Open Access Article
Open Access ArticleCobalt doped BiVO4 with rich oxygen vacancies for efficient photoelectrochemical water oxidation†
Guoquan Liua,
Fei Li *a,
Yong Zhua,
Jiayuan Lia and
Licheng Sun
*a,
Yong Zhua,
Jiayuan Lia and
Licheng Sun ab
ab
aState Key Laboratory of Fine Chemicals, DUT-KTH Joint Education and Research Center on Molecular Devices, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: lifei@dlut.edu.cn
bDepartment of Chemistry, School of Engineering Sciences in Chemistry, Biotechnology and Health, KTH Royal Institute of Technology, Stockholm 10044, Sweden
First published on 3rd August 2020
Abstract
A facile electrodeposition method was developed to prepare Co-BiVO4 thin films with rich oxygen vacancies. The resulting photoanode exhibited a photocurrent density of 3.5 mA cm−2 1.23 V (vs. reversible hydrogen electrode (RHE), AM 1.5 G), which is over two times higher than that of undoped BiVO4.
Solar-driven photoelectrochemical (PEC) hydrogen production is a promising method towards clean energy. Among the candidate semiconductors for PEC water oxidation, monoclinic bismuth vanadate (BiVO4) as a promising photoanode material has attracted extensive attention due to its appropriate band gap (2.4–2.6 eV) with a VB edge position of ∼2.40 V vs. RHE, enabling absorption up to 9.2% of the photon flux in the solar spectrum.1–7 However, the solar hydrogen conversion efficiency of BiVO4 is still limited by its high charge recombination and poor surface reaction kinetics.8–12
Defect engineering has been widely used in electrocatalytic and photocatalytic water oxidation.13–16 The presence of defects can play an important role in leaking more active sites and increasing the active centers.17–19 For example, ultrathin spinel structured nanosheets with rich oxygen deficiencies have been evidenced as an excellent catalyst for electrocatalytic water oxidation. The presence of oxygen vacancy was found to increase the synergistic interplay between the number and the reactivity of active sites.20 Wang and co-workers prepared an efficient Co3O4 electrocatalyst with rich oxygen vacancies by plasma-engraving, where the oxygen vacancies on Co3O4 surface improve the electronic conductivity and create more active sites for OER.21 Pan and co-workers utilized multi-step deposition to fabricate multi-layer BiVO4 films with rich oxygen vacancies, which drastically enhances the photocatalytic activity of water oxidation.22 On the other hand, doping foreign elements is a promising strategy to tune the electrical properties of semiconductor materials.23–26 During the doping process, heteroatom dopants with different valence and size may play an additional role in introducing and stabilizing the oxygen vacancies.27,28 For instance, Jiang and Miao synthesized Zn-doped CoOOH with the abundance of oxygen vacancies and demonstrated that the oxygen vacancies were formed when the lower valence-state Zn occupies the lattice site of Co.29 Ding and co-workers also report that Mo doping on BiVO4 surface may introduce surface oxygen quasi-vacancies based on density functional theory computations. The presence of the oxygen vacancies was suggested to improve the adsorption of water molecules and enhance photocatalytic activity.30
Motivated by the above results, we report here the fabrication of the oxygen-vacancy-rich BiVO4 photoanode by means of cobalt doping. In this strategy, the dopant Co2+ cations partially occupy the lattice sites of Bi3+ in BiVO4 and the BiVO4 crystals tend to maintain the charge balance by forming oxygen vacancies. Theoretical calculations support that the formation energy of oxygen vacancies is significantly reduced in cobalt doped BiVO4 (Co-BiVO4). The resulting oxygen vacancies could potentially act as catalytic active sites to promote OER performance. As a result, the cobalt doped BiVO4 film gave a photocurrent density of 3.5 mA cm−2 at 1.23 V vs. RHE with a negatively shifted onset potential of 250 mV under AM 1.5 G (100 mW cm−2) solar illumination. The photocurrent reported here is the highest value among the doped BiVO4 electrodes without the assistance of co-catalysts.
