Open Access Article
Open Access ArticleEnhanced photodegradation of diphenhydramine in aqueous solution containing natural sand particles†
Chunlin Yia,
Lihong Songa,
Qingfeng Wu *a,
Zhaohui Li
*a,
Zhaohui Li *b,
Weibin Zhanga and
Ke Yinc
*b,
Weibin Zhanga and
Ke Yinc
aSchool of Physics and Optoelectronic Engineering, Yangtze University, 1 Nanhuan Road, Jingzhou, Hubei 434023, China. E-mail: wqfscience@aliyun.com
bDepartment of Geosciences, University of Wisconsin-Parkside, 900 Wood Road, Kenosha, WI 53144, USA. E-mail: li@uwp.edu
cFaculty of Earth Sciences, China University of Geosciences, 388 Lumo Road, Wuhan, Hubei 430074, China
First published on 4th May 2020
Abstract
Understanding the effects of natural solid particles on the phototransformation of pharmaceuticals in aqueous environments is very important, but studies on this are still limited. In this study, natural sands were selected as a solid particle model due to their wide distribution in surface waters during the rainy season, and the phototransformation of diphenhydramine (DP) in the presence of the sands was investigated. The kinetic studies showed that the natural sands exhibited significant photocatalytic activity for the DP photodegradation, and the activity varied depending on their sources. Scavenging experiments and electron paramagnetic resonance analysis demonstrated that O2−˙ and ˙OH were produced in the irradiated natural sand systems, and O2−˙ played a more important role than ˙OH in the photodegradation of DP. The results obtained from H2O2 treatment and deoxygenation experiments verified that the generation of radicals was mainly attributed to the low content of natural organic matter (NOM) in the sands. The possible reaction mechanism was that the NOM in the sands was excited and became triplet-state NOM after irradiation, and then induced the generation of free radicals through an electron transfer mechanism, resulting in DP oxidation. This work indicated that natural sand particles were a key factor affecting the phototransformation of drugs, and should be considered in evaluating their fate in natural waters.
1. Introduction
Extensive use of pharmaceutically active compounds (PhACs) has resulted in their frequent detection in natural waters.1–3 Although they were generally detected at low concentrations (ng L−1 to μg L−1), the long-term presence of PhACs in aquatic environments might provoke potential adverse effects on ecosystems and human health.4–6 Therefore, it is of great importance to investigate the transformation and fate of PhACs in the aqueous environment.Natural water system is a main reservoir of PhACs, where they undergo various degradation pathways to reduce their environmental concentrations, including biotic (bioaccumulation, biodegradation) and abiotic (sorption, hydrolysis, photolysis, oxidation) processes. For many pharmaceuticals, phototransformation is expected to be a significant attenuation process in natural waters. Currently, many studies have been conducted to investigate the photolysis of pharmaceuticals in aqueous solution. However, little work was done to the photolysis of pharmaceuticals in the presence of natural solid particles, and the information about the effects of natural solid particles on photoreaction and its mechanisms was still very limited. In fact, natural water bodies often contain a certain amount of suspended solid particles, such as sand, sediment, and organic substances. The presence of solid particles may influence the photolysis process via adsorption, light attenuation, light scattering, or act as a catalyst. Therefore, it is important to investigate the phototransformation of pharmaceuticals in the presence of solid particles in evaluating the fate of pharmaceuticals in aquatic environments.
Diphenhydramine (DP) belongs to the class of ethanolamine H1 receptor antagonist. It is the active ingredient of Benadryl, and commonly used in treatment of allergies, hives, itching and insomnia since 1946.7 DP has been commonly detected in wastewater influent and effluent,8 surface water,9 soil,10–12 and sediment.13 In response to the emergence of DP in water, several studies have been performed to investigate the removal of DP via photochemical processes, such as photolytic, heterogeneous photocatalytic, and photo-Fenton degradation. About 26% of the initial 5 μM of DP was removed under the UV fluence of 1272 mJ cm−2, and considerably higher removal of DP was obtained with the addition of 0.29 mM H2O2 under ultraviolet (UV) photolysis and UV/H2O2 advanced oxidation.14 In the presence of nitrate and humic substance (HS) under simulated solar light, hydroxyl radicals induced by nitrate and the triplet-state HS were the main reactive species responsible for DP degradation.15 Degradation of DP by TiO2 photocatalysis under different radiation sources: UVC, black blue lamps (BLB), simulated solar radiation (SB) and solar radiation (CPC) showed that the most efficient degradation and highest mineralization obtained after 60 min irradiation were 44.8% of DP removal in BLB and 9.0% of mineralization in SB.16 The addition of H2O2 drastically improved the photocatalytic process, obtaining 100% DP degradation and 28.6% total organic carbon (TOC) reduction in UVC system. In another study, the degradation of DP via photo-Fenton process was investigated, and 100% of DP elimination and 38.5% of TOC reduction were achieved, showing the high efficiency and mineralization.17 Moreover, the photodegradation of DP catalyzed by some synthetic materials was also reported.18,19 Nevertheless, the phototransformation of DP in water in the presence of natural solid particles have not yet been reported.
