Open Access Article
Open Access ArticleSynthesis of Schiff and Mannich bases of new s-triazole derivatives and their potential applications for removal of heavy metals from aqueous solution and as antimicrobial agents†
Zeinab A. Hoziena,
Ahmed F. M. EL-Mahdy *ab,
Ahmad Abo Markeb
*ab,
Ahmad Abo Markeb a,
Laila S. A. Alia and
Hassan A. H. El-Sherief*a
a,
Laila S. A. Alia and
Hassan A. H. El-Sherief*a
aChemistry Department, Faculty of Science, Assiut University, Egypt. E-mail: dr_hassanahmed@yahoo.com
bDepartment of Materials and Optoelectronic Science, National Sun Yat-sen University, Kaohsiung, 80424, Taiwan. E-mail: ahmedelmahdy@mail.nsysu.edu.tw
First published on 27th May 2020
Abstract
An efficient, simple, and one-pot double Mannich reaction was performed for the synthesis of cyclized 2-methyl-6-substituted-6,7-dihydro-5H-s-triazolo[5,1-b]-1,3,5-thiadiazines via a reaction of 5-methyl-1H-s-triazole-3-thiol (1) with formaldehyde and primary aliphatic amines in ethanol at room temperature, while with primary aromatic amines, uncyclized 3-methyl-1-((substituted-amino)methyl)-1H-s-triazole-5-thiols were produced. Under Mannich reaction conditions, 4-amino-3-methyl-s-triazole-5-thiol (8) reacted with formaldehyde only in boiling ethanol or at room temperature to afford 3-methyl-5,6-dihydro-s-triazolo[3,4-b]-1,3,4-thiadiazole without incorporation of secondary amine. Furthermore, after reaction of compound 8 with aromatic aldehydes under different reaction conditions, uncyclized Schiff's bases were produced. Therefore, reaction of these Schiff's bases with primary or secondary amines with formaldehyde in ethanol at room temperature afforded the corresponding Mannich bases 13–14. The structures of all new compounds were confirmed using spectral analysis. Furthermore, most of the synthesized derivatives showed high efficiency for removal of Pb2+, Cd2+, Ca2+, and Mg2+ from aqueous solutions, as well as antimicrobial activity.
1. Introduction
The therapeutic importance of s-triazole derivatives has been documented in the numerous roles they play as antimicrobial,1–4 antiviral,5 antifungal,6 anti-inflammatory,7 antitumoral8,9 anticonvulsant,10,11 antidepressant,12 hypoglycemic,13 antihypertensive,14 analgesic,15 plant growth regulating,16 and anticoagulant agents.17 For instance, commonly prescribed drugs that possess an s-triazole nucleus are ribavirin, rizatriptan, alprazolam, which are used as antiviral, antimigraine, and anxiolytic agents, respectively, as well as fluconazole and itraconazole, which are used as antifungal agents.18–22 In addition, derivatives of s-triazole have been used for environmental,23 industrial,24 and agricultural applications.25–27Due to the strong biological and medical activities of s-triazole moieties and their widespread use, it is necessary to synthesise new s-triazole derivatives in a shorter amount of time with minimum steps under mild reaction conditions. Therefore, we selected the attractive Mannich reaction to quickly synthesise various derivatives of s-triazole under mild conditions with high yield. In the last decade, the Mannich reaction has been highly utilized as one of the potential multi component reactions (MCRs) due to its performance in the building of carbon–nitrogen (C–N) and carbon–carbon (C–C) single bonds.28 It is well-known that compounds with one active hydrogen atom undergo a single Mannich reaction, while compounds with two neighboring active hydrogen atoms undergo a double Mannich reaction.29–31 Recently, we reported the triple Mannich reaction of 6-amino-2-(ethylthio)pyrimidin-4(3H)-one, which contains three active hydrogen atoms, and the quadruple Mannich reaction of 6-amino-2-thioxo-2,3-dihydropyrimidin-4(1H)-one, which contains four active hydrogen atoms.32,33
Biologically, the efficient route for the modification of biologically active compounds is the amino alkylation of aromatic substrates by Mannich reaction.34 Therefore, several applications of Mannich bases have been reported as potential biological agents. For example, Mannich bases can be used as anticancer, antimalarial, antitubercular, vasorelaxant, and analgesic drugs.35–40 In addition, a literature survey reveals that the incorporation of N-methylpiperazine, piperidine, or morpholine rings into Mannich bases results in antimicrobial activity.41,42 Furthermore, we reported the application of a double Mannich reaction as an efficient route for the synthesis of biologically active triazolothiadiazine,30 pyrimidothiadiazine,43 pyrazolopyrimidine,44 and thiadiazino-benzimidazole.45 Moreover, one of the most concise and effective approaches to the preparation of the biologically active s-triazolo-1,3,5-thiadiazines is based on the double Mannich-type condensation of 2-mercaptoazole or -azine derivatives with primary amines and an excess of formaldehyde.46
Although several pollutants have been registered as contaminants of emerging concern in water, no studies have been reported for the applications of triazole Mannich base compounds in water treatment processes. When metal and heavy metal ions exist in water above the maximum contaminated levels, environmental problems can result, according to the World Health Organization (WHO) and the U.S. Environmental Protection Agency (EPA).47,48 As a result of these facts, this work presents the synthesis of some new 2-methyl-6-substituted-6,7-dihydro-5H-s-triazolo[5,1-b]-1,3,5-thiadiazines, 3-methyl-1-((substituted-amino)methyl)-1H-s-triazole-5-thiol, 3-methyl-5,6-dihydro-s-triazolo[3,4-b]-1,3,4-thiadiazole, and Mannich bases possessing both the s-triazole nucleus as well as morpholine, N-methylpiperazine, or piperidine moieties and the investigation of their biological activities as well as their efficiency for the removal of metal and heavy metal ions from water.
2. Results and discussion
2.1. Chemistry
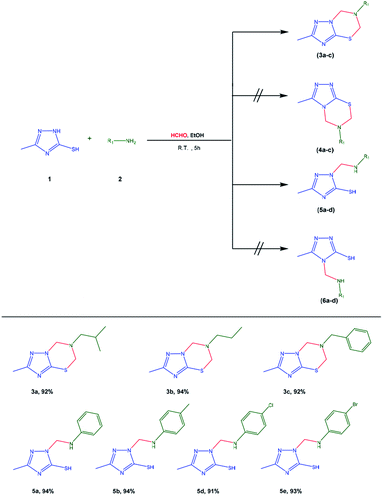
In the context of our progressive research on the preparation of creative heterocyclic frameworks through minimum steps and under mild reaction conditions,49–51 including the Mannich reaction, with this study, we endeavored to investigate the behavior of 5-methyl-1H-s-triazole-3-thiol (1) and 4-amino-5-methyl-4H-s-triazole-3-thiol (8) as nucleophiles with both primary aliphatic, aromatic, and secondary amines. Interestingly, when compound 1 was allowed to react with one equivalent of a primary aliphatic amine such as an isobutyl amine, propyl amine, or benzyl amine 2 and excess of aqueous formaldehyde (37%) either in boiling ethanol or at room temperature for 5 hours, single products with unproven structures were produced (Scheme 1). In addition to mass spectroscopy, Fourier transform infrared (FTIR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy confirmed that the products might be cyclized 2-methyl-6-substituted-6,7-dihydro-5H-s-triazolo[5,1-b]-1,3,5-thiadiazines (3a–c) or other isomeric products such as 3-methyl-6-substituted-6,7-dihydro-5H-s-triazolo[3,4-b]-1,3,5-thiadiazines (4a–c). The FTIR spectra of the isolated products revealed the appearance of specific absorption bands at wavenumbers of 2960–2870, 1618–1580, and 1495–1427 cm−1 for C–H aliphatic, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C
N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration bonds, respectively. The isolated product from the Mannich reaction using benzylamine as a nucleophile exhibited an absorption band at 3030 cm−1 that was attributed to C–H aromatic vibration bonds. In addition, all isolated products lacked the characteristic NH absorption band. The 1H-NMR spectra of the isolated products showed singlet signals at 4.60–4.69 and 5.05–5.09 ppm that corresponded to two methylene groups, confirming the reaction of compound 1 with two equivalent molecules of formaldehyde and the occurrence of a double-Mannich reaction. The other protons of CH3 triazole appeared at the expected chemical shift of δ = 2.35–2.38 ppm. The isolated product from isobutyl amine exhibited signals at δ = 0.94–0.96, 1.67–1.98, and 2.54 ppm for CH(C
C vibration bonds, respectively. The isolated product from the Mannich reaction using benzylamine as a nucleophile exhibited an absorption band at 3030 cm−1 that was attributed to C–H aromatic vibration bonds. In addition, all isolated products lacked the characteristic NH absorption band. The 1H-NMR spectra of the isolated products showed singlet signals at 4.60–4.69 and 5.05–5.09 ppm that corresponded to two methylene groups, confirming the reaction of compound 1 with two equivalent molecules of formaldehyde and the occurrence of a double-Mannich reaction. The other protons of CH3 triazole appeared at the expected chemical shift of δ = 2.35–2.38 ppm. The isolated product from isobutyl amine exhibited signals at δ = 0.94–0.96, 1.67–1.98, and 2.54 ppm for CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2, C
3)2, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2, and C
(CH3)2, and C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH protons, while the isolated products from propyl and benzyl amines exhibited signals at δ = 0.96 ppm for CH2C
2–CH protons, while the isolated products from propyl and benzyl amines exhibited signals at δ = 0.96 ppm for CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3, 1.60 ppm for C
3, 1.60 ppm for C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3, 2.73 ppm for N–C
2CH3, 2.73 ppm for N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2, 3.93 ppm for C
2CH2, 3.93 ppm for C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2-Ph, and 7.29–7.37 ppm for aromatic protons. The 13C-NMR spectra further confirmed the performance of the double Mannich reaction by the appearance of two signals at δ = 68.03 and 58.33 ppm for the methylene carbons. The mass spectra of the products from isobutyl, propyl, and benzyl amines revealed a molecular ion peak of 212.08 [M+], 198.02 [M+], and 246.09 [M+], respectively. All these findings confirmed the performance of the double Mannich reaction, while expecting that the isolated products were 3a–c or 4a–c.
