Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoinitiated decarboxylative C3-difluoroarylmethylation of quinoxalin-2(1H)-ones with potassium 2,2-difluoro-2-arylacetates in water†
Yanhui Gaoa,
Lulu Zhaoa,
Tianyi Xiangc,
Pinhua Li *a and
Lei Wang
*a and
Lei Wang *ab
*ab
aKey Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education, Department of Chemistry, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China. E-mail: pphuali@126.com; leiwang@chnu.edu.cn
bDepartment of Chemistry, Advanced Research Institute, Taizhou University, Taizhou, Zhejiang 318000, P. R. China
cCollege of Pharmacy, Shenyang Pharmaceutical University, Shenyang, 110016, P. R. China
First published on 12th March 2020
Abstract
An efficient and green strategy for the preparation of C3-difluoroarylmethylated quinoxalin-2(1H)-one via a visible-light-induced decarboxylative C3-difluoroarylmethylation of quinoxalin-2(1H)-one with potassium 2,2-difluoro-2-arylacetate in water at room temperature was developed. This photoinduced reaction generated the desired products in good yields under simple and mild conditions.
Introduction
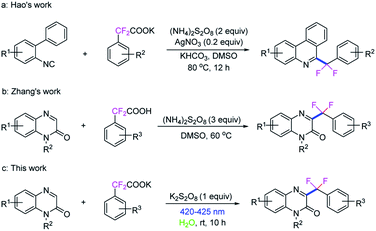
Organic compounds containing fluorine have been widely found in pharmaceuticals, agrochemicals and materials.1 The incorporation of fluorine atoms could remarkably change the physical and biological properties of its parent compounds, such as lipophilicity, stability, and bioavailability.2 Among various fluoroalkyl groups, the benzylic difluoromethylene group (ArCF2) has attracted much attention in medicinal chemistry, due to the fact that the ArCF2 moiety has unique stability, and isosteric properties as an ethereal oxygen atom or a carbonyl group.3 So, it is of great value for the construction of fluorinated molecules, especially in the designed structure of drugs. Traditionally, difluoromethylene groups are introduced into the molecular skeleton by a deoxyfluorination of aldehydes or ketones with aminosulfur trifluorides, XeF2, or F2.4 Most recently, transition metals including Cu-, Pd-, and Ni-catalyzed difluoroalkylation reactions have been developed.5 As a distinct type of difluorobenzylic compound, difluoroalkylated arenes are present in many bioactive compounds. Therefore, the exploration of practical and broadly applicable methods for the introduction of the ArCF2 group into target molecules is in high demand. α,α-Difluoroarylacetic acids and their salts are inexpensive, easy to store and simple to handle fluorine-containing regents, can be readily converted to a variety of useful ArCF2-containing compounds. Recently, the decarboxylative coupling of gem-difluoroarylacetic acids and their salts have been well established. For example, Wu's group reported a direct decarboxylative alkynylation of α,α-difluoroarylacetic acids under transition metal-free conditions.6 Hashmi's group developed a silver-catalyzed decarboxylative alkynylation of α,α-difluoroarylacetic acids with ethynyl-benziodoxolone reagents,7 and Hao's group disclosed a silver-catalyzed decarboxylative difluoroarylmethylation of difluoroacetates with isocyanides for constructing 6-gem-difluoromethylenated phenanthridines (Scheme 1a).8 Very recently, Wan and Hao's group demonstrated a palladium(II)-catalyzed decarboxylative meta-selective C–H difluoromethylation of arenes from easily accessible difluoroacetic acids, and then a Ag-catalyzed minisci C–H difluoromethylarylation of N-heteroarenes was also developed by the group.9 Despite these achievements, it is still desirable to develop practical and mild synthetic methods for the preparation of CF2-containing scaffolds.Quinoxalin-2(1H)-ones, especially the C3-functionalized derivatives are important moieties in pharmaceuticals and materials (Fig. 1).10 In the past few years, the C3-functionalizations of quinoxalin-2(1H)-ones have become a hot topics,11 including C3-arylation,12 C3-alkylation,13 C3-acylation,14 C3-amination,15 C3-phosphonation,16 C3-alkoxylation,17 C3-sulfenylation18 and C3-di/trifluoromethylation,19 have been extensive investigated. More recently, Zhang et al. reported a decarboxylative C3-difluoroarylmethylation of quinoxalin-2(1H)-ones with α,α-difluoroarylacetic acids in the presence of (NH4)2S2O8 (3.0 equiv.) in DMSO at 60 °C for 18 h (Scheme 1b),20 while an excessive dose of oxidant and high temperature was still need in this transformation.
As we all known, visible-light photocatalysis as a powerful tool for organic synthesis21 have met to the demands of reaction economy, operational simplicity and environmental friendliness. The organic transformations under visible-light irradiation in the absence of additional photocatalysts have received considerable attention, providing a challenging but meaningful direction for further photochemistry research. Because of our interest in visible-light-induced organic reactions without the photosensitizer,22 we here wish to describe a simple and efficient method for the direct C3-difluoroarylmethylation of quinoxalin-2(1H)-ones with potassium 2,2-difluoro-2-arylacetates via photochemical process without the additional photosensitizer in water under ambient conditions (Scheme 1c).