In view of the wide distribution of natural sands in surface water during the rainy season, we investigated the photodegradation of DP in the presence of two types of natural sands: desert sand (DS) and sea sand (SS) in aqueous solution. A series of experiments were systematically designed to elucidate the photolysis mechanisms. The main objects of this work were (1) to examine the possible catalytic effects of natural sands on the photodegradation of DP in aqueous solution; (2) to evaluate the relative importance of each reactive species produced in the photoreactions; and (3) to elucidate the intrinsic mechanisms for the photocatalytic activities of natural sands. This study will help understand the role of natural sands in the phototransformation of pharmaceuticals in aquatic environments.
2. Experimental
2.1 Materials
The natural sand samples SS and DS were collected from Shenzhen ocean beach and Tenggeli desert in China, respectively (Fig. S1†). The diphenhydramine hydrochloride (DP) was obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China), with a purity >98%. Acetonitrile, acetic acid, triethylamine, isopropanol (IPA) and p-benzoquinone (BQ) were of high-performance liquid chromatography (HPLC) grade, and purchased from J&K Chemical Co., Ltd (Beijing, China). The spin trapping reagent 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was obtained from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). All aqueous solutions were prepared with Milli-Q ultrapure water.2.2 Pre-treatment and characterization of natural sand samples
The natural sands were first sieved to obtain a uniform 60–100 mesh sized sand particles. Then, the sand samples were washed with ultrapure water until the dust was completely removed, dried at 60 °C for 4 h, and stored in a desiccator for further use. In order to maintain the natural attributes of sand samples, no chemical treatments were performed. The X-ray diffraction (XRD) patterns of the washed sand samples were recorded at 2θ 5° to 90° using Bruker D8 advanced XRD measuring instrument equipped with CuKα radiation. The collected XRD data were analyzed using MDI Jade 6.5 software. The results indicated that the basic components of the DS were quartz, albite, and gismondine, and the predominant component of the SS was quartz (Fig. S2†). The elemental compositions of natural sands were determined using a Rigaku-ZSX Primus II X-ray fluorescence (XRF) spectrometer. In order to quantify the content of natural organic matter (NOM) in the sand samples, the TOC content was measured by a TOC analyzer (LECO CS230, America). The results of XRF and TOC analyses were shown in Table 1.| Composition | % content | |
|---|---|---|
| SS | DS | |
| SiO2 | 93.3 | 77.4 |
| Al2O3 | 2.15 | 10.6 |
| CaO | 2.95 | 2.9 |
| K2O | 0.93 | 2.79 |
| Fe2O3 | 0.502 | 2.16 |
| Na2O | — | 2.14 |
| MgO | — | 1.37 |
| TiO2 | — | 0.266 |
| TOC | 0.019 | 0.011 |
2.3 Photolysis experiments
Photoreaction was conducted in a PR22-25 photochemical reactor (PerfectLight, China) equipped with a 300 W xenon light source (PLS-SXE300UV) and a water-cooling system DC-0506 (HengPing, Shanghai). The spectral output of the xenon light source ranged from 300 to 800 nm, and the irradiation entering the photoreactor was about 120 mW cm−2. During the irradiation, the suspension was stirred continuously, and the reaction temperature was kept at 4 °C by circulating water. To minimize water loss due to evaporation, the reactor was made airtight by sealing a quartz glass plate on the open top of the vessel. In each experiment, 1.5 g sand sample and 200 mL DP solution at a concentration of 40 mg L−1 were added to the 250 mL reactor, and then magnetically stirred for 60 min. After equilibration, the suspension was irradiated under simulated solar light. At the given time intervals, a 2 mL aliquot of the suspension was taken out, filtered through a membrane (0.45 μm), and analyzed by HPLC. Dark control experiments were performed in the same manner as for regular experiments except that they were not exposed to light. Duplicate experiments were performed in parallel to check the results.The concentration of DP in water was quantified by a Shimadzu high performance liquid chromatography (LC-20) equipped with a C18 ODS reversed phase column (4.6 mm × 150 mm, 5 μm) and a SPD20 UV detector. The mobile phase consisted of 25 mM acetic acid and acetonitrile (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v), with pH adjusted to 6.0 ± 0.1 using triethylamine. Isocratic elution was performed at a flow rate 0.8 mL min−1. The injection volume was 20 μL. Oven temperature was maintained at 30 °C and the detector wavelength was set at 220 nm.
