalytical activity at all. The photocatalytic activity of Ag2(Mo0.95Cr0.05)O4 cuboctahedra turned out to be the higher in comparison to β-Ag2MoO4 cuboctahedra the longer the wavelength of the irradiated light is. The facet-, composition- and wavelength-depending photocatalytic activity generally contributes to the fundamental understanding of photocatalysis. The partial incorporation of chromate into molybdate-based photocatalysts and the resulting shift of the optical absorption are shown for the first time and can be of general interest for visible-light-driven photocatalysis of metal molybdates and tungstates.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft (DFG) for funding of equipment. Moreover, L. W. acknowledges the Studienstiftung des deutschen Volkes for scholarship.Notes and references
- (a) L. Han, S. Cai, M. Gao, J. Hasegawa, P. Wang, J. Zhang, L. Shi and D. Zhang, Chem. Rev., 2019, 119, 10916–10976 CrossRef CAS; (b) A. M. Kaczmarek and R. van Deun, Chem. Soc. Rev., 2013, 42, 8835–8848 RSC.
- (a) H. S. Kim, J. B. Cook, H. Lin, J. S. Ko, S. H. Tolbert, V. Ozolins and B. Dunn, Nat. Mater., 2017, 16, 454–460 CrossRef CAS PubMed; (b) X. Hu, W. Zhang, X. Liu, Y. Mei and Y. Huang, Chem. Soc. Rev., 2015, 44, 2376–2404 RSC; (c) C. Wang, L. Wu, H. Wang, W. Zuo, Y. Li and J. Liu, Adv. Funct. Mater., 2015, 25, 3524–3533 CrossRef CAS; (d) R. Wu, J. Zhang, Y. Shi, D. Liu and B. Zhang, J. Am. Chem. Soc., 2015, 137, 6983–6986 CrossRef CAS PubMed.
- (a) T. Guo, Y. Lin, W. J. Zhang, J. S. Hong, R. H. Lin, X. P. Wu, J. Li, C. H. Lu and H. H. Yang, Nanoscale, 2018, 10, 1607–1612 RSC; (b) W. Ouyang, Small, 2017, 13, 2177 Search PubMed; (c) W. Wang, J. Qin, Z. Yin and M. Cao, ACS Nano, 2016, 10, 10106–10116 CrossRef CAS PubMed; (d) J. Han, C. McBean, L. Wang, J. Hoy, C. Jaye, H. Liu, Z. Q. Li, M. Y. Sfeir, D. A. Fischer and G. T. Taylor, Chem. Mater., 2015, 27, 778–792 CrossRef CAS; (e) Y. Ma, Y. Jia, Z. Jiao, M. Yang, Y. Qi and Y. Bi, Chem. Commun., 2015, 51, 6655–6658 RSC; (f) A. Kudo, M. Steinberg, A. J. Bard, A. Campion, M. A. Fox, T. E. Mallouk, S. E. Webber and J. W. White, Catal. Lett., 1990, 5, 61–66 CrossRef CAS.
- (a) Y. Park, K. J. Mcdonald and K.-S. Choi, Chem. Soc. Rev., 2013, 42, 2321–2337 RSC; (b) A. Walsh, D. J. Payne, R. G. Egdell and G. W. Watson, Chem. Soc. Rev., 2011, 40, 4455–4463 RSC.
- (a) B. Qian, X. Li and Z. Song, J. Am. Ceram. Soc., 2018, 101, 4425–4429 CrossRef CAS; (b) J. Liu, Y. Li, Z. Li, J. Ke, H. Xiao and Y. Hou, Catal. Today, 2018, 314, 2–9 CrossRef CAS; (c) A. Etogo, R. Liu, J. Ren, L. Qi, C. Zheng, J. Ning, Y. Zhong and Y. Hu, J. Mater. Chem. A, 2016, 4, 13242–13250 RSC.
- (a) D. Mukherjee and B. M. Reddy, Catal. Today, 2018, 309, 227–235 CrossRef CAS; (b) H. Y. Kim, M. S. Hybertsen and P. Liu, Nano Lett., 2017, 17, 348–354 CrossRef CAS PubMed; (c) A. Trovarelli and J. Llorca, ACS Catal., 2017, 7, 4716–4735 CrossRef CAS.
- (a) S. K. Meena, N. L. Heda, G. Arora, L. Meena and B. L. Ahuja, Phys. B, 2019, 560, 236–243 CrossRef CAS; (b) M. Wu, H. Lv, T. Wang, Z. Ao, H. Sun, C. Wang, T. An and S. Wang, Catal. Today, 2018, 315, 205–212 CrossRef CAS.