Results and discussion
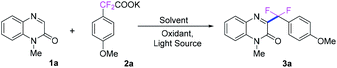
First, N-methyl-quinoxalin-2(1H)-one (1a) and potassium α,α-difluoro-2-(4-methoxyphenyl)acetate (2a) were used as the model substrates to optimize the reaction conditions, and the results were shown in Table 1. When the model reaction was conducted with 1.0 equivalent of K2S2O8 in DCE at room temperature under the irradiation of blue LED (420–425 nm) for 10 h, only trace amount of the desired product 3a was detected (Table 1, entry 1). To improve yield of the product, a number of solvents were examined. Organic solvents, such as DCE, DMSO, acetone and CH3CN show all negative effect to the reaction. To our delight, H2O exhibits excellent reactivity, delivering good yield of product 3a in 91% yield. However, co-solvents (DCE/H2O and CH3CN/H2O in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) give poor reactivity (Table 1, entries 2–7). The structure of 3a was characterized by 1H, 13C and 19F NMR, and the structure of 3i was further confirmed by X-ray single crystal analysis.24 In the absence of visible-light irradiation, no desired product was formed (Table 1, entry 8). A number of oxidants were also tested for the model reaction, and the results indicated that (NH4)2S2O8 is another effective oxidant, while no reactivity of BPO, DCP, BI-OH, TBHP and DTBP, and less reactivity of BQ, H2O2, generating 3a in 54% and 47% yields, respectively (Table 1, entries 9–16). When the model reaction was performed in the presence of oxygen atmosphere without K2S2O8, only 31% yield of 3a was isolated (Table 1, entry 17). It is worth noting that the desired product 3a was also obtained in 90% yield when the reaction was performed under a nitrogen atmosphere, which indicates that oxygen is not required in this transformation (Table 1, entry 18). Subsequently, the wavelength of light source was investigated and blue LED (420–425 nm) was the best choice for the reaction. When the wavelength was less than 420–425 nm or more than 420–425 nm, the results exhibited the less reactivity (Table 1, entries 19–23). Moreover, when sodium α,α-difluoro-2-(4-methoxyphenyl)acetate and α,α-difluorophenylacetic acid were used as substrate, the product 3a was obtained in 89% and 86% yield, respectively (entries 24–25). The loading of oxidant, the ratio of 1a to 2a, as well as the reaction time were optimized, which are also summarized in Table 1 (entries 26–28).
1 volume ratio) give poor reactivity (Table 1, entries 2–7). The structure of 3a was characterized by 1H, 13C and 19F NMR, and the structure of 3i was further confirmed by X-ray single crystal analysis.24 In the absence of visible-light irradiation, no desired product was formed (Table 1, entry 8). A number of oxidants were also tested for the model reaction, and the results indicated that (NH4)2S2O8 is another effective oxidant, while no reactivity of BPO, DCP, BI-OH, TBHP and DTBP, and less reactivity of BQ, H2O2, generating 3a in 54% and 47% yields, respectively (Table 1, entries 9–16). When the model reaction was performed in the presence of oxygen atmosphere without K2S2O8, only 31% yield of 3a was isolated (Table 1, entry 17). It is worth noting that the desired product 3a was also obtained in 90% yield when the reaction was performed under a nitrogen atmosphere, which indicates that oxygen is not required in this transformation (Table 1, entry 18). Subsequently, the wavelength of light source was investigated and blue LED (420–425 nm) was the best choice for the reaction. When the wavelength was less than 420–425 nm or more than 420–425 nm, the results exhibited the less reactivity (Table 1, entries 19–23). Moreover, when sodium α,α-difluoro-2-(4-methoxyphenyl)acetate and α,α-difluorophenylacetic acid were used as substrate, the product 3a was obtained in 89% and 86% yield, respectively (entries 24–25). The loading of oxidant, the ratio of 1a to 2a, as well as the reaction time were optimized, which are also summarized in Table 1 (entries 26–28).