2-Ph, and 7.29–7.37 ppm for aromatic protons. The 13C-NMR spectra further confirmed the performance of the double Mannich reaction by the appearance of two signals at δ = 68.03 and 58.33 ppm for the methylene carbons. The mass spectra of the products from isobutyl, propyl, and benzyl amines revealed a molecular ion peak of 212.08 [M+], 198.02 [M+], and 246.09 [M+], respectively. All these findings confirmed the performance of the double Mannich reaction, while expecting that the isolated products were 3a–c or 4a–c.
Recently, Haijian et al. reported the synthesis of s-triazolo[3,4-b]-1,3,5-thiadiazines through the double Mannich reaction of 5-aryl-2H-s-triazole-3(4H)-thiones with polyformaldehyde and S-(−)-α-phenylethylamine in the presence of hydrogen chloride as an acidic catalyst.52 The formation of s-triazolo[3,4-b]-1,3,5-thiadiazines rather than s-triazolo[5,1-b]-1,3,5-thiadiazines due to the protonation of highly basic N-2 directed the Mannich cyclization step to occur on N-4. Consequently, this reported result supports the formation of products 3a–c due to the higher nucleophilicity of N-2 as compared to N-4 in the neutral condition, and therefore, precluded the formation of products 4a–c as previously reported.30,43
In comparison, after treatment of compound 1 with primary aromatic amines such as aniline, p-toluidine, p-chloroaniline, and p-bromoaniline 2 under the same conditions, single products of the uncyclized 3-methyl-1-((substituted-amino)methyl)-1H-s-triazole-5-thiols (5a–d) rather than 3-methyl-4-((substituted-amino)methyl)-4H-s-triazole-3-thiol (6a–d) were produced due to the higher nucleophilicity of N-2 as compared to N-4 in the neutral condition (Scheme 1). The FTIR spectra of compounds 5a–d were characterized by the appearance of absorption bands at wavenumbers of 3369–3340, 3100–3034, 2987–2914, and 1594–1570 cm−1 assigned to NH, aromatic C–H, aliphatic C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration bonds. In addition, the 1H-NMR spectra of compounds 5a–d showed two characteristic signals: a doublet at δ = 5.37–5.52 ppm for the methylene (CH2–NH) group and a triplet signal at δ = 6.79–7.13 ppm for the secondary amine (CH2–NH) group, confirming the performance of the singlet Mannich reaction and the reaction of compound 1 with one equivalent molecule of formaldehyde. The other protons for SH and methyl groups appeared at δ = 12.60–13.25 ppm (exchange with D2O) and 2.18–2.40 ppm, respectively. As an example, the mass spectra of the obtained compounds 5a, 5c, and 5d revealed a molecular ion peak at the value of 220 [M+], 254 [Cl35, M+], 256 [Cl37, M+ + 2] and 298 [Br79, M+], 300 [Br81, M+ + 2], respectively. Similarly, the treatment of compound 1 with secondary amine such as morpholine under Mannich reaction conditions resulted in the production of 3-methyl-1-(morpholinomethyl)-1H-s-triazole-5-thiol (7), as shown in Scheme S1.† The FTIR spectrum of compound 7 exhibited several absorbance bands at 3100, 2983, and 1569 cm−1 for the SH, aliphatic C–H, and C
N vibration bonds. In addition, the 1H-NMR spectra of compounds 5a–d showed two characteristic signals: a doublet at δ = 5.37–5.52 ppm for the methylene (CH2–NH) group and a triplet signal at δ = 6.79–7.13 ppm for the secondary amine (CH2–NH) group, confirming the performance of the singlet Mannich reaction and the reaction of compound 1 with one equivalent molecule of formaldehyde. The other protons for SH and methyl groups appeared at δ = 12.60–13.25 ppm (exchange with D2O) and 2.18–2.40 ppm, respectively. As an example, the mass spectra of the obtained compounds 5a, 5c, and 5d revealed a molecular ion peak at the value of 220 [M+], 254 [Cl35, M+], 256 [Cl37, M+ + 2] and 298 [Br79, M+], 300 [Br81, M+ + 2], respectively. Similarly, the treatment of compound 1 with secondary amine such as morpholine under Mannich reaction conditions resulted in the production of 3-methyl-1-(morpholinomethyl)-1H-s-triazole-5-thiol (7), as shown in Scheme S1.† The FTIR spectrum of compound 7 exhibited several absorbance bands at 3100, 2983, and 1569 cm−1 for the SH, aliphatic C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration bonds. The 1H-NMR spectrum of compound 7 showed a singlet signal at 4.99 ppm corresponding to the methylene group, in addition to the other methyl, (N–(CH2)2, O–(CH2)2), and thiol protons at δ = 2.35, 2.76, 3.70, and 12.50 ppm, respectively. The mass spectrum of the obtained compound 7 exhibited a molecular ion peak at the value of 214.03 [M+].
N vibration bonds. The 1H-NMR spectrum of compound 7 showed a singlet signal at 4.99 ppm corresponding to the methylene group, in addition to the other methyl, (N–(CH2)2, O–(CH2)2), and thiol protons at δ = 2.35, 2.76, 3.70, and 12.50 ppm, respectively. The mass spectrum of the obtained compound 7 exhibited a molecular ion peak at the value of 214.03 [M+].
We examined the behavior of 4-amino-3-methyl-s-triazole-5-thiol (8) towards the Mannich reaction through the treatment of compound 8 with secondary amine such as morpholine or piperidine (9) and excess of aqueous formaldehyde (37%) either in boiling ethanol or at room temperature for 5 hours. The reaction proceeded to yield a single product, and FTIR and 1H-NMR spectroscopy indicated that it was 3-methyl-5,6-dihydro-s-triazolo[3,4-b]-1,3,4-thiadiazole (10), which highlighted the performance of the Mannich reaction between the amino (NH2) and thiol (SH) groups of the s-triazole moiety without the incorporation of secondary amine molecules (Scheme 2). This result was further confirmed by the reaction of compound 8 with formaldehyde refluxing in ethanol or in acetic acid in the presence of sulfuric acid as catalyst to afford the same product 10. The FTIR spectrum of compound 10 exhibited adsorption bands at 3236 (NH), 2989 (aliphatic C–H), and 1586 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), while its 1H-NMR spectrum exhibited a doublet signal at δ = 5.34 ppm that was attributed to the methylene group, in addition to the other NH and methyl protons at δ = 7.38 and 2.31 ppm, respectively. The mass spectrum of the obtained compound 10 showed a molecular ion peak at the value of 140 [M+].