40, v/v), with pH adjusted to 6.0 ± 0.1 using triethylamine. Isocratic elution was performed at a flow rate 0.8 mL min−1. The injection volume was 20 μL. Oven temperature was maintained at 30 °C and the detector wavelength was set at 220 nm.
To further evaluate the role of reactive species in the phototransformation of DP, reactive oxygen species (ROS) scavengers including IPA (˙OH scavenger) and BQ (O2−˙ scavenger) were used for the inhibition experiments. The photoreactions were also compared in the conditions with and without N2 purging. In order to determine free radicals in the photoreactions, the DMPO-free radical adducts were detected at room temperature by a JEOL JES-FA200 electron paramagnetic resonance spectrometer with center field of 3227.67 G, microwave frequency at 9054.62 MHz, and power at 0.998 mW.
3. Results and discussion
3.1 Enhanced photodegradation of DP in the presence of natural sands
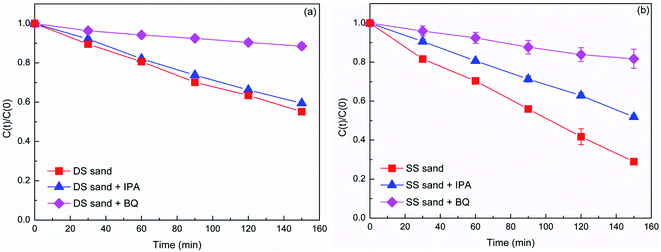
Fig. 1 illustrates photodegradation of DP against time in the presence and absence of natural sands. DP in aqueous solution was degraded under simulated solar light, and the degradation of DP increased with the prolonging of irradiation time. After 150 min irradiation, about 23% of DP was decomposed. A recent study by López et al.16 reported DP conversions of 32.5, 2.5, and 1.4% after 60 min irradiation under UVC, simulated solar light, and solar light, respectively. Compared with the aqueous solution, the presence of the DS and SS sands significantly enhanced the photodegradation of DP, exhibiting notable photocatalytic activity. In contrast, the photocatalytic activity of the SS sand was much higher than that of the DS sand. The difference in the photocatalytic activity of natural sands might be attributed to their different physicochemical properties, which could determine whether they act as a photocatalyst or an inhibitor. Some researchers pointed out that the presence of solid particles in aqueous solution could promote photodegradation via energy transfer reactions and efficient light scattering, or suppress it through excited-state quenching and light shielding.20–22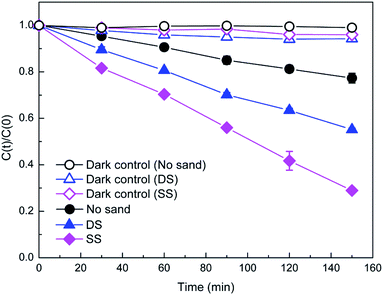 | ||
| Fig. 1 Photodegradation of DP (40 mg L−1) with and without the presence of natural sands (1.5 g) under simulated solar light. | ||
The photodegradation of DP was well described with the pseudo-first order kinetic model (R2 > 0.98), and the calculated pseudo-first order rate constants are summarized in Table 2. The estimated half-lives (t1/2) decreased from 400 min in the absence of sands to 182 and 93 min in the presence of DS and SS, respectively. Apparently, the presence of natural sands could affect the persistence of pharmaceuticals in the aqueous environment. Furthermore, different types of sand exhibited different effects. Hence the role of natural solid particles such as natural sands in the phototransformation of pharmaceuticals should be evaluated more comprehensively.