| Entry | Solvent | Oxidant | Light source | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: N-methyl-quinoxalin-2(1H)-one (1a, 0.10 mmol), potassium 2,2-difluoro-2-(4-methoxyphenyl)acetate (2a, 0.15 mmol), oxidant (1.0 equiv.), solvent (3.0 mL) at room temperature under light irradiation (1.5 W) in air for 10 h.b Isolated yield. NR = no reaction.c Oxygen balloon instead of K2S2O8.d Nitrogen atmosphere.e Sodium α,α-difluoro-2-(4-methoxyphenyl) acetate was instead of 2a.f α,α-Difluorophenylacetic acid was instead of 2a.g K2S2O8 (0.75 equiv.).h K2S2O8 (1.5 equiv.).i 2a (0.1 mmol, 1.0 equiv.).j 2a (0.2 mmol, 2.0 equiv.).k 8 h.l 12 h. | ||||
| 1 | DCE | K2S2O8 | 420–425 nm | Trace |
| 2 | DMSO | K2S2O8 | 420–425 nm | Trace |
| 3 | Acetone | K2S2O8 | 420–425 nm | NR |
| 4 | CH3CN | K2S2O8 | 420–425 nm | NR |
| 5 | H2O | K2S2O8 | 420–425 nm | 91 |
| 6 | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
K2S2O8 | 420–425 nm | 42 |
| 7 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
K2S2O8 | 420–425 nm | <5 |
| 8 | H2O | K2S2O8 | In dark | 0 |
| 9 | H2O | BPO | 420–425 nm | Trace |
| 10 | H2O | DCP | 420–425 nm | Trace |
| 11 | H2O | BI-OH | 420–425 nm | Trace |
| 12 | H2O | TBHP | 420–425 nm | NR |
| 13 | H2O | DTBP | 420–425 nm | NR |
| 14 | H2O | (NH4)2S2O8 | 420–425 nm | 82 |
| 15 | H2O | BQ | 420–425 nm | 54 |
| 16 | H2O | H2O2 | 420–425 nm | 47 |
| 17 | H2O | O2 | 420–425 nm | 31c |
| 18 | H2O | K2S2O8 | 420–425 nm | 90d |
| 19 | H2O | K2S2O8 | 380–385 nm | 75 |
| 20 | H2O | K2S2O8 | 390–395 nm | 74 |
| 21 | H2O | K2S2O8 | 410–415 nm | 84 |
| 22 | H2O | K2S2O8 | 450–455 nm | 86 |
| 23 | H2O | K2S2O8 | Sunlight | 65 |
| 24 | H2O | K2S2O8 | 420–425 nm | 89e |
| 25 | H2O | K2S2O8 | 420–425 nm | 86f |
| 26 | H2O | K2S2O8 | 420–425 nm | 64g, 90h |
| 27 | H2O | K2S2O8 | 420–425 nm | 61i, 91j |
| 28 | H2O | K2S2O8 | 420–425 nm | 75k, 89l |
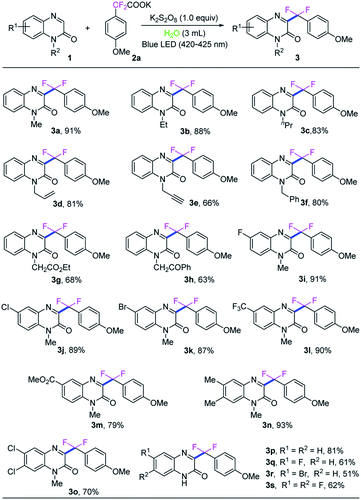
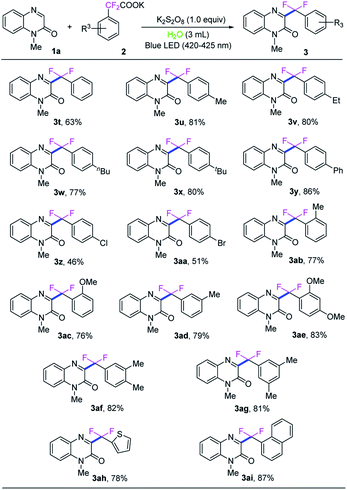
With the optimized reaction conditions in hand, we next investigated the generality of this direct C3-difluoroarylmethylation reaction. A variety of N-substituted quinoxalin-2(1H)-ones were subjected to the reaction, and the results are listed in Scheme 2. In general, all the selected quinoxalin-2(1H)-one derivatives could reacted with potassium 2,2-difluoro-2-(4-methoxyphenyl)acetate (2a) very smoothly under the standard conditions, indicating a broad tolerance of substituted groups including an electron-donating and an electron-withdrawing group on aromatic rings of quinoxalin-2(1H)-ones. Initial studies were focused on various N-protected quinoxalin-2(1H)-ones and the desired products 3a–3h were obtained in good to excellent yields. Next, a series of quinoxalin-2(1H)-ones bearing substituents on the benzene ring were also investigated under the optimal reaction conditions. In general, the C6-position substituted quinoxalin-2(1H)-ones bearing an electron-deficient group (F, Cl, Br, CF3, CO2CH3) could generate the desired products 3i–3m in good to excellent yields (79–91%). Moreover, the dimethyl-substituted substrate 1n, was compatible with the reaction as well, and provided the desired product 3n in 93% yield, while the dichloro-substituted substrate 1o furnished the product 3o in a lower yield of 70%. It should be noted that quinoxalin-2(1H)-ones without protecting group were also well matched with the transformation, while the expected products 3p–3s were obtained in middle yields because of the poor solubility of the starting materials and products in water and commonly used organic solvents.