N), while its 1H-NMR spectrum exhibited a doublet signal at δ = 5.34 ppm that was attributed to the methylene group, in addition to the other NH and methyl protons at δ = 7.38 and 2.31 ppm, respectively. The mass spectrum of the obtained compound 10 showed a molecular ion peak at the value of 140 [M+].
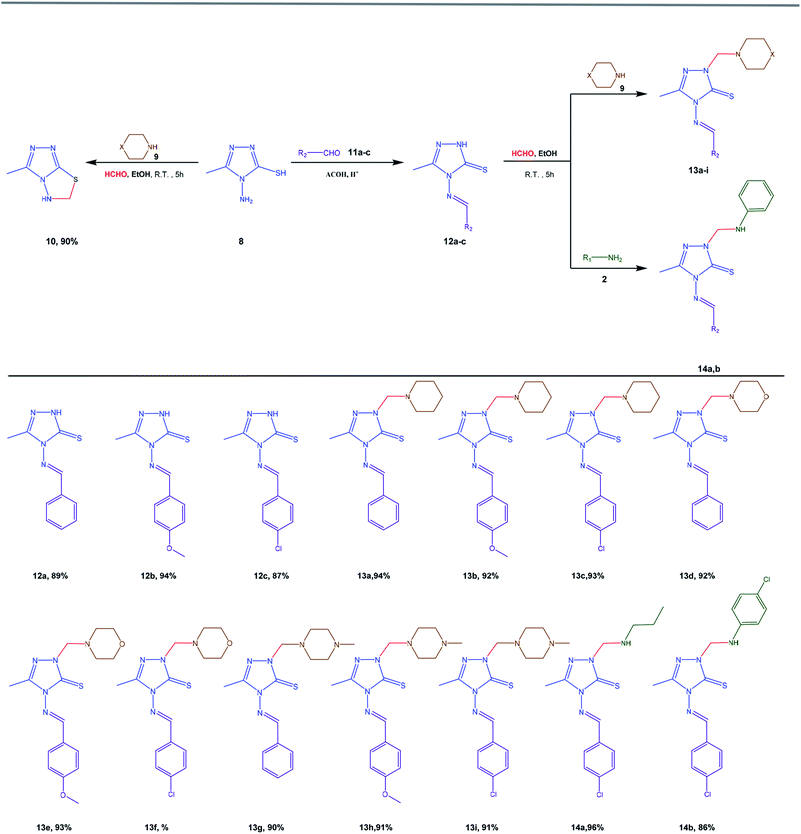 | ||
| Scheme 2 Mannich reaction of 4-amino-5-methyl-4H-s-triazole-3-thiol (8) and its benzylidene derivatives (12a–c). | ||
In the condensation reaction of compound 8 with aromatic aldehydes such as benzaldehyde, p-methoxybenzaldehyde, and p-chlorobenzaldehyde (11a–c) either in boiling acetic acid or at room temperature, uncyclized Schiff's base 4-arylidenamino-3-methyl-s-triazole-5-thiones (12a–c) were produced, as shown in Scheme 2. Several trials were attempted to cyclize the Schiff's bases to s-triazolo[3,4-b]thiadiazoles, using different reaction conditions as previously reported, but they failed.53 As a result of this condensation reaction of compound 8, the product 12 contains one active hydrogen atom (NH) that might undergo a single Mannich reaction. Therefore, when treated with formaldehyde and secondary amine such as morpholine, piperidine, or N-methylpiperazine (9) in ethanol at room temperature, compounds 12a–c afforded 4-arylideneamino-3-methyl-1-(N-morpholinomethyl)-s-triazole-5-thiones (13d–f) and 4-arylid eneamino-3-methyl-1-(N-methylpiperazinomethyl)-s-triazole-5-thiones (13g–i), respectively (Scheme 2). In addition, in the reaction of 4-((4-chlorobenzylidene)amino)-3-methyl-2,4-dihydro-3H-s-triazole-5-thione (12c) with primary amine such as propylamine or p-chloroaniline (2) under the same conditions, 4(4-chlorobenzylideneamino)-3-methyl-1-((propyl-amino)methyl)-1H-s-triazole-3-thione and 4(4-chlorobenzy-lideneamino)-3-methyl-1-((4-chlorophenylamino)methyl)-1H-s-triazole-3-thione (14a,b) were afforded, respectively (Scheme 2).
The IR spectra of compounds 12a–c lacked NH2 absorption bands and exhibited absorption bands at 3110–3102, 3080–3056, 2960–2942, and 1656–1586 cm−1 for the NH, aromatic C–H, aliphatic C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration bonds, respectively, while compounds 13a–i lacked NH absorption bands and showed absorption bands at 3090–3015, 2968–2809, and 1610–1525 cm−1 that were attributed to aromatic C–H, aliphatic C–H, and C
N vibration bonds, respectively, while compounds 13a–i lacked NH absorption bands and showed absorption bands at 3090–3015, 2968–2809, and 1610–1525 cm−1 that were attributed to aromatic C–H, aliphatic C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration bonds, respectively. In addition, compounds 14a,b exhibited the NH absorption band at 3389–3235 cm−1. The 1H-NMR spectrum of compounds 12a–c showed singlet signals at δ = 2.33–2.37, 9.70–10.00, and 13.57–13.77 (exchange with D2O) ppm for the methyl (CH3), imine (N
N vibration bonds, respectively. In addition, compounds 14a,b exhibited the NH absorption band at 3389–3235 cm−1. The 1H-NMR spectrum of compounds 12a–c showed singlet signals at δ = 2.33–2.37, 9.70–10.00, and 13.57–13.77 (exchange with D2O) ppm for the methyl (CH3), imine (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), and amine (NH) groups, respectively, as well as the other aromatic protons that appeared at δ = 7.14–7.92 ppm, while compound 12b exhibited an additional singlet signal at δ = 3.68 ppm that was attributed to the methoxy (OCH3) group. The 1H-NMR spectra of compounds 13a–i showed a singlet signal at δ = 5.00–5.13 ppm corresponding to the methylene (N–CH2–N) group, in addition to the other protons at δ = 2.40–2.48 ppm for the CH3 group, 7.01–7.88 ppm for the aromatic protons, and 9.70–10.60 ppm for the imine (–N
CH), and amine (NH) groups, respectively, as well as the other aromatic protons that appeared at δ = 7.14–7.92 ppm, while compound 12b exhibited an additional singlet signal at δ = 3.68 ppm that was attributed to the methoxy (OCH3) group. The 1H-NMR spectra of compounds 13a–i showed a singlet signal at δ = 5.00–5.13 ppm corresponding to the methylene (N–CH2–N) group, in addition to the other protons at δ = 2.40–2.48 ppm for the CH3 group, 7.01–7.88 ppm for the aromatic protons, and 9.70–10.60 ppm for the imine (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) group.
CH) group.