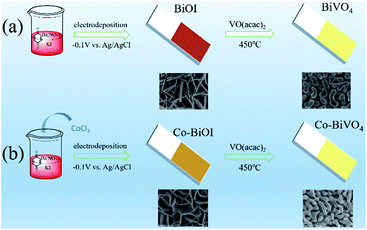
As shown in Scheme 1, electrodeposition of a solution containing p-benzoquinone, KI and Bi(NO3)3 gave perpendicularly oriented nanoflakes of BiOI (Fig. 1a).31 By addition of CoCl2 as a dopant, a potato-chip-like nanoflakes BiOI was obtained (Fig. 1c), the thin nanoflakes with curved wall are interconnected to form a reticulated porous microstructure. Both precursors gave interconnected nano-worms BiVO4 after heating in a muffle furnace at 450 °C (Fig. 1b and d).
 | ||
| Fig. 1 SEM image (a) BiOI, (b) BiVO4, (c) Co-BiOI, (d) Co-BiVO4, (e) the corresponding elemental mapping images of Co-BiVO4, (f) HRTEM image of Co-BiVO4. | ||
The Energy Dispersive X-ray Spectroscopy (EDS) in Fig. 1e confirms the homogeneous distribution of Co elements in Co-BiVO4 nano-worms. Fig. S1† shows the EDS results of 10Co-BiVO4 (abbreviated as Co-BiVO4), indicating the representative BiVO4 photoanode has Co atoms around 1.5 at% of the doping level. The high-resolution electron microscopy (HRTEM) revealed a lattice spacing of 0.26 nm, which is a well agreement with the (200) facets for monoclinic BiVO4 (Fig. 1f).
As shown in Fig. 2a, both undoped and doped films showed similar XRD patterns indexed to monoclinic BiVO4, implying that the introduction of Co dopant did not induce phase transformation in BiVO4. In Fig. S2,† the magnified analysis of the XRD pattern shows that the Bragg peak of BiVO4 after Co-doping shifts to a higher value of 2θ, which is in good agreement with the unit lattice shrinkage according to the Bragg equation (2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ).28 The shift of characteristic diffraction peaks indicates that Co is incorporated into the BiVO4 matrix and causes BiVO4 lattice distortion, which is consistent with the previous reports.12 The Raman spectra are also indicative of the monoclinic scheelite crystal structure for BiVO4 and Co-BiVO4 (Fig. 2b), with characteristic signals at 330, 365, 720 and 827 cm−1. The peaks at 330 and 365 cm−1 corresponded to the asymmetric and symmetric deformation modes of the VO43− tetrahedron (δas(VO3−) and δs(VO3−)), respectively.22 The peak at 827 cm−1 was due to the symmetric stretching mode of the V–O bands (νs(V–O)). No other diffraction peaks attributed to other phases or impurities were found.
θ = nλ).28 The shift of characteristic diffraction peaks indicates that Co is incorporated into the BiVO4 matrix and causes BiVO4 lattice distortion, which is consistent with the previous reports.12 The Raman spectra are also indicative of the monoclinic scheelite crystal structure for BiVO4 and Co-BiVO4 (Fig. 2b), with characteristic signals at 330, 365, 720 and 827 cm−1. The peaks at 330 and 365 cm−1 corresponded to the asymmetric and symmetric deformation modes of the VO43− tetrahedron (δas(VO3−) and δs(VO3−)), respectively.22 The peak at 827 cm−1 was due to the symmetric stretching mode of the V–O bands (νs(V–O)). No other diffraction peaks attributed to other phases or impurities were found.