Subsequently, the universality of potassium difluoroarylacetates were further explored, as shown in Scheme 3. Various para-substituted potassium α,α-difluoroarylacetates were used as difluoroarylmethylation reagent to react with N-methyl-quinoxalin-2(1H)-one (1a) to afford the corresponding difluoroarylmethylated quinoxalin-ones 3t–3aa in moderate to high yields. Generally, potassium α,α-difluoroarylacetates with electron-donating groups (3u–3y) gave higher yields than those with electron-withdrawing groups (3z–3aa). Compared with the corresponding 4-substituted potassium α,α-difluoroarylacetates. 2-Methyl, 2-methoxyl and 3-methyl substitutions in the arene ring of potassium α,α-difluoroarylacetates could provide the corresponding products in slightly lower yields. Moreover, the di-substituted substrates were also compatible with the reaction as well, and provided the desired product 3ae–3ag in good yields. To our delight, when the heterocyclic potassium difluoroarylacetate was employed, the transformation could also proceed smoothly and the corresponding product 3ah in 78% yield. In particular, naphthyl-based substrate was also examined and showed good reactivity in the reaction, providing 3ai in 87% yield.
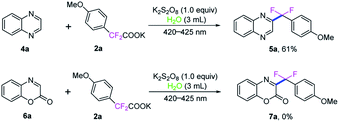
It is important to note that quinoxaline (4a) could also been involved in this direct C3-difluoroarylmethylation reaction, which reacted with potassium 2,2-difluoro-2-(4-methoxyphenyl)acetate (2a) under the standard conditions, providing the corresponding product 5a in 61% yield. However, when 2H-benzo[b][1,4]oxazin-2-one (6a) was employed in this transformation, no desired product 7a was detected (Scheme 4).
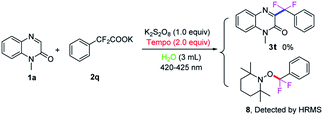
To further clarify the mechanism of this transformation, the control experiment was conducted, as shown in Scheme 5. When the model reaction was carried out in the presence of radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO, 2.5 equiv.) under the standard conditions, no desired product was found, suggesting that a radical process might involve in the reaction. An aryl difluoromethyl radical 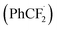 was trapped with TEMPO under standard reaction conditions to generate the corresponding adduct 8, which was detected by HRMS analysis.
was trapped with TEMPO under standard reaction conditions to generate the corresponding adduct 8, which was detected by HRMS analysis.
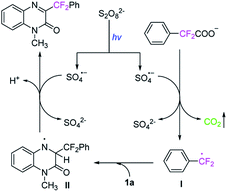
On the basis of above experimental results and relevant literature,23 a plausible mechanism is proposed in Scheme 6. The radical anion SO4−˙ was firstly generated from K2S2O8 under visible-light irradiation. With the assistance of radical anion SO4−˙, the potassium 2,2-difluoro-2-phenylacetate 2t undergoes a decarboxylation process to generate a radical intermediate I, releasing carbon dioxide. Then the radical intermediate 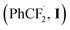 attacks quinoxalin-2(1H)-one 1a at C3-position to generate the radical intermediate II, which is further undergoes single-electron oxidation by loss of H+ to afford the product 3t.
attacks quinoxalin-2(1H)-one 1a at C3-position to generate the radical intermediate II, which is further undergoes single-electron oxidation by loss of H+ to afford the product 3t.
Conclusions
In summary, we have developed an efficient and environment-friendly synthetic protocol for the preparation of C3-difluoroarylmethylated quinoxalin-2(1H)-one via a visible-light-induced decarboxylative difluoroarylmethylation of quinoxalin-2(1H)-one with potassium 2,2-difluoro-2-arylacetate in water under simple and mild conditions. The reaction proceeds smoothly at room temperature afford the corresponding products in moderate to good yields with a broad substituent group tolerance. Further application of this photo-generated difluoroarylmethyl radical to other organic transformations and a detailed mechanistic study are underway in our laboratory.Experimental section
General remarks
The 1H NMR, 13C NMR and 19F NMR spectra were recorded on a 400 MHz or a 600 MHz Bruker FT-NMR spectrometer (400/100/376 MHz or 600/150/564 MHz, respectively). All chemical shifts are given as δ value (ppm) with reference to tetramethylsilane (TMS) as an internal standard. The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; m, multiplet; q, quartet. The coupling constants, J, are reported in hertz (Hz). High resolution mass spectroscopy data of the product were collected on an Agilent Technologies 6540 UHD Accurate-Mass Q-TOF LC/MS (ESI). Melting points (uncorrected) were obtained on WRS-1B digital melting point apparatus. The quinoxalin-2(1H)-ones and potassium 2,2-difluoro-2-(4-methoxyphenyl)acetates were prepared according to the reported literature.8,19a All the solvents and commercially available reagents were purchased from commercial suppliers. Products were purified by flash chromatography on 200–300 mesh silica gels, SiO2.Typical procedure for the photoinitiated decarboxylative C3-difluoroarylmethylation
A 5 mL oven-dried reaction vessel equipped with a magnetic stirrer bar was charged with N-methyl-quinoxalin-2(1H)-one (1a, 0.10 mmol), potassium 2,2-difluoro-2-(4-methoxyphenyl)acetate (2a, 0.15 mmol), K2S2O8 (0.10 mmol) and H2O (3.0 mL). The reaction vessel was exposed to a blue LED (420–425 nm, 1.5 W) irradiation at room temperature in air with stirring for 10 h. After completion of the reaction, the mixture was extracted with ethyl acetate and concentrated to yield the crude product, which was further purified by flash chromatography (silica gel, petroleum ether/ethyl acetate = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 9
1 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product 3a.