Compounds 13a–c showed signals at δ = 1.27–1.69 ppm for three methylene (3CH2 piperidine) groups and at δ = 2.77–2.80 ppm for two methylene (N(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2)2 piperidine) groups. Compounds 13d–f showed signals at δ = 2.70–2.85 and 3.60–3.71 ppm that were attributed to the methylene N(CH2)2 and O(CH2)2 protons of the morpholine moiety, while compounds 13g–i showed signals at δ = 2.44–2.89 and 2.27–2.28 ppm for four methylene (N(CH2)4N) and (N–CH3) protons of the piperazine moiety. In addition, compounds 13b, 13e, and 13h showed a singlet signal at δ = 3.88–4.00 ppm that was attributed to the methoxy group. Furthermore, the 1H-NMR spectra of compounds 14a,b were characterized by the appearance of signals at δ = 2.38–2.41 ppm for the methyl (CH3), δ = 5.30–5.40 ppm for the NH group, δ = 5.52–5.53 ppm for the methylene (N–CH2–N), and δ = 10.46–10.52 ppm for the imine (N
2)2 piperidine) groups. Compounds 13d–f showed signals at δ = 2.70–2.85 and 3.60–3.71 ppm that were attributed to the methylene N(CH2)2 and O(CH2)2 protons of the morpholine moiety, while compounds 13g–i showed signals at δ = 2.44–2.89 and 2.27–2.28 ppm for four methylene (N(CH2)4N) and (N–CH3) protons of the piperazine moiety. In addition, compounds 13b, 13e, and 13h showed a singlet signal at δ = 3.88–4.00 ppm that was attributed to the methoxy group. Furthermore, the 1H-NMR spectra of compounds 14a,b were characterized by the appearance of signals at δ = 2.38–2.41 ppm for the methyl (CH3), δ = 5.30–5.40 ppm for the NH group, δ = 5.52–5.53 ppm for the methylene (N–CH2–N), and δ = 10.46–10.52 ppm for the imine (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) group, in addition to the other protons for propyl and phenyl groups.
CH) group, in addition to the other protons for propyl and phenyl groups.
The 13C-NMR spectrum of the isolated product 12a, as an example, exhibited signals at 163.78, 161.68, 148.86, and 11.22 ppm that were attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, C
S, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, N
N, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, and methyl carbons and other expected aromatic carbons. The 13C-NMR spectra of isolated products 13a, 13d, and 13h showed signals at δ = 162.86, 147.08, and 69.80 ppm corresponding to C
CH, and methyl carbons and other expected aromatic carbons. The 13C-NMR spectra of isolated products 13a, 13d, and 13h showed signals at δ = 162.86, 147.08, and 69.80 ppm corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, N
S, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, and methylene (N–CH2–N) carbons, in addition to the piperidine, morpholine, and N-methylpiperazine signals at δ = 52.79 (N(CH2)2), 25.94 (3CH2), 66.50 (O(CH2)2), 50.87 (N(CH2)2) and 54.90(2CH2), and 50.40 (2CH2), respectively. Moreover, compound 13h exhibited two additional signals at δ = 55.50 and 46.01 ppm that were attributed to the methoxy (–O–CH3) and methyl (N–CH3) carbons, respectively. Finally, the mass spectra of compounds 13a–i and 14a,b showed the expected molecular ion peaks at m/z = 315 [M+], 345.30 [M+], 372 [M + Na], 317 [M+], 347 [M+] 351.00 [M+], 330 [M+], 360.08 [M+], 364.93 [M+], 323 [M+] and 392 [Cl35, M+], respectively.
CH, and methylene (N–CH2–N) carbons, in addition to the piperidine, morpholine, and N-methylpiperazine signals at δ = 52.79 (N(CH2)2), 25.94 (3CH2), 66.50 (O(CH2)2), 50.87 (N(CH2)2) and 54.90(2CH2), and 50.40 (2CH2), respectively. Moreover, compound 13h exhibited two additional signals at δ = 55.50 and 46.01 ppm that were attributed to the methoxy (–O–CH3) and methyl (N–CH3) carbons, respectively. Finally, the mass spectra of compounds 13a–i and 14a,b showed the expected molecular ion peaks at m/z = 315 [M+], 345.30 [M+], 372 [M + Na], 317 [M+], 347 [M+] 351.00 [M+], 330 [M+], 360.08 [M+], 364.93 [M+], 323 [M+] and 392 [Cl35, M+], respectively.
2.2. Proposed mechanisms
The proposed mechanism of the obtained products 3a–c and 5a–d is illustrated in Scheme S2.† In the first step, a typical condensation reaction between the primary aliphatic amines with formaldehyde forms imines 15, which are attached by (NH-1) of the starting compound 1 to provide the uncyclized intermediates 16a–c. These intermediates 16a–c reacted with another molecule of formaldehyde followed by dehydration and intramolecular cyclization to generate the cyclized compounds 3a–c. The stability of the uncyclized intermediates 5a–d with primary aromatic amines may be due to the resonance with the aryl moiety. Compound 10 can be formed through the nucleophilic attack of the amino (NH2) group of the starting compound 8 with formaldehyde followed by intramolecular cyclization, as shown in Scheme S3.†2.3. Screening of heavy metals and metal ion removal from aqueous solution
In this work, the compounds produced from the interaction of 5-methyl-1H-s-triazole-3-thiol (1) with primary aliphatic and aromatic amines were investigated for their ability to remove Pb2+, Cd2+, Ca2+, and Mg2+ from aqueous solution. Fig. 1 illustrates that the removal percentage of metal and heavy metal ions was increased when the size of the side chain increased (3a–c). For example, 74.30%, 73.61%, and 72.29% of the Pb2+ in solution was removed using 3c, 3a, and 3b, respectively. The removal efficiencies for Cd2+ were found to be 70.95%, 72.00%, and 72.24% for 3b, 3a, and 3c, respectively. In addition, 62.45%, 61.26%, and 53.92% of Ca2+, and 71.42%, 70.48%, and 70.27% of Mg2+ were removed using 3c, 3a, and 3b, respectively.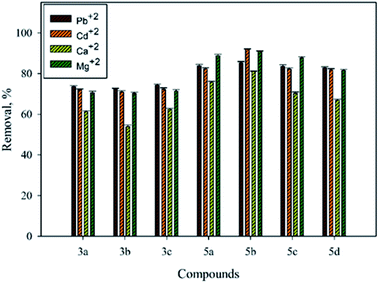 | ||
| Fig. 1 Effect of the aliphatic and aromatic primary amine on the removal efficiencies of Pb2+, Cd2+, Ca2+, and Mg2+. | ||
The efficiencies were enhanced when compound 1 interacted with primary aromatic amines. Fig. 1 illustrates that the presence of a donating group such as methyl in the para position of the aromatic amine 5b resulted in higher removal in comparison with the absence of one in 5a, with 85.26%, 92.00%, 81.00%, and 91.00% removal using 5b and 83.76%, 82.36%, 75.58%, and 88.81% removal using 5a for Pb2+, Cd2+, Ca2+, and Mg2+, respectively. However, a decrease in the removal efficiency was observed when a withdrawing group such as a chloro or bromo group was in the para position. Also, a bromo group diminished the capacities of metal and heavy metal ion removal in comparison with the chloro group, as presented in Fig. 1. For instance, the efficacy of 5c was found to be 83.55%, 82.20%, 70.28%, and 87.73%, while 5d shows 82.74%, 81.81%, 66.87%, and 81.50% removal of Pb2+, Cd2+, Ca2+, and Mg2+, respectively.
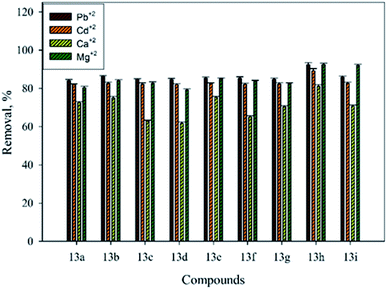
Likewise, high capacity of removal was observed using compound 7, where 84.24%, 82.34%, 72.17%, and 80.77% removal of Pb2+, Cd2+, Ca2+, and Mg2+, respectively, was demonstrated. Furthermore, to evaluate the efficiency of Mannich base derivatives produced by the reaction of 4-arylidenamino-3-methyl-s-triazole-5-thiones with secondary amines such as morpholine, piperidine, and N-methylpiperazine, or with primary amines such as propylamine, and p-chloroaniline, the removal of Pb2+, Cd2+, Ca2+, and Mg2+ was tested. Fig. 2 shows the heavy metals and metal ions removed from aqueous solution using Mannich base derivative molecules and batch mode experiments. High efficiencies were obtained with lead, cadmium, and magnesium, while medium efficiency was found with calcium.