| Reaction condition | KDP (min−1) | R2 | t1/2 (min) |
|---|---|---|---|
| No sand | (1.73 ± 0.02) × 10−3 | 0.999 | 400.7 |
| DS sand | (3.80 ± 0.05) × 10−3 | 0.999 | 182.4 |
| SS sand | (7.40 ± 0.04) × 10−3 | 0.986 | 93.7 |
| DS sand + IPA | (3.52 ± 0.07) × 10−3 | 0.998 | 196.9 |
| DS sand + BQ | (0.74 ± 0.07) × 10−3 | 0.961 | 936.7 |
| SS sand + IPA | (4.3 ± 0.3) × 10−3 | 0.984 | 161.2 |
| SS sand + BQ | (1.41 ± 0.06) × 10−3 | 0.993 | 491.6 |
| DS sand treated with H2O2 | (2.34 ± 0.08) × 10−3 | 0.994 | 296.2 |
| SS sand treated with H2O2 | (1.82 ± 0.08) × 10−3 | 0.989 | 380.9 |
| DS sand + N2 | (6.68 ± 0.02) × 10−3 | 0.997 | 103.7 |
| SS sand + N2 | (1.6 ± 0.2) × 10−2 | 0.927 | 43.3 |
3.2 Study of reactive species
Generally, photo-induced ROS, such as singlet oxygen (1O2), superoxide anion radical (O2−˙), and hydroxyl radical (˙OH) were considered as the main reactive species in photocatalytic processes.23,24 As such, it could be speculated that the enhanced transformation of DP might also be due to the ROS produced in the natural sand system under irradiation. In order to determine the ROS in the present irradiation system, scavenger experiments were employed. In this study, the role of 1O2 in the DP degradation was excluded due to the inappreciable or too weak reactions between the amine drugs (including DP) and 1O2.15 Therefore, the determination of reactive species was focused on O2−˙ and ˙OH. Fig. 2 illustrated the photodegradation of DP in the presence of BQ (O2−˙ scavenger) and IPA (˙OH scavenger). For the DS system, the presence of BQ significantly suppressed the DP photodegradation, but the addition of IPA only showed a slightly inhibitory effect, indicating a more important role played by O2−˙ than by ˙OH. In the case of SS system, the drastic inhibition from the two radical scavengers implied the importance of both O2−˙ and ˙OH in the photoreaction. In addition, the rate constant for DP degradation in the presence of BQ is smaller than that in aqueous phase, which could be attributed to the light shielding of sand particles after free radical quenching (Table 2).The EPR spin-trapping technique, which is useful for detecting radical species, was deployed to further confirm the role of O2−˙ and ˙OH in the photocatalytic systems. The characteristic peaks of DMPO-O2−˙ adducts are observed in both natural sand systems after irradiation (Fig. 3). However, no signal was detected in the dark. With the increasing irradiation time, the intensity of peaks increased gradually. The evidence confirmed the generation of O2−˙ in the photocatalytic system of DS and SS. Comparatively, the signal in SS system is stronger than that in DS systems (Fig. S3†), indicating more O2−˙ generated in the SS system, which could partially interpret the larger rate constant for DP photodegradation in the SS system. Meanwhile, a quartet of signals with relative intensities of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from the DMPO–OH adducts were detected in DS and SS systems during the reaction process, implying that the ˙OH was formed in the catalytic systems, which is consistent with the results on the inhibitory effects of IPA (Fig. 4).
1 from the DMPO–OH adducts were detected in DS and SS systems during the reaction process, implying that the ˙OH was formed in the catalytic systems, which is consistent with the results on the inhibitory effects of IPA (Fig. 4).