1) to give the desired product 3a.
Characterization data for products
Conflicts of interest
There are no conflicts to declareAcknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (21772062), the Young Talent Key Project of Anhui Province (170808J02) and the Scientific Research Project of Anhui Provincial Education Department (KJ2015TD002) for financial support of this work.Notes and references
- (a) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (b) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed; (c) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS PubMed.
- (a) F. Alonso, I. P. Beletskaya and M. Yus, Chem. Rev., 2004, 104, 3079 CrossRef CAS PubMed; (b) R. Chinchilla and C. Najera, Chem. Rev., 2007, 107, 874 CrossRef CAS PubMed; (c) G. K. S. Prakash and J. Hu, Acc. Chem. Res., 2007, 40, 921 CrossRef CAS PubMed; (d) J. Liu, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799 CrossRef CAS PubMed; (e) X.-L. Qiu, X.-H. Xu and F.-L. Qing, Tetrahedron, 2010, 66, 789 CrossRef CAS; (f) B. Godoi, R. F. Schumacher and G. Zeni, Chem. Rev., 2011, 111, 2937 CrossRef CAS PubMed; (g) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529 CrossRef CAS PubMed; (h) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (i) C. Hollingworth and V. Gouverneur, Chem. Commun., 2012, 48, 2929 RSC.
- (a) G. M. Blackburn, D. A. England and F. J. Kolkmann, J. Chem. Soc., Chem. Commun., 1981, 930 RSC; (b) Y. Xu, J. Aoki, K. Shimizu, M. Umezu-Goto, K. Hama, Y. Takanezawa, S. Yu, G. B. Mills, H. Arai, L. Qian and G. D. Prestwich, J. Med. Chem., 2005, 48, 3319 CrossRef CAS PubMed; (c) J. Zheng, Y. Li, L. Zhang, J. Hu, G. J. Meuzelaar and H. J. Federsel, Chem. Commun., 2007, 5149 RSC; (d) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529 CrossRef CAS PubMed; (e) P. S. Fier and J. F. Hartwig, Angew. Chem., Int. Ed., 2013, 52, 2092 CrossRef CAS; (f) Y. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov and S. Saphier, J. Med. Chem., 2017, 60, 797 CrossRef CAS.
- (a) J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793 CrossRef CAS; (b) T. Umemoto, R. P. Singh, Y. Xu and N. Saito, J. Am. Chem. Soc., 2010, 132, 1819 CrossRef PubMed; (c) P. Bannwarth, D. Gree and R. Gree, Tetrahedron Lett., 2010, 51, 2413 CrossRef CAS; (d) A. Khalaf, D. Gree, H. Abdallah, N. Jaber, A. Hachem and R. Gree, Tetrahedron, 2011, 67, 3881 CrossRef CAS; (e) Y. Li, K. A. Wheeler and R. Dembinski, Org. Biomol. Chem., 2012, 10, 2395 RSC.
- (a) Z. Feng, F. Chen and X. Zhang, Org. Lett., 2012, 14, 1938 CrossRef CAS PubMed; (b) Q.-Q. Min, Z. Yin, Z. Feng, W.-H. Guo and X. Zhang, J. Am. Chem. Soc., 2014, 136, 1230 CrossRef CAS PubMed; (c) Y.-B. Yu, G.-Z. He and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 10457 CrossRef CAS PubMed; (d) Z. Feng, Q.-Q. Min, Y.-L. Xiao, B. Zhang and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 1669 CrossRef CAS; (e) Y.-L. Xiao, W.-H. Guo, G.-Z. He, Q. Pan and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 9909 CrossRef CAS PubMed; (f) Y.-L. Xiao, B. Zhang, Z. Feng and X. Zhang, Org. Lett., 2014, 16, 4822 CrossRef CAS PubMed; (g) Z. Feng, Q.-Q. Min, H.-Y. Zhao, J.-W. Gu and X. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1270 CrossRef CAS; (h) Y.-L. Xiao, Q. Pan and X. Zhang, Acta Chim. Sin., 2015, 76, 387 Search PubMed; (i) Y. L. Xiao, Q. Q. Min, C. Xu, R. W. Wang and X. Zhang, Angew. Chem., Int. Ed., 2016, 55, 5837 CrossRef CAS PubMed.
- X. Li, S. Li, S. Sun, F. Yang, W. Zhu, Y. Zhu, Y. Wu and Y. Wu, Adv. Synth. Catal., 2016, 358, 1699 CrossRef CAS.
- F. Chen and A. S. K. Hashmi, Org. Lett., 2016, 18, 2880 CrossRef CAS PubMed.
- W. Wan, G. Ma, J. Li, Y. Chen, Q. Hu, M. Li, H. Jiang, H. Deng and J. Hao, Chem. Commun., 2016, 52, 1598 RSC.