The efficiency of removal of Pb2+ by these Mannich base derivatives varied between 84.18–92.40%. The highest removal values were observed when a methoxy group was located in the para position of the benzaldehyde; then 92.40%, 86.24%, and 85.39% removal was obtained by using 13h, 13b, and 13e, respectively. The Mannich base derivatives bearing a chloro group in the para position of benzaldehyde showed medium efficiency, where 85.99%, 85.31%, and 84.59% removal was achieved using 13i, 13f, and 13c, respectively. Low removal in the case of unsubstituted benzaldehyde was found, with 84.81%, 84.56%, and 84.18% removal using 13g, 13d, and 13a, respectively. Also, the compounds of Mannich bases under investigation demonstrated high efficiency for the removal of Cd2+. The substitution of the methoxy group in the para position of benzaldehyde increased the removal efficiency of cadmium from 81.91–82.39% to 82.42–89.21%. For instance, the removal efficiency of Cd2+ when the methoxy group was present was found to be 82.66%, 82.42%, and 89.21% by 13b, 13e, and 13h, respectively.
The same efficiency for 13a and 13d (81.91%) was observed without any withdrawing group in the para position of benzaldehyde. The presence of a chloro group in the para position of benzaldehyde decreased the efficiency of Cd2+ removal when compared with the methoxy group, and was 82.13%, 82.17%, and 82.77% by 13c, 13f, and 13i, respectively. Interestingly, high and medium efficiencies were demonstrated for the removal of the metal ions responsible for water hardness (Ca2+ and Mg2+). For the removal of Mg2+, the tested Mannich bases exhibited high efficiency with values ranging between 79.16% and 92.49%, while for Ca2+, the removal varied from 61.75% to 81.01%. However, the highest efficiencies were obtained for both cases of metal ion removal when the methoxy group was in the para position. For example, 81.01%, 75.33%, and 74.97% removal for Ca2+ and 92.49%, 85.02%, and 83.98% removal for Mg2+ were obtained when Mannich bases based on 13h, 13e, and 13b, respectively were used. In the case of the chloro substituent group in the para position, the capacities to remove Ca2+ and Mg2+ ions were reduced. Then, 70.71%, 65.04%, and 62.89% removal of calcium and 92.14%, 83.71%, and 82.76% removal of magnesium from aqueous solution was attained utilizing 13i, 13f, and 13c, respectively. In general, without substitution in the para position of benzaldehyde, the removal efficiencies were lower than those of substituent ones with methoxy and chloro groups. This could be attributed to the presence of the donating group, as the methoxy group enhances the electron density of the molecule.
Moreover, the removal efficiency of heavy metals and metal ions varied by the type of amino group, e.g., primary or secondary. As shown in Fig. 3, high efficiency was found when propyl amine (14a) was used, where 93.92%, 87.74%, 85.43%, and 75.89% removal was obtained for Mg2+, Pb2+, Cd2+, and Ca2+, respectively. This could be attributed to the presence of the propyl amino (CH3CH2CH2NH–) group, which facilitates the coordination between the metal and heavy metal ions and Mannich base molecules. Comparatively, when secondary amine, such as N-methylpiperazine, was used (13i), reduction of the removal efficiency was observed. Furthermore, 14b shows that the lowest efficiency was due to the presence of withdrawal groups (chloro) in the para position. For example, 92.14%, 86.00%, 82.77%, and 70.81% removal occurred when 13i was used, and 83.24%, 84.43%, 82.40%, and 70.65% removal was measured when 14a was used.
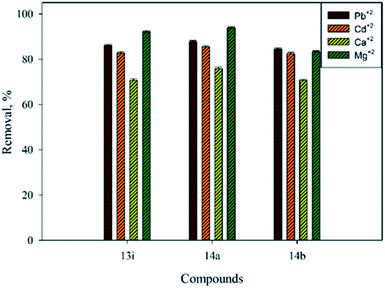 | ||
| Fig. 3 Comparison of the removal efficiencies for Pb2+, Cd2+, Ca2+, and Mg2+ using primary and secondary amines of Mannich base compounds. | ||
Compound 10 exhibited the lowest values, with 21.06%, 7.41%, 9.09%, and 30.00% removal for Pb2+, Cd2+, Ca2+, and Mg2+, respectively. This could be attributed to the cyclization between compound 8 and formaldehyde. In accordance with the results obtained for these Mannich base compounds, it was observed that some molecules had a tendency to coordinate with heavy metals; Pb2+ and Cd2+ tended to coordinate with metal ions, and Mg2+ and Ca2+ tended to coordinate with both metals and heavy metals. This could be attributed to the nature of heavy metals and metal ions, and the capacity of the Mannich base compounds to complex several metals and heavy metals. The different nature of these molecules may also be an important factor.
The pH is a critical parameter for metal and heavy metal ion adsorption from aqueous solution.54 Therefore, in this study, the adsorption experiments were carried out at pH 6.0. At acidic pH, lower than 6, the compounds under investigation were protonated, which decreased the interaction between the heavy metals and metal ions and the adsorbent molecules. In addition, when the pH was higher than 6.0, the metals and heavy metals could be precipitated in hydroxide form [M(OH)2]. Hence, the selected pH was 6.0, where surface groups such as the imino group became deprotonated and able to chelate the heavy metals and metal ions, as shown in eqn (1). This ensured that only ionic species existed in solution (Pb2+, Cd2+, Ca2+, and Mg2+) and eliminated the interference of hydroxide precipitation at pH above 6.0. Additionally, the highest removal efficiency could be obtained by avoiding the strong electrostatic repulsion that normally occurred at lower pH values.
| M2+(aq) + x[L](org) ↔ [MLx](org) | (1) |
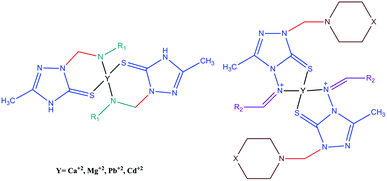
Therefore, a possible mechanism is the formation of coordination sites to receive the heavy metals and metal ions between an imine group and the thione of the s-triazole moiety, as shown in Fig. 4. The ability to form a complex is strongly affected by the nature of the substituent group and its position. Each substituent group has a specific effect on the coordination site that may increase or decrease the coordination capacity of the molecules. Thus, the efficiency in removing heavy metals and metal ions from aqueous solution is influenced by the electronic effect of substituents.
Further study is required to confirm the mechanism of metal and heavy metal ion removal from water. For instance, X-ray structural analysis and phase purity of the formed complex is required, and FTIR spectroscopy is required to confirm the binding sites through the functional group of the synthesized compounds that attaches to the metal/heavy metal ions. Additionally, thermogravimetric analysis/differential thermal analysis (TGA/DTA) is useful for predicting the loss of water molecules, as well as the UV/Vis technique for identifying the stoichiometry of the formed complex.
2.4. Antimicrobial activity
Derivatives of s-triazoles are considered as potent antimicrobial agents.55 Antimicrobial activity was evaluated in vitro using three strains of bacteria (Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa) and three strains of fungi (Aspergillus niger, A. flavus, and A. fumigatus). The inhibition zones (mm) of the screened compounds were compared with those of chloramphenicol and clotrimazole, which were used as a reference for the antibacterial and antifungal tests, respectively. The well diffusion method was used because the inhibition zone diameters were clear with good definition. Also, an advantage of this method is that when the inoculum is added to the culture medium that is then poured into the plate, homogenous distribution and growth of the strain is obtained, which facilitates measurement of the inhibition zone diameters after 24 h.56Table 1 presents the antibacterial and antifungal activities of the synthesized compounds. In determining the efficiency of the compounds against the bacteria, the activity was higher with Gram-negative bacteria in comparison to Gram-positive bacteria. For instance, the inhibition zones ranged from 29 to 2 mm, and 28 to 2 mm for Gram (−ve) and Gram (+ve) bacteria, respectively. Compound 3b showed the highest activity, with inhibition zones of 29 mm, 28 mm, and 21 mm against Pseudomonas aeruginosa (−ve), Bacillus subtilis (+ve), and Escherichia coli (−ve), respectively. Compounds 7, 3a, 3c, and 5a exhibited great activity with inhibition zones of 20, 19, and 18 mm towards Escherichia coli (−ve), while compounds 5b and 5c and showed low activity. In the case of Pseudomonas aeruginosa (−ve) and Bacillus subtilis (+ve), compounds 3a, 3c, 5a, 5b, 7, 5c, and 5d were found to have strong activity compared to chloramphenicol, with inhibition zones of 14 mm and 13 mm, respectively (Fig. 5).