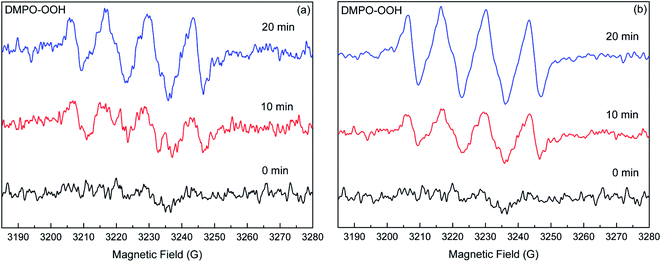 | ||
| Fig. 3 EPR spectral changes of the DMPO–O2−˙ adducts generated in the DS (a) and SS (b) systems under simulated solar light irradiation. | ||
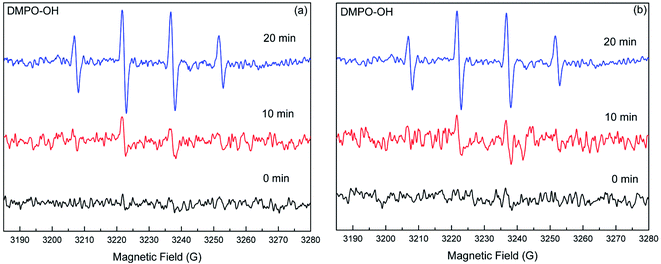 | ||
| Fig. 4 EPR spectral changes of the DMPO–˙OH adducts generated in the DS (a) and SS (b) systems under simulated solar light irradiation. | ||
Based on the radical scavenger experiments and the EPR analyses, it could be concluded that the O2−˙ and ˙OH were the main reactive species responsible for the enhanced transformation of DP in the natural sand systems. In the SS system, the strong signals of DMPO–O2−˙ and DMPO–OH adducts combined with the notable inhibition from radical scavengers demonstrated that the O2−˙ and ˙OH were the key factors contributing to the sand-enhanced photodegradation of DP. For the DS system, the information obtained from scavenger experiments and EPR analyses indicated that the O2−˙ played a dominant role in the DP transformation relative to the ˙OH.
3.3 Mechanisms for photocatalytic activity of natural sands
Silica was the main component of natural sands, and there were also some photoactive components such as iron oxide and titanium dioxide in the DS (Table 1). Apart from inorganic components, natural sands usually contain a small amount of NOM, such as HS. The TOC contents determined in DS and SS were about 0.011 and 0.019%, respectively (Table 1). As the major component of natural sands, silica itself could not enhance the photodegradation of DP, but inhibited it through light shielding (Fig. S4†). Thus, it could be concluded that the generation of free radicals in the natural sand systems was due to the photoactive components (iron oxide, titanium dioxide) or NOM.As is well known, the presence of iron oxide and titanium dioxide in aqueous solution could induce the generation of O2−˙ and ˙OH under irradiation. Thus, part of O2−˙ and ˙OH free radicals generated in the DS system could be attributed to these oxides. Besides, iron ions released from the natural sands could also induce the generation of free radicals under irradiation. To ascertain the role of iron ions, their content in aqueous phase after the experimentation was determined by inductively coupled plasma-mass spectrometry (ICP-MS). The results indicated that the iron ion contents in DS and SS systems were 0.104 and 0.091 ng mL−1, respectively. Considering that the iron ion content is too low, the role of iron ion in the generation of free radicals can be neglected. As for the role of NOM in the generation of free radicals in natural sand systems, it needs to be further confirmed. HS are important photosensitizers and can produce a series of reactive species including ˙OH, 1O2, O2−˙, eaq− (hydrated electron), and the triplet excited state HS under irradiation, but it could also act as a quencher of free radical under some conditions.25–28 In order to probe the role of NOM in the generation of free radicals, the photolysis of DP in the presence of the natural sands treated with H2O2 was performed. Removal of NOM significantly decreased the DP degradation (Fig. 5). The rate constant for the photodegradation of DP in the SS system was 7.40 × 10−3 min−1. However, after NOM removal, the rate constant decreased to 1.82 × 10−3 min−1, which was approximate to that in aqueous solution (1.73 × 10−3 min−1). This result suggested that the NOM played a dominant role in the generation of reactive radicals. For the DS, a decrease of the rate constant from 3.80 × 10−3 to 2.34 × 10−3 min−1 after NOM removal indicated the important contribution of NOM to the generation of free radicals (Table 2). Considering the rate constant after NOM removal was greater than that in aqueous solution, it can be inferred that the NOM was not the only factor that induced the generation of reactive radicals. The iron oxide and titanium dioxide in the DS also contributed to it.