- (a) H. Zhao, G. Ma, X. Xie, Y. Wang, J. Hao and W. Wan, Chem. Commun., 2019, 55, 3927 RSC; (b) X. Xie, Y. Zhang, J. Hao and W. Wan, Org. Biomol. Chem., 2020, 18, 400 RSC.
- (a) D. S. Lawrence, J. E. Copper and C. D. Smith, J. Med. Chem., 2001, 44, 594 CrossRef CAS; (b) U. J. Rise, H. W. M. Preppie, N. H. Haul, S. Handschuh, G. Mihm, J. M. Stassen, W. Wienen and H. Nar, Bioorg. Med. Chem. Lett., 2003, 13, 2297 CrossRef; (c) A. Cartaa, M. Lorigaa, S. Pirasa, G. Pagliettia, P. La Collab, B. Busonerab, G. Collub and R. Loddo, Med. Chem., 2006, 2, 113 CrossRef; (d) L. Xun, Y. K. Hui, L. W. Lu and X. W. Fang, Drugs Future, 2006, 31, 979 CrossRef; (e) B. Wu, Y. Yang, X. Qin, S. Zhang, C. Jing, C. Zhu and B. Ma, ChemMedChem, 2013, 8, 1913 CrossRef CAS; (f) S. N. Khattab, S. A. H. Abdel Moneim, A. A. Bekhit, A. M. El Massry, S. Y. Hassan, A. El-Faham, H. E. Ali Ahmed and A. Amer, Eur. J. Med. Chem., 2015, 93, 308 CrossRef CAS PubMed; (g) H. S. Dutta, A. Ahmad, A. A. Khan, M. Kumar, Raziullah and D. Koley, Adv. Synth. Catal., 2019, 361, 5534 CrossRef.
- For selected reviews, see: (a) Q. Ke, G. Yan, J. Yu and X. Wu, Org. Biomol. Chem., 2019, 17, 5863 RSC; (b) P. Mao, J. Zhu, J. Yuan, L. Yang, Y. Xiao and C. Zhang, Chin. J. Org. Chem., 2019, 39, 1529 CrossRef; (c) S. Monika and S. Selvakumar, Synthesis, 2019, 51, 4113 CrossRef.
- (a) Y.-Y. Han, Z.-J. Wu, X.-M. Zhang and W.-C. Yuan, Tetrahedron Lett., 2010, 51, 2023 CrossRef CAS; (b) A. Carrër, J.-D. Brion, S. Messaoudi and M. Alami, Org. Lett., 2013, 15, 5606 CrossRef; (c) K. Yin and R. Zhang, Org. Lett., 2017, 19, 1530 CrossRef CAS PubMed; (d) S. Paul, J. H. Ha, G. E. Park and Y. R. Lee, Adv. Synth. Catal., 2017, 359, 1515 CrossRef CAS; (e) J. Yuan, S. Liu and L. Qu, Adv. Synth. Catal., 2017, 359, 4197 CrossRef CAS; (f) H. I. Jung, J. H. Lee and D. Y. Kim, Bull. Korean Chem. Soc., 2018, 39, 1003 CrossRef CAS; (g) S. J. Kwon, H. I. Jung and D. Y. Kim, ChemistrySelect, 2018, 3, 5824 CrossRef CAS; (h) L. Wang, P. Bao, W. Liu, S. Liu, C. Hu, H. Yue, D. Yang and W. Wei, Chin. J. Org. Chem., 2018, 38, 3189 CrossRef CAS; (i) K. Yin and R. Zhang, Synlett, 2018, 29, 597 CrossRef CAS; (j) B. Ramesh, C. R. Reddy, G. R. Kumar and B. V. S. Reddy, Tetrahedron Lett., 2018, 59, 628 CrossRef CAS; (k) M. Noikham, T. Kittikool and S. Yotphan, Synthesis, 2018, 50, 2337 CrossRef CAS; (l) S. Paul, H. D. Khanal, C. D. Clinton, S. H. Kim and Y. R. Lee, Org. Chem. Front., 2019, 6, 231 RSC.