| Compounds | Escherichia coli (−ve) | Pseudomonas aeruginosa (−ve) | Bacillus subtilis (+ve) | Aspergillus niger | Aspergillus flavus | Aspergillus fumigatus |
|---|---|---|---|---|---|---|
| 3a | 19 | 27 | 25 | 15 | 10 | 12 |
| 3b | 21 | 29 | 28 | 14 | 12 | 15 |
| 3c | 18 | 26 | 25 | 12 | 8 | 13 |
| 5a | 18 | 23 | 23 | 12 | 12 | 15 |
| 5b | 15 | 20 | 21 | 11 | 11 | 12 |
| 5c | 14 | 17 | 20 | 12 | 10 | 14 |
| 5d | 10 | 15 | 18 | 10 | — | 12 |
| 7 | 20 | 20 | 27 | 13 | 8 | 11 |
| 10 | 13 | 20 | 13 | — | — | — |
| 13a | — | — | — | 12 | — | 14 |
| 13b | — | — | — | 12 | 8 | 10 |
| 13c | 24 | 22 | — | 2 | 4 | — |
| 13d | 6 | 8 | — | 8 | — | 10 |
| 13e | 22 | 18 | 2 | 6 | — | 10 |
| 13f | 0 | 2 | 6 | 8 | 2 | 10 |
| 13g | 14 | 10 | — | 20 | 8 | 16 |
| 13h | 6 | 14 | — | 16 | — | 10 |
| 13i | 10 | — | — | 22 | — | 16 |
| 14a | 2 | — | — | 30 | — | 26 |
| 14b | 4 | 2 | — | 14 | 12 | 18 |
| Chloramphenicol | 16 | 14 | 13 | — | — | — |
| Clotrimazole | — | — | — | 20 | 25 | 30 |
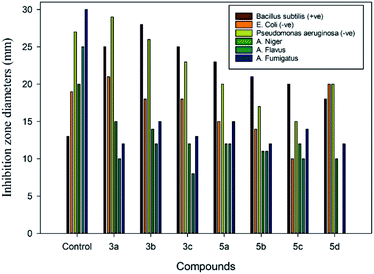 | ||
| Fig. 5 Comparison of inhibition zone diameters for the antimicrobial activity of the synthesized compounds 3a–c and 5a–d at a concentration of 2.5 μg/0.1 mL. | ||
In measuring the efficiencies of the Mannich base compounds against the bacteria, the highest activity was observed with Gram-negative bacteria in comparison to Gram-positive bacteria. For instance, the inhibition zones ranged from 24 to 2 mm, and 13 to 2 mm for Gram (−ve) and Gram (+ve) bacteria, respectively. Compounds 13c, 13e, and 10 exhibited excellent activity towards both Escherichia coli (−ve), and Pseudomonas aeruginosa (−ve), as shown in Table 1 and Fig. 6. Moreover, moderate activity was shown using compounds 13d, 13g, 13h, and 14b, while low activity was observed for 13f, 13i, and 14a. Furthermore, compounds 3a and 13b did not exhibit any activity against Escherichia coli (−ve) or Pseudomonas aeruginosa (−ve). Only compounds 10, 13e, and 13f exhibited activity against Bacillus subtilis (+ve) compared to clotrimazole, with an inhibition zone of 13 mm.
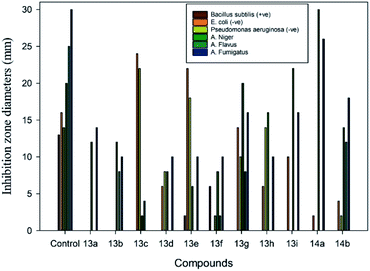 | ||
| Fig. 6 Comparison of inhibition zone diameters for the antimicrobial activity of the synthesized compounds 13a–i and 14a,b at a concentration of 2.5 μg/0.1 mL. | ||
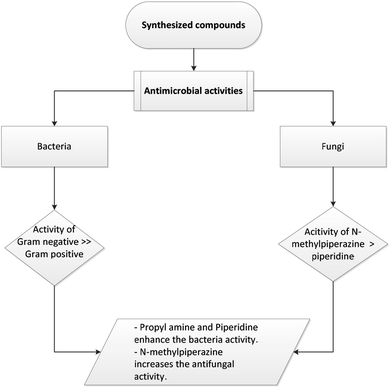
In the same manner, all the synthesized compounds showed noteworthy antifungal activity against different strains of fungi, as shown in Table 1 and Fig. 5. Compounds 3a–c, 5a–d, and 7 were active against A. niger, A. flavus, and A. fumigatus. However, compound 5d did not show any activity toward A. flavus. Interestingly, almost all Mannich base compounds exhibited antifungal activity against A. niger, A. flavus, and A. fumigatus, as shown in Table 1 and Fig. 6. Compounds 13g, 13h, and 14a exhibited high activity, with inhibition zones of 20, 22, and 30 mm against A. niger, respectively. Additionally, compounds 14a and 14b exhibited significant activity against A. fumigatus, while moderate activity was found using compounds 13a, 13b, 13d, 13f, and 13h. Compounds 13c and 13e presented the lowest activity against all species of fungi under investigation. Fig. 7 illustrates a simple explanation of the antimicrobial mechanism of action for the synthesized compounds.
3 Conclusions
We achieved an efficient one pot synthesis of the cyclized 2-methyl-6-substituted-6,7-dihydro-5H-s-triazolo[5,1-b]-1,3,5-thiadiazines via a double Mannich reaction of readily available 5-methyl-1H-s-triazole-3-thiol, formaldehyde, and primary aliphatic amines in ethanol at room temperature rather than other isomeric products such as 3-methyl-6-substituted-6,7-dihydro-5H-s-triazolo[3,4-b]-1,3,5-thiadiazines. By using primary aromatic amines, uncyclized 3-methyl-1-((substituted-amino)methyl)-1H-s-triazole-5-thiols rather than 3-methyl-4-((substituted-amino)methyl)-4H-s-triazole-3-thiol were obtained. Under the same reaction conditions, we investigated the behavior of 4-amino-3-methyl-s-triazole-5-thiol while undergoing the Mannich reaction through its treatment with secondary amine in boiling ethanol or at room temperature for 5 hours, and only 3-methyl-5,6-dihydro-s-triazolo[3,4-b]-1,3,4-thiadiazole was obtained without incorporation of secondary amine.Additionally, after treatment of 4-amino-3-methyl-s-triazole-5-thiol with aromatic aldehydes either in boiling acetic acid or at room temperature, uncyclized Schiff's bases were produced. Mannich bases were then obtained by reaction of uncyclized Schiff's bases with both primary and secondary amines under Mannich reaction conditions. The reaction products were easily isolated by filtration in very good to excellent yield and then purified by recrystallization with the proper solvent. All the synthesized products were used to remove Pb2+, Cd2+, Ca2+, and Mg2+ from aqueous solution, with remarkable results. For example, 70.27–93.92%, 72.29–92.40%, 70.95–92.00%, and 53.92–89.00% removal values were obtained for the removal of Mg2+, Pb2+, Cd2+, and Ca2+, respectively. Compound 10 exhibited the lowest values of removal at 21.06%, 7.41%, 9.09%, and 30.00% for Pb2+, Cd2+, Ca2+, and Mg2+, respectively.
Our synthesized compounds exhibited biological activity against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Aspergillus niger, A. flavus, and A. fumigatus. Therefore, these compounds have dual functions and can be used in water remediation, and also for medical and pharmaceutical applications. In comparison with other materials reported in the literature, these adsorbents are highly competitive, as they have high removal efficiencies and promising features for potential applications. Further studies will focus on the adsorption mechanism and the effect of other parameters on our synthesized Mannich base compounds.