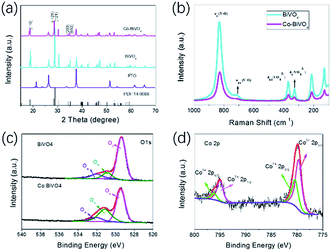 | ||
| Fig. 2 (a) XRD patterns of BiVO4, Co-BVO4. (b) Raman spectra of pristine BiVO4 and Co-BiVO4 films. High-resolution XPS spectra of (c) O 1s and (d) Co 2p for the Co-BiVO4 electrode. | ||
To investigate the chemical compositions and their oxidation states, the Co-BiVO4 film was characterized by X-ray photoelectron spectroscopy (XPS). The Bi 4f spectrum is shown in Fig. S1 (ESI†). The peaks located at 159.2 eV (Bi 4f7/2) and 164.5 eV (Bi 4f5/2) give evidence to the presence of Bi3+. Based on the V 2p spectrum (Fig. S2, ESI†), two peaks of V 2p at 516.8 eV (V 2p3/2) and 524.4 eV (V 2p1/2) were assigned to V5+. The peak at either 780.1 or 795.2 eV in the high-resolution spectrum of Co 2p (Fig. 2d) was deconvoluted into the 2p3/2 and 2p1/2 orbitals for Co2+ and Co3+, respectively, indicating that Co2+ was partially oxidized into cobalt(III) oxide on the surface of Co-BiVO4.32 The high-resolution O 1s spectra of Co-BiVO4 and BiVO4 samples were fitted into three components at 529.7, 531.3, 532.1 eV (Fig. 2c), corresponding to the O2− species in the lattice (OL), hydroxyl groups bound to metal cations in the oxygen-deficient region (OV) and the chemisorbed or dissociated oxygen species from water (OC).5 We further explored the effect of different doping concentrations of Co on the oxygen vacancy. As shown in Fig. S5,† the concentration of oxygen vacancy increases with the concentration of doped cobalt. This observation highlights a key role of cobalt in promoting the forming of oxygen vacancies.
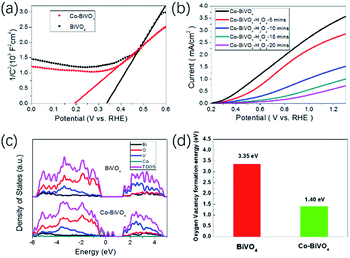
The performance of PEC water splitting was measured in 0.5 M borate buffer (pH 9.3) employing a three-electrode configuration with BiVO4 as the working electrode, a platinum wire as the counter electrode and Ag/AgCl as the reference electrode. The PEC water oxidation activities of Co-BiVO4 photoanodes with different Co doping concentrations were shown in Fig. 3a. In general, all Co-doped BiVO4 photoanodes show higher photocurrents than the undoped one. However, photocurrent density of the 12.5Co-BiVO4 electrode is lower than that of 10Co-BiVO4 (abbreviated as Co-BiVO4) electrode. This observation implies that an excess of oxygen vacancies produced by Co doping might become the recombination centers for the electron–hole pairs. When Co-BiVO4 was used as the photoanode, the onset potential for water oxidation was negatively shifted by 250 mV and a notably enhanced photocurrent density of 3.5 mA cm−2 at 1.23 V vs. RHE was obtained. The value acquired here was compared with the data in the literature, which is among the best performance for the doped BiVO4 without co-catalysts reported so far (Table S1, ESI†). Additionally, a photocurrent over 2 mA cm−2 at 0.7 V vs. RHE was obtained in an extended period of light illumination for 1 h (Fig. S6, ESI†). The slow decrease in the photocurrent is related to the oxidation and consumption of the oxygen vacancies during the PEC reaction.28 Based on the LSV plots, the maximum applied bias photon-to-current efficiency (ABPE) for the Co-BiVO4 was calculated to be 1.05% at 0.7 VRHE (Fig. 3b), compared to 0.2% for the undoped BiVO4. The incident photon-to-current conversion efficiency (IPCE) as a function of wavelength under 1.23 VRHE was presented in Fig. 3c. Over the visible light range, the IPCE values for Co-BiVO4 reached its maximum of 59% at 460 nm, well matching the corresponding UV-vis absorption spectrum (Fig. S7, ESI†).
Electrochemical impedance spectroscopy (EIS) was conducted at 1.23 V vs. RHE under the same conditions used for PEC measurements. According to the Nyquist plots (Fig. 3d), the Co-BiVO4 film featured a smaller resistance than the pristine BiVO4, demonstrating an enhanced conductivity and improved interfacial charge transfer by including cobalt dopants. The surface charge separation efficiency ηsurface was investigated in the presence of 0.5 M sodium sulfite as the hole scavenger. The Co-BiVO4 film exhibited a photocurrent density of 6.10 mA cm−2 with a charge separation efficiency of 56% at 1.23 V vs. RHE (Fig. S8†). By contrast, the pure BiVO4 electrode showed a poor charge separation of 39% (Fig. S9, ESI†). This result fully agrees with the trend shown by EIS measurements.