- (a) W. Wei, L. Wang, H. Yue, P. Bao, W. Liu, C. Hu, D. Yang and H. Wang, ACS Sustainable Chem. Eng., 2018, 6, 17252 CrossRef CAS; (b) L. Yang, P. Gao, X. Duan, Y. Gu and L. Guo, Org. Lett., 2018, 20, 1034 CrossRef CAS PubMed; (c) S. Toonchue, L. Sumunnee, K. Phomphrai and S. Yotphan, Org. Chem. Front., 2018, 5, 1928 RSC; (d) J. Yuan, J. Fu, J. Yin, Z. Dong, Y. Xiao, P. Mao and L. Qu, Org. Chem. Front., 2018, 5, 2820 RSC; (e) J. Fu, J. Yuan, Y. Zhang, Y. Xiao, P. Mao, X. Diao and L. Qu, Org. Chem. Front., 2018, 5, 3382 RSC; (f) L. Hu, J. Yuan, J. Fu, T. Zhang, L. Gao, Y. Xiao, P. Mao and L. Qu, Eur. J. Org. Chem., 2018, 4113 CrossRef CAS; (g) L. Liu, N. Pan, W. Sheng, L. Su, L. Liu, J. Dong, Y. Zhou and S. Yin, Adv. Synth. Catal., 2019, 361, 4126 CrossRef CAS; (h) L. Xie, L. Jiang, J. Tan, Y. Wang, X. Xu, B. Zhang, Z. Cao and W. He, ACS Sustainable Chem. Eng., 2019, 7, 14153 CrossRef CAS; (i) Z. Yan, B. Sun, X. Zhang, X. Zhuang, J. Yang, W. Su and C. Jin, Chem.–Asian J., 2019, 14, 3344 CrossRef CAS; (j) F. Lian, K. Xu, W. Meng, H. Zhang, Z. Tan and C. Zeng, Chem. Commun., 2019, 55, 14685 RSC; (k) L. Wang, J. Zhao, Y. Sun, H. Zhang and Y. Zhang, Eur. J. Org. Chem., 2019, 6935 CrossRef CAS; (l) W. Zhang, Y. Pan, C. Yang, L. Chen, X. Li and J. Cheng, J. Org. Chem., 2019, 84, 7786 CrossRef CAS; (m) W. Xue, Y. Su, K. Wang, R. Zhang, Y. Feng, L. Cao, D. Huang and Y. Hu, Org. Biomol. Chem., 2019, 17, 6654 RSC; (n) H. Zhang, J. Xu, M. Zhou, J. Zhao, P. Zhang and W. Li, Org. Biomol. Chem., 2019, 17, 10201 RSC; (o) D. Zheng and A. Studer, Org. Lett., 2019, 21, 325 CrossRef CAS PubMed; (p) Y. Gu, X. Duan, L. Chen, Z. Ma, P. Gao and L. Guo, Org. Lett., 2019, 21, 917 CrossRef CAS PubMed; (q) L. Xie, S. Peng, T. Fan, Y. Liu, M. Sun, L. Jiang, X. Wang, Z. Cao and W. He, Sci. China: Chem., 2019, 62, 460 CrossRef CAS; (r) B. Zhao, X. Kong and B. Xu, Tetrahedron Lett., 2019, 60, 2063 CrossRef CAS; (s) J. Wang, B. Sun, L. Zhang, T. Xu, Y. Xie and C. Jin, Org. Chem. Front., 2020, 7, 113 RSC.
- (a) X. Zeng, C. Liu, X. Wang, J. Zhang, X. Wang and Y. Hu, Org. Biomol. Chem., 2017, 15, 8929 RSC; (b) J.-W. Yuan, J.-H. Fu, S.-N. Liu, Y.-M. Xiao, P. Mao and L.-B. Qu, Org. Biomol. Chem., 2018, 16, 3203 RSC.
- (a) A. V. Gulevskaya, O. N. Burov, A. F. Pozharskii, M. E. Kletskii and I. N. Korbukova, Tetrahedron, 2008, 64, 696 CrossRef CAS; (b) Y. Li, M. Gao, L. Wang and X. Cui, Org. Biomol. Chem., 2016, 14, 8428 RSC; (c) T. T. Hoang, T. A. To, V. T. T. Cao, A. T. Nguyen, T. T. Nguyen and N. T. S. Phan, Catal. Commun., 2017, 101, 20 CrossRef CAS; (d) A. Gupta, M. S. Deshmukh and N. Jain, J. Org. Chem., 2017, 82, 4784 CrossRef CAS PubMed; (e) W. Wei, L. Wang, P. Bao, Y. Shao, H. Yue, D. Yang, X. Yang, X. Zhao and H. Wang, Org. Lett., 2018, 20, 7125 CrossRef CAS PubMed; (f) L. Sumunnee, C. Pimpasri, M. Noikham and S. Yotphan, Org. Biomol. Chem., 2018, 16, 2697 RSC; (g) K.-J. Li, K. Xu, Y.-G. Liu, C.-C. Zeng and B.-G. Sun, Adv. Synth. Catal., 2019, 361, 1033 CrossRef CAS; (h) Q. Yang, Z. Yang, Y. Tan, J. Zhao, Q. Sun, H.-Y. Zhang and Y. Zhang, Adv. Synth. Catal., 2019, 361, 1662 CrossRef CAS; (i) J. Yuan, J. Zhu, J. Fu, L. Yang, Y. Xiao, P. Mao, X. Du and L. Qu, Org. Chem. Front., 2019, 6, 925 RSC; (j) J.-W. Yuan, J.-L. Zhu, B. Li, L.-Y. Yang, P. Mao, S.-R. Zhang, Y.-C. Li and L.-B. Qu, Org. Biomol. Chem., 2019, 17, 10178 RSC; (k) L.-Y. Xie, J.-L. Hu, Y.-X. Song, G.-K. Jia, Y.-W. Lin, J.-Y. He, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 19993 CrossRef CAS; (l) T. Guo, C.-C. Wang, X.-H. Fu, Y. Liu and P.-K. Zhang, Org. Biomol. Chem., 2019, 17, 3333 RSC.