Conflicts of interest
There are no conflicts to declare.References
- I. K. Jassim, W. Kh. Jassim, S. A. Alsatar and A. H. Mohammed, Synthesis and characterization of some new of thiazolidine, 1,2,4-triazole, 1,3,4-thiadiazole, semicarbazide, oxazoline and a study of their biological activity, karbala J. Pharm. Sci., 2012, 3, 213–222 Search PubMed.
- P. K. Shukla, N. Soni, A. Verma and A. K. Jha, Synthesis, characterization and in vitro biological evaluation of a series of 1,2,4-triazoles derivatives & triazole based Schiff bases, Der Pharma Chem., 2014, 6, 153–160 Search PubMed.
- I. Küçükgüzel, S. G. Küçükgüzel, S. Rollas and M. Kiraz, Some 3-thioxo/alkylthio-1,2,4-triazoles with a substituted thiourea moiety as possible antimycobacterials, Bioorg. Med. Chem. Lett., 2001, 11, 1703–1707 CrossRef.
- S. Eswaran, A. V. Adhikari and N. S. Shetty, Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety, Eur. J. Med. Chem., 2009, 44(11), 4637–4647 CrossRef CAS PubMed.
- E. De Clercq, Antiviral drugs in current clinical use, J. Clin. Virol., 2004, 30(2), 115–133 CrossRef CAS PubMed.
- J. Xu, Y. Cao, J. Zhang, Sh. Yu, Y. Zou, X. Chai, Q. Wu, D. Zhang, Y. Jiang and Q. Sun, Design, synthesis and antifungal activities of novel 1,2,4-triazole derivatives, Eur. J. Med. Chem., 2011, 46(7), 3142–3148 CrossRef CAS PubMed.
- M. W. Akhter, M. Z. Hassan and M. Amir, Synthesis and pharmacological evaluation of 3-diphenylmethyl-6-substituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles: a condensed bridgehead nitrogen heterocyclic system, Arab. J. Chem., 2014, 7(6), 955–963 CrossRef CAS.
- K. S. Bhat, B. Poojary, D. J. Prasad, P. Naik and B. S. Holla, Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety, Eur. J. Med. Chem., 2009, 44(12), 5066–5070 CrossRef CAS PubMed.
- Y. A. Al-Soud, M. N. Al-Dweri and N. A. Al-Masoudi, Synthesis, antitumor and antiviral properties of some 1,2,4-triazole derivatives, Il Farmaco, 2004, 59(10), 775–783 CrossRef CAS PubMed.
- I. Küçükgüzel, S. G. Küçükgüzel, S. Rollas, G. Ötük-Sanıs, O. Özdemir, I. Bayrak, T. Altug and J. P. Stables, Synthesis of some 3-(arylalkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4H-1,2,4-triazole derivatives and their anticonvulsant activity, Il Farmaco, 2004, 59(11), 893–901 CrossRef PubMed.
- N. F. Eweiss, A. A. Bahajaj and E. A. Elsherbini, Synthesis of heterocycles. Part VI. Synthesis and antimicrobial activity of some 4-amino-5-aryl-1,2,4-triazole-3-thiones and their derivatives, J. Heterocycl. Chem., 1986, 23(5), 1451–1458 CrossRef CAS.
- J. M. Kane, M. W. Dudley, S. M. Sorensen and F. P. Miller, 2,4-Dihydro-3H-1,2,4-triazole-3-thiones as potential antidepressant agents, J. Med. Chem., 1988, 31(6), 1253–1258 CrossRef CAS PubMed.
- M. Y. Mhasalkar, M. H. Shah, S. T. Nikam and C. V. Deliwala, 4-Alkyl-5-aryl-4H-1,2,4-triazole-3-thiols as hypoglycemic agents, J. Med. Chem., 1970, 13(4), 672–674 CrossRef CAS.
- J. Liu, Q. Liu, X. Yang, Sh. Xu, H. Zhang, R. Bai, H. Yao, J. Jiang, M. Shen, X. Wu and J. Xu, Design, synthesis, and biological evaluation of 1,2,4-triazole bearing 5-substituted biphenyl-2-sulfonamide derivatives as potential antihypertensive candidates, Bioorg. Med. Chem., 2013, 21(24), 7742–7751 CrossRef CAS.
- M. Amir and K. Shikha, Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives, Eur. J. Med. Chem., 2004, 39(6), 535–545 CrossRef CAS.
- R. A. Fletcher, G. Hofstra and J.-g. Gao, Comparative fungitoxic and plant growth regulating properties of triazole derivatives, Plant Cell Physiol., 1986, 27(2), 367–371 CAS.
- W. Khalid, A. Badshah, A. Khan, H. Nadeem and S. Ahmed, Synthesis, characterization, molecular docking evaluation, antiplatelet and anticoagulant actions of 1,2,4 triazole hydrazone and sulphonamide novel derivatives, Chem. Cent. J., 2018, 12(1), 11 CrossRef CAS PubMed.
- A. M. Isloor, B. Kalluraya and P. Shetty, Regioselective reaction: Synthesis, characterization and pharmacological studies of some new Mannich bases derived from 1,2,4-triazoles, Eur. J. Med. Chem., 2009, 44(9), 3784–3787 CrossRef CAS PubMed.
- V. Padmavathi, P. Thriveni, G. S. Reddy and D. Deepti, Synthesis and antimicrobial activity of novel sulfone-linked bis heterocycles, Eur. J. Med. Chem., 2008, 43(5), 917–924 CrossRef CAS PubMed.
- M. Amir, H. Kumar and S. A. Javed, Condensed bridgehead nitrogen heterocyclic system: Synthesis and pharmacological activities of 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives of ibuprofen and biphenyl-4-yloxy acetic acid, Eur. J. Med. Chem., 2008, 43(10), 2056–2066 CrossRef CAS PubMed.
- K. Sztanke, T. Tuzimski, J. Rzymowska, K. Pasternak and M. Kandefer-Szerszeń, Synthesis, determination of the lipophilicity, anticancer and antimicrobial properties of some fused 1,2,4-triazole derivatives, Eur. J. Med. Chem., 2008, 43(2), 404–419 CrossRef CAS.
- C. Kuş, G. Ayhan-Kılcıgil, S. Zbey, F. B. Kaynak, M. Kaya, T. Coban and B. Can-Eke, Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class, Bioorg. Med. Chem., 2008, 16(8), 4294–4303 CrossRef.
- C. Kavaklı, P. A. Kavaklı and O. Güven, Preparation and characterization of glycidyl methacrylate grafted 4-amino-1,2,4-triazole modified nonwoven fiber adsorbent for environmental application, Radiat. Phys. Chem., 2014, 94, 111–114 CrossRef.
- S. Murtaza, M. S. Akhtar, F. Kanwal, A. Abbas, S. Ashiq and S. Shamim, Synthesis and biological evaluation of Schiff bases of 4-aminophenazone as an anti-inflammatory, analgesic and antipyretic agent, J. Saudi Chem. Soc., 2017, 21, s359–s372 CrossRef CAS.
- M. Hanif, M. Saleem, M. T. Hussain, N. H. Rama, S. Zaib, M. A. M. Aslam, P. G. Jonesd and J. Iqbal, Synthesis, urease inhibition, antioxidant and antibacterial studies of some 4-amino-5-aryl-3H-1,2,4-triazole-3-thiones and their 3,6-disubstituted 1,2,4-triazolo[3,4-b]1,3,4-thiadiazole derivatives, J. Braz. Chem. Soc., 2012, 23, 854–860 CrossRef CAS.
- J. K. Shneine and Y. H. Alaraji, Chemistry of 1, 2, 4-triazole: a review article, Int. J. Sci. Res., 2013, 5(13), 1411–1423 Search PubMed.
- H. A. H. El-Sherief, Z. A. Hozien, A. F. M. El-Mahdy and A. A. O. Sarhan, One pot synthesis and reactions of novel 5-amino[1,3]thiazolo[3,2-b][1,2,4]triazoles, Arkivoc, 2011,(10), 71–84 Search PubMed.