The flat band potentials (Efb) and the charge carrier density of pristine BiVO4 and Co-BiVO4 were investigated by Mott–Schottky analysis (MS). Since the charge carrier density was inversely proportional to the slopes of Mott–Schottky plots (Fig. 4a and Table S2, ESI†). The increase of donor density is expected to improve the electrical conductivity and reduce the internal charge transfer resistance.33 Moreover, the negative shift of Efb from 0.34 to 0.19 V after Co2+ doping, which is an advantage for electron accumulation and transport.28
To imply the effect of oxygen vacancies on water oxidation, Co-doped BiVO4 photoanode was placed in an electrolyte containing hydrogen peroxide that can efficiently eliminate the oxygen vacancies on electrode. Within a period of 20 minutes, the photocurrent for water oxidation was found to gradually fall from 3.5 to 0.7 mA cm−2 at 1.23 V vs. RHE (Fig. 4b), providing direct evidence to the correlation between oxygen vacancy and water oxidation activity.34
In order to further understand the relationship between Co2+ doping and oxygen vacancies, the density functional theory (DFT) calculations were performed (see ESI† for details).36,37 As shown in the density of states (DOSs) curves in Fig. 4c, the majority of Co 3d orbit stays in the valence band, and there is a small overlap between the Co 3d orbit and the O 2p orbit. Thus, the interaction between Co and the neighbor O atom is weaker than Bi and O atoms at the same sites, making the breaking of Co–O bond much easier.29 Consequently, more oxygen vacancies were created in Co-BiVO4 over the pure BiVO4. The calculated formation energy of oxygen vacancies for Co-BiVO4 was 1.40 eV, which is smaller than that for the pristine BiVO4 (3.35 eV), supporting our assumption that cobalt doping is an efficient approach for creating oxygen vacancies on BiVO4 (Fig. 4d).
To get insight into the crucial role of the oxygen vacancies of the catalyst in OER,20,35 the adsorption energy of H2O molecules was studied by DFT calculations. Fig. S10† indicates that the adsorption energy for a surface with oxygen vacancy is −0.43 eV, which is lower than the oxygen vacancy-free surface with an adsorption energy of −0.28 eV. A possible explanation for the enhancement of absorption energy is that oxygen vacancy would enhance the exposure of surface Bi atoms, which have been suggested to be the active sites for H2O adsorption.28,30
In conclusion, we have successfully fabricated a cobalt-doped BiVO4 with rich oxygen vacancies by a facile electrodeposition method. The DFT calculation evidenced that cobalt-doping facilitates the formation of oxygen vacancies by reducing their formation energy. The resulting Co-BiVO4 photoanode exhibited significantly improved PEC performance than the pure BiVO4 due to reduced H2O adsorption energy in catalysis and increased carries density that boosting the electrical conductivity of the electrode. Our results are important in demonstrating the correlations between vacancies and foreign element doping in PEC water splitting, which may inspire the further development of photoelectrodes with high efficiency.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21872016), the Swedish Research Council (2017-00935), the LiaoNing Revitalization Talents Program (XLYC1807125), the Swedish Energy Agency, and K&A Wallenberg Foundation.References
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- Q. Meng, B. Zhang, L. Fan, H. Liu, M. Valvo, K. Edstrom, M. Cuartero, R. De Marco, G. A. Crespo and L. Sun, Angew. Chem., Int. Ed., 2019, 58, 2–9 CrossRef.
- H. Ren, T. Dittrich, H. Ma, J. N. Hart, S. Fengler, S. Chen, Y. Li, Y. Wang, F. Cao, M. Schieda, Y. H. Ng, Z. Xie, X. Bo, P. Koshy, L. R. Sheppard, C. Zhao and C. C. Sorrell, Adv. Mater., 2019, 31, 1807204 CrossRef PubMed.
- M. García-Tecedor, D. Cardenas-Morcoso, R. Fernández-Climent and S. Giménez, Adv. Mater. Interfaces, 2019, 6, 1900299 CrossRef.
- S. Wang, P. Chen, Y. Bai, J. H. Yun, G. Liu and L. Wang, Adv. Mater., 2018, 30, 1800486 CrossRef PubMed.
- Y. Peng, G. V. Govindaraju, D. K. Lee, K. S. Choi and T. L. Andrew, ACS Appl. Mater. Interfaces, 2017, 9, 22449–22455 CrossRef CAS PubMed.