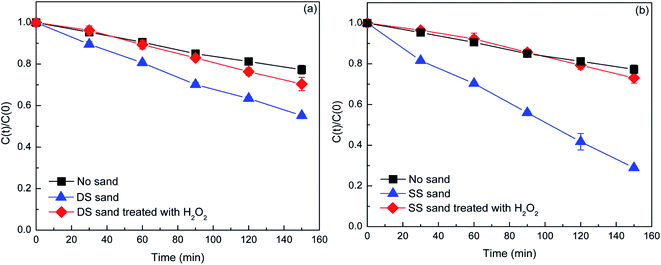 | ||
| Fig. 5 The contrast of the transformation of DP (40 mg L−1) in the presence of DS (a) and SS (b) (1.5 g) before and after H2O2 treatment. | ||
Generally, NOM induces the generation of free radicals via the triplet-state NOM excited by irradiation. To determine whether this process occurred in the natural sand system, deoxygenation experiments were performed. As dissolved oxygen (DO) is a very efficient triple-state quencher of organic compounds,29 the photolysis of DP was drastically enhanced in humic acid (HA) and fulvic acid (FA) solution after removing the DO.15 Based on this, it was concluded that the HS and FA triplet states were the main reactive species in the photochemical reaction, and an electron transfer mechanism for the reaction between the HS triplet states and amine drugs was proposed. Fig. 6 shows the contrast of the DP photodegradation in the solution with and without deoxygenation. Obviously, the reactions in DS and SS systems were enhanced in the deoxygenated solutions. The rate constant increased to 6.68 × 10−3 and 1.6 × 10−2 min−1 in the DS and SS systems, respectively. This result verified that the triplet-state NOM played an important role in DP degradation. Combined with the study of active species, it could be concluded that the triplet-state NOM acted more as a reactive inducer of free radicals than as a reactant in direct DP oxidation. After irradiation, the NOM was excited and became a triplet-state NOM, and then induced the generation of free radicals through electron transfer mechanism. The exclusion of DO reduced the quench of triplet-state NOM, and consequently increased the generation of free radicals resulting in enhanced degradation of DP. As shown in Table 1, the SS has much lower levels of iron oxide and titanium dioxide than the DS, but the NOM content of SS was nearly twice that of DS. Meanwhile, the SS exhibited much higher photocatalytic activity, suggesting that the NOM played a more important role than the iron oxide and titanium dioxide in the generation of free radicals.
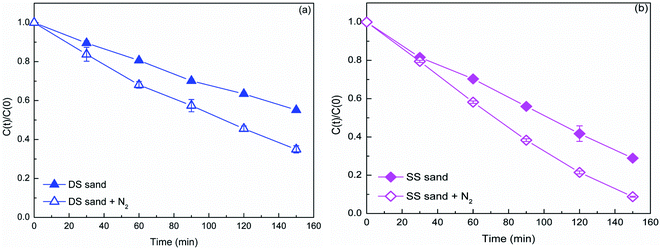 | ||
| Fig. 6 The transformation of DP (40 mg L−1) in the presence of DS (a) and SS (b) (1.5 g) in the deoxygenated solution. | ||
4. Conclusion
Understanding the effects of natural solid particles on the photolysis of pharmaceuticals was very limited. For the first time, the photolysis of DP in aqueous solution in the presence of natural sands was investigated. The results demonstrated that the presence of DS and SS greatly promoted the photodegradation of DP. Different types of natural sands showed different photocatalytic activities, which was attributed to their different compositions. The results obtained from the designed experiments demonstrated the O2−˙ and ˙OH induced by NOM in natural sands was mainly responsible for the enhanced DP degradation. The mechanisms for photocatalytic activity of natural sands could be elucidated as follows. After irradiation, the NOM in natural sands was excited and became a triplet-state NOM, and then induced the generation of free radicals through electron transfer mechanism, resulting in the degradation of DP. This work suggested that the presence of natural sands could significantly influence the phototransformation of drugs in aqueous solution. Therefore, as an important factor, the natural solid particles should be considered in evaluating the fate of drugs in the natural waters, especially in rainy season.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 41403083), the Key Project of Science and Technology of Hubei Provincial Department of Education (Grant D20141305) and Natural Science of Foundation of Hubei Province, China (Grant 2019CFB225).References
- Q. Bu, B. Wang, J. Huang, S. Deng and H. Yu, J. Hazard. Mater., 2013, 262, 189–211 CrossRef CAS PubMed.
- L. Arpin-Pont, M. J. M. Bueno, E. Gomez and H. Fenet, Environ. Sci. Pollut. Res., 2016, 23, 4978–4991 CrossRef PubMed.
- Y. Yang, Y. S. Ok, K. H. Kim, E. E. Kwon and Y. F. Tsang, Sci. Total Environ., 2017, 596–597, 303–320 CrossRef CAS PubMed.
- P. K. Jjemba, Ecotoxicol. Environ. Saf., 2006, 63, 113–130 CrossRef CAS PubMed.
- S. A. Ortiz de Garciá, G. P. Pinto, P. A. García-Encina and R. Irusta-Mata, Ecotoxicology, 2014, 23, 1517–1533 CrossRef PubMed.