- (a) M. Gao, Y. Li, L. Xie, R. Chauvin and X. Cui, Chem. Commun., 2016, 52, 2846 RSC; (b) Y. Kim and D. Y. Kim, Tetrahedron Lett., 2018, 59, 2443 CrossRef CAS; (c) C. Hu, G. Hong, C. Zhou, Z.-C. Tang, J.-W. Han and L.-M. Wang, Asian J. Org. Chem., 2019, 8, 2092 CrossRef CAS; (d) K.-J. Li, Y.-Y. Jiang, K. Xu, C.-C. Zeng and B.-G. Sun, Green Chem., 2019, 21, 4412 RSC.
- (a) J. Xu, H. Yang, H. Cai, H. Bao, W. Li and P. Zhang, Org. Lett., 2019, 21, 469 CrossRef PubMed; (b) Q. Yang, X. Han, J. Zhao, H.-Y. Zhang and Y. Zhang, J. Org. Chem., 2019, 84, 11417 CrossRef CAS PubMed; (c) J. Zhou, P. Zhou, T. Zhao, Q. Ren and J. Li, Adv. Synth. Catal., 2019, 361, 5371 CrossRef CAS; (d) L. Zhao, L. Wang, Y. Gao, Z. Wang and P. Li, Adv. Synth. Catal., 2019, 361, 5363 CrossRef CAS.
- (a) Q.-H. Teng, Y. Yao, W.-X. Wei, H.-T. Tang, J.-R. Li and Y.-M. Pan, Green Chem., 2019, 21, 6241 RSC; (b) L.-Y. Xie, Y.-L. Chen, L. Qin, Y. Wen, J.-W. Xie, J.-X. Tan, Y. Huang, Z. Cao and W.-M. He, Org. Chem. Front., 2019, 6, 3950 RSC.
- (a) S. Liu, Y. Huang, F.-L. Qing and X.-H. Xu, Org. Lett., 2018, 20, 5497 CrossRef CAS; (b) L. Wang, Y. Zhang, F. Li, X. Hao, H.-Y. Zhang and J. Zhao, Adv. Synth. Catal., 2018, 360, 3969 CrossRef CAS; (c) L. Wang, H. Liu, F. Li, J. Zhao, H.-Y. Zhang and Y. Zhang, Adv. Synth. Catal., 2019, 361, 2354 CrossRef CAS; (d) Z. Wei, S. Qi, Y. Xu, H. Liu, J. Wu, H. Li, C. Xia and G. Duan, Adv. Synth. Catal., 2019, 361, 5490 CrossRef CAS; (e) J. Wang, B. Sun, L. Zhang, T. Xu, Y. Xie and C. Jin, Asian J. Org. Chem., 2019, 8, 1942 CrossRef CAS; (f) G.-Y. Dou, Y.-Y. Jiang, K. Xu and C.-C. Zeng, Org. Chem. Front., 2019, 6, 2392 RSC.
- G. Hong, J. Yuan, J. Fu, G. Pan, Z. Wang, L. Yang, Y. Xiao, P. Mao and X. Zhang, Org. Chem. Front., 2019, 6, 1173 RSC.
- For selected reviews, see: (a) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (b) L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC; (c) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (d) M. D. Kärkäs, J. A. Porco and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683 CrossRef PubMed; (e) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035 CrossRef CAS PubMed; (f) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed; (g) J. Chen, X. Hu, L. Lu and W. Xiao, Chem. Soc. Rev., 2016, 45, 2044 RSC; (h) J. Chen, X. Hu, L. Lu and W. Xiao, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed; (i) J. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel and G. A. Molander, Acc. Chem. Res., 2016, 49, 1429 CrossRef CAS PubMed; (j) I. Ghosh, L. Marzo, A. Das, R. Shaikh and B. König, Acc. Chem. Res., 2016, 49, 1566 CrossRef CAS PubMed; (k) M. N. Hopkinson, A. Tlahuext-Aca and F. Glorius, Acc. Chem. Res., 2016, 49, 2261 CrossRef CAS.
- (a) L. Zhao, P. Li, X. Xie and L. Wang, Org. Chem. Front., 2018, 5, 1689 RSC; (b) L. Zou, P. Li, B. Wang and L. Wang, Chem. Commun., 2019, 55, 3737 RSC.
- (a) Y. Zhao, B. Huang, C. Yang and W. Xia, Org. Lett., 2016, 18, 3326 CrossRef CAS PubMed; (b) D. Yang, G. Li, C. Xing, W. Cui, K. Li and W. Wei, Org. Chem. Front., 2018, 5, 2974 RSC.
- X-Ray single crystal structure 3i (CCDC number: 1961424).†
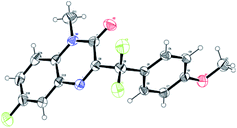 .
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1961424. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra02059a |
| This journal is © The Royal Society of Chemistry 2020 |