- M. Huang, C. Li, L. Zhang, Q. Chen, Z. Zhen, Z. Li and H. Zhu, Adv. Energy Mater., 2018, 8, 1802198 CrossRef.
- F. Yu, F. Li, T. Yao, J. Du, Y. Liang, Y. Wang, H. Han and L. Sun, ACS Catal., 2017, 7, 1868–1874 CrossRef CAS.
- B. B. Zhang, X. J. Huang, H. Y. Hu, L. J. Chou and Y. P. Bi, J. Mater. Chem. A, 2019, 7, 4415–4419 RSC.
- S. Wang, T. He, J.-H. Yun, Y. Hu, M. Xiao, A. Du and L. Wang, Adv. Funct. Mater., 2018, 28, 1802685 CrossRef.
- S. Wang, P. Chen, J. H. Yun, Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2017, 56, 8500–8504 CrossRef CAS PubMed.
- B. Zhang, H. Zhang, Z. Wang, X. Zhang, X. Qin, Y. Dai, Y. Liu, P. Wang, Y. Li and B. Huang, Appl. Catal., B, 2017, 211, 258–265 CrossRef CAS.
- H. Tan, Z. Zhao, W. B. Zhu, E. N. Coker, B. Li, M. Zheng, W. Yu, H. Fan and Z. Sun, ACS Appl. Mater. Interfaces, 2014, 6, 19184–19190 CrossRef CAS PubMed.
- Y. Liu, C. Ma, Q. Zhang, W. Wang, P. Pan, L. Gu, D. Xu, J. Bao and Z. Dai, Adv. Mater., 2019, 31, 1900062 CrossRef PubMed.
- R. Liu, Y. Wang, D. Liu, Y. Zou and S. Wang, Adv. Mater., 2017, 29, 1701546 CrossRef PubMed.
- Y. Zhao, Y. Zhao, R. Shi, B. Wang, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2019, 31, 1806482 CrossRef PubMed.
- L. Zhuang, L. Ge, Y. Yang, M. Li, Y. Jia, X. Yao and Z. Zhu, Adv. Mater., 2017, 29, 1606793 CrossRef PubMed.
- W. J. Xu, F. L. Lyu, Y. C. Bai, A. Q. Gao, J. Feng, Z. X. Cai and Y. D. Yin, Nano Energy, 2018, 43, 110–116 CrossRef CAS.
- K. Zhang, G. Zhang, J. Qu and H. Liu, Small, 2018, 14, 1802760 CrossRef PubMed.
- J. Bao, X. Zhang, B. Fan, J. Zhang, M. Zhou, W. Yang, X. Hu, H. Wang, B. Pan and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 7399–7404 CrossRef CAS PubMed.
- L. Xu, Q. Jiang, Z. Xiao, X. Li, J. Huo, S. Wang and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 5277–5281 CrossRef CAS PubMed.
- J.-M. Wu, Y. Chen, L. Pan, P. Wang, Y. Cui, D. Kong, L. Wang, X. Zhang and J.-J. Zou, Appl. Catal., B, 2018, 221, 187–195 CrossRef CAS.
- H. Miaoyan, B. Juncao, X. Wei, H. Chao and Z. Ruiqin, J. Mater. Chem. A, 2018, 6, 3602–3609 RSC.
- M. N. Shaddad, P. Arunachalam, J. Labis, M. Hezam and A. M. Al-Mayouf, Appl. Catal., B, 2019, 244, 863–870 CrossRef CAS.
- S. Qin, M.-L. Sebastián, T. Pengyi, F. Cristina, R. M. Joan, B. Zhaoyong, W. Hui and A. Teresa, ACS Catal., 2018, 8, 3331–3342 CrossRef.
- L. Han, S. Dong and E. Wang, Adv. Mater., 2016, 28, 9266–9291 CrossRef CAS PubMed.
- X. Yang, A. J. Fernandez-Carrion, J. Wang, F. Porcher, F. Fayon, M. Allix and X. Kuang, Nat. Commun., 2018, 9, 4484 CrossRef PubMed.
- Q. G. Pan, K. R. Yang, G. L. Wang, D. D. Li, J. Sun, B. Yang, Z. Q. Zou, W