- M. Arend, B. Westermann and N. Risch, Moderne varianten der Mannich-reaktion, Angew. Chem., 1998, 110(8), 1096–1122 CrossRef.
- A. F. M. El-Mahdy and S.-W. Kuo, Direct synthesis of poly(benzoxazine imide) from an ortho-benzoxazine: its thermal conversion to highly cross-linked polybenzoxazole and blending with poly(4-vinylphenol), Polym. Chem., 2018, 9, 1815–1826 RSC.
- A. M. Mahmoud, H. A. H. El-Sherif, O. M. A. Habib and A. A. O. Sarhan, Synthesis and studies of triazolothiadiazines. An approach toward new biologically active heterocyclic compounds, Phosphorus Sulfur Relat. Elem., 2007, 182(8), 1757–1766 CrossRef CAS.
- H. R. Abuzeid, A. F. M. EL-Mahdy, M. M. M. Ahmed and S. Kuo, Triazine-functionalized covalent benzoxazine framework for direct synthesis of N-doped microporous carbon, Polym. Chem., 2019, 10, 6010–6020 RSC.
- A. F. M. El-Mahdy and H. A. H. El-Sherief, An efficient and rapid intramolecular cyclization of a quadruple Mannich reaction for one-pot synthesis of pentaazaphenalenes and their antimicrobial activities, RSC Adv., 2016, 6(94), 92134–92143 RSC.
- A. F. M. EL-Mahdy, H. A. H. El-Sherief and Z. A. Hozien, Convenient one-pot four-component synthesis of 6,8-disubstituted-5,6,7,8-tetrahydropyrimido[4,5-d]pyrimidin-4(3H)-ones via a triple Mannich Reaction, Aust. J. Chem., 2019, 72(7), 542–554 CrossRef CAS.
- M. Tramontini and L. Angiolini, Further advances in the chemistry of Mannich bases, Tetrahedron, 1990, 46(6), 1791–1837 CrossRef CAS.
- B. Shivarama Holla, B. Veerendra, M. K. Shivananda and B. Poojary, Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles, Eur. J. Med. Chem., 2003, 38(7), 759–767 CrossRef CAS PubMed.
- F. Lopes, R. Capela, J. O. Goncaves, P. N. Horton, M. B. Hursthouse, J. Iley, C. M. Casimiro, J. Bomd and R. Moreira, Amidomethylation of amodiaquine: antimalarial N-Mannich base derivatives, Tetrahedron Lett., 2004, 45(41), 7663–7666 CrossRef CAS.
- S. Joshi, N. Khosla and P. Tiwari, In vitro study of some medicinally important Mannich bases derived from antitubercular agent, Bioorg. Med. Chem., 2004, 12(3), 571–576 CrossRef CAS PubMed.
- M. G. Ferlin, G. Chiarelotto, F. Antonucci, L. Caparrotta and G. Froldi, Mannich bases of 3H-pyrrolo[3,2-f]quinoline having vasorelaxing activity, Eur. J. Med. Chem., 2002, 37(5), 427–434 CrossRef CAS PubMed.
- W. Malinka, P. Swiatek, B. Filipek, J. Sapa, A. Jezierska and A. Koll, Synthesis, analgesic activity and computational study of new isothiazolopyridines of Mannich base type, Il Farmaco, 2005, 60(11), 961–968 CrossRef CAS PubMed.
- M. Ashok, B. S. Holla and B. Poojary, Convenient one pot synthesis and antimicrobial evaluation of some new Mannich bases carrying 4-methylthiobenzyl moiety, Eur. J. Med. Chem., 2007, 42(8), 1095–1101 CrossRef CAS PubMed.
- H. Bayrak, A. Demirbas, N. Demirbas and S. A. Karaoglu, Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities, Eur. J. Med. Chem., 2009, 44(11), 4362–4366 CrossRef CAS.
- H. Bayrak, A. Demirbas, S. A. Karaoglu and N. Demirbas, Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities, Eur. J. Med. Chem., 2009, 44(3), 1057–1066 CrossRef CAS PubMed.
- H. A. H. El-Sherief, Z. A. Hozien, A. F. M. El-Mahdy and A. A. O. Sarhan, Intramolecular cyclization of Mannich reaction for synthesis of pyrimido[2,1-b]-1,3,5-thiadiazines, J. Heterocycl. Chem., 2010, 47(6), 1294–1302 CrossRef CAS.
- Z. A. Hozien, A. A. O. Sarhan, H. A. H. El-Sherief and A. M. Mahmoud, A convenient one-pot Synthesis of pyrazolo[3,4-d]pyrimidines and s-triazolo[3,4-b][l,3,5]thiadiazines, Z. Naturforsch. B Chem. Sci., 1997, 52, 1401–1412 CAS.
- A. A. O. Sarhan, S. H. Abdel-Hafez, H. A. H. El -Sherief and T. Aboel-Fadl, Utility and synthetic uses of Mannich reaction: an efficient route for synthesis of thiadiazino-[1,3,5][3,2-a]benzimidazoles, Synth. Commun., 2006, 36(8), 987–996 CrossRef CAS.
- V. V. Dotsenko, K. A. Frolov and S. G. Krivokolysko, Synthesis of partially hydrogenated 1,3,5-thiadiazines by Mannich reaction, Chem. Heterocycl. Compd., 2015, 51(2), 109–127 CrossRef CAS.
- WHO, Guidelines for drinking-water quality, 4th edn, December 15th, 2019, Available from: https://www.who.int/water_sanitation_health/publications/dwq-guidelines-4/en/? Search PubMed.
- EPA, Drinking Water Contaminants – Standards and Regulations, December 15th, 2019, Available from: https://www.epa.gov/dwstandardsregulations Search PubMed.
- H. A. H. El-Sherief, Z. A. Hozien, A. F. M. El-Mahdy and A. A. O. Sarhan, Novel method for the synthesis of s-triazolo[3,4-b][1,3,4]thiadiazines, Synthesis, 2010, 15, 2636–2642 CrossRef.
- A. F. M. EL-Mahdy, Y. H. Hung, T. H. Mansoure, H. H. Yu, Y. S. Hsu, K. C. Wu and S. W. Kuo, Synthesis of [3 + 3] β-Ketoenamine-Tethered Covalent Organic Frameworks (COFs) for High-Performance Supercapacitance and CO2 Storage, J. Taiwan Inst. Chem. E, 2019, 103, 199–208 CrossRef CAS.
- A. F. M. El-Mahdy, Y. H. Hung, T. H. Mansoure, H. H. Yu, T. Chen and S. W. Kuo, A hollow microtubular triazine- and benzobisoxazole-based covalent organic framework presenting sponge-like shells that functions as a high-performance supercapacitor, Chem.–Asian J., 2019, 14, 1429–1435 CrossRef CAS PubMed.
- S. Haijian, S. Haoxin and W. Zhongyi, Efficient one-pot synthesis of s-triazolo[3,4-b]-[1,3,5]thiadiazines containing a chiral side chain by double Mannich type reaction, J. Heterocycl. Chem., 2001, 38, 929–932 CrossRef.
- A. M. El-sayed and A. Khodairy, Synthesis of new fused and spiro heterocycles derived from 4-amino-5-mercapto-3-trifluoromethyl-1,2,4-triazole, Phosphorus Sulfur Relat. Elem., 1998, 132(1), 41–52 CrossRef CAS.
- L. P. Lingamdinne, Y. Chang, J. Yang, J. Singh, E. Choi, M. Shiratani, J. R. Koduru and P. Attri, Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals, Chem. Eng. J., 2017, 307, 74–84 CrossRef CAS.
- A. A. El-Emam, A. S. Al-Tamimi, M. A. Al-Omar, K. A. Alrashood and E. E. Habib, Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones, Eur. J. Med. Chem., 2013, 68, 96–102 CrossRef CAS PubMed.
- S. Magaldi, S. Mata-Essayag, C. H. de Capriles, C. Perez, M. T. Colella, C. Olaizola and Y. Ontiveros, Well diffusion for antifungal susceptibility testing, Int. J. Infect. Dis., 2004, 8(1), 39–45 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02872j |
| This journal is © The Royal Society of Chemistry 2020 |