- (a) C. A. Oliveira, D. P. Volanti, A. E. Nogueira, C. A. Zamperini, C. E. Vergani and E. Longo, Mater. Des., 2017, 115, 73–81 CrossRef CAS; (b) J. V. Kumar, R. Karthik, S.-M. Chen, V. Muthuraj and C. Karuppiah, Sci. Rep., 2016, 6, 34149 CrossRef CAS PubMed; (c) A. Beltrán, L. Gracia, E. Longo and J. Andrés, J. Phys. Chem. C, 2014, 118, 3724–3732 CrossRef.
- (a) R. Kohlmuller and J.-P. Faurie, Bull. Soc. Chim. Fr., 1968, 11, 4379–4382 Search PubMed; (b) C. H. B. Ng and W. Y. Fan, Cryst. Growth Des., 2015, 15, 3032–3037 CrossRef CAS.
- Z. Wang, K. Dai, C. Liang, J. Zhang and G. Zhu, Mater. Lett., 2017, 196, 373–376 CrossRef CAS.
- (a) M. T. Fabbro, C. C. Foggi, L. P. S. Santos, L. Gracia, A. Perrin, C. Perrin, C. E. Vergani, A. L. Machado, J. Andrés and E. Cordoncillo, Dalton Trans., 2016, 45, 10736–10743 RSC; (b) D. Xu, B. Cheng, J. Zhang, W. Wang, J. Yu and W. Ho, J. Mater. Chem. A, 2015, 3, 20153–20166 RSC; (c) F. S. Cunha, J. C. Sczancoski, I. C. Nogueira, V. G. de Oliveira, S. M. C. Lustosa, E. Longo and L. S. Cavalcante, CrystEngComm, 2015, 17, 8207–8211 RSC; (d) A. F. Gouveia, J. C. Sczancoski, M. M. Ferrer, A. S. Lima, M. R. M. C. Santos, M. S. Li, R. S. Santos, E. Longo and L. S. Cavalcante, Inorg. Chem., 2014, 53, 5589–5599 CrossRef CAS PubMed; (e) Z. Li, X. Chen and Z.-L. Xue, Sci. China: Chem., 2013, 56, 443–450 CrossRef CAS.
- Y.-C. Chen, Y.-K. Hsu, R. Popescu, D. Gerthsen, Y.-G. Lin and C. Feldmann, Nat. Commun., 2018, 9, 1–11 CrossRef PubMed.
- P. Schmitt, N. Brem, S. Schunk and C. Feldmann, Adv. Funct. Mater., 2011, 21, 3037–3046 CrossRef CAS.
- (a) Y.-C. Chen, Y.-G. Lin, L.-C. Hsu, A. Tarasov, P.-T. Chen, M. Hayashi, J. Ungelenk, Y.-K. Hsu and C. Feldmann, ACS Catal., 2016, 6, 2357–2367 CrossRef CAS; (b) J. Ungelenk and C. Feldmann, Chem. Commun., 2012, 48, 7838–7840 RSC.
- H. Dong, Y.-C. Chen and C. Feldmann, Green Chem., 2015, 17, 4107–4132 RSC.
- (a) M. Rycenga, C. M. Cobley, J. Zeng, W. Li, C. H. Moran, Q. Zhang, D. Qin and Y. Xia, Chem. Rev., 2011, 111, 3669–3712 CrossRef CAS PubMed; (b) B. D. Evanoff and G. Chumanov, J. Phys. Chem. B, 2004, 108, 13957–13962 CrossRef.
- R. W. G. Wyckoff, J. Am. Chem. Soc., 1922, 44, 1994–1998 CrossRef CAS.
- M. L. Hackert and R. A. Jacobson, J. Solid State Chem., 1971, 3, 364–368 CrossRef CAS.
- S. Ouyang, Z. Li, Z. Ouyang, T. Yu, J. Ye and Z. Zou, J. Phys. Chem. C, 2008, 112, 3134–3141 CrossRef CAS.
- (a) Y.-Y. Song and G.-C. Wang, J. Phys. Chem. C, 2018, 122, 21500–21513 CrossRef CAS; (b) Y. Wang, J. Yang, R. Gu, L. Peng, X. Guo, N. Xue, Y. Zhu and W. Ding, ACS Catal., 2018, 8, 6419–6425 CrossRef CAS; (c) L. Liu, Z. Jiang, L. Fang, H. Xu, H. Zhang, X. Gu and Y. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 27736–27744 CrossRef CAS PubMed.
- J. Li, F. Liu and Y. Li, New J. Chem., 2018, 42, 12054–12061 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Data related to photoluminescence and shape of catalyst before and after photocatalysis. See DOI: 10.1039/d0ra02953j |
| This journal is © The Royal Society of Chemistry 2020 |