- L. Cizmas, V. K. Sharma, C. M. Gray and T. J. McDonald, Environ. Chem. Lett., 2015, 13, 381–394 CrossRef CAS PubMed.
- J. P. Berninger, B. Du, K. A. Connors, S. A. Eytcheson, M. A. Kolkmeier, K. N. Prosser, T. W. Valenti Jr, C. K. Chambliss and B. W. Brooks, Environ. Toxicol. Chem., 2011, 30, 2065–2072 CrossRef CAS PubMed.
- B. Du, A. E. Price, W. C. Scott, L. A. Kristofco, A. J. Ranirez, C. K. Chambliss, J. C. Yederman and B. W. Brooks, Sci. Total Environ., 2014, 466–467, 976–984 CrossRef CAS PubMed.
- P. E. Stackelberg, E. T. Furlong, M. T. Meyer, S. D. Zaugg, A. K. Henderson and D. B. Reissman, Sci. Total Environ., 2004, 329, 99–113 CrossRef CAS PubMed.
- E. Topp, M. W. Sumarah and L. Sabourin, Sci. Total Environ., 2012, 439, 136–140 CrossRef CAS PubMed.
- C. A. Kinney, E. T. Furlong, S. L. Werner and J. D. Cahill, Environ. Toxicol. Chem., 2009, 25, 317–326 CrossRef PubMed.
- C. Wu, A. L. Spongberg, J. D. Witter, M. Fang, A. Ames and K. P. Czajkowski, Clean: Soil, Air, Water, 2010, 38, 230–237 CAS.
- I. Ferrer, C. E. Heine and E. M. Thurman, Anal. Chem., 2014, 76, 1437–1444 CrossRef PubMed.
- F. Yuan, C. Hu, X. Hu, J. Qu and M. Yang, Water Res., 2009, 43, 1766–1774 CrossRef CAS PubMed.
- Y. Chen, C. Hu, X. Hu and J. Qu, Environ. Sci. Technol., 2009, 43, 2760–2765 CrossRef CAS PubMed.
- N. López, P. Marco, J. Giménez and S. Esplugas, Appl. Catal., B, 2018, 220, 497–505 CrossRef.
- N. López, S. Plaza, A. Afkhami, P. Marco, J. Giménez and S. Esplugas, Chem. Eng. J., 2017, 318, 112–120 CrossRef.
- S. Morales-Torres, L. M. Pastrana-Martínez, J. L. Figueiredo, J. L. Faria and A. M. T. Silva, Appl. Surf. Sci., 2013, 275, 361–368 CrossRef CAS.
- L. M. Pastrana-Martínez, N. Pereira, R. Lima, J. L. Faria, H. T. Gomes and A. M. T. Silva, Chem. Eng. J., 2015, 261, 45–52 CrossRef.
- G. C. Miller and R. G. Zepp, Water Res., 1978, 1, 453–459 Search PubMed.
- T. Katagi, J. Agr. Food Chem., 1993, 41, 2178–2183 CrossRef CAS.
- R. Mathew and S. U. Khan, J. Agr. Food Chem., 1996, 41, 3996–4000 CrossRef.
- Y.-H. Chiu, T.-F. M. Chang, C.-Y. Chen, M. Sone and Y.-J. Hsu, Catalysts, 2019, 9, 430 CrossRef CAS.
- M.-J. Fang, C.-W. Tsao and Y.-J. Hsu, J. Phys. D: Appl. Phys., 2020, 53, 143001 CrossRef CAS.
- W. R. Haag and J. Hoigné, Environ. Sci. Technol., 1986, 20, 341–348 CrossRef CAS PubMed.
- J. V. Goldstone and B. M. Voelker, Environ. Sci. Technol., 2000, 34, 1043–1048 CrossRef CAS.
- R. G. Zepp, A. M. Braun, J. Hoigné and J. A. Leenheer, Environ. Sci. Technol., 1987, 21, 485–490 CrossRef CAS PubMed.
- P. Boule, M. Bolte and C. Richard, The Handbook of Environmental Chemistry, 1999, Springer, pp. 204–205 Search PubMed.
- S. Halladja, A. ter Halle, J. P. Aguer, A. Boulkamh and C. Richard, Environ. Sci. Technol., 2007, 41, 6066–6073 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02019b |
| This journal is © The Royal Society of Chemistry 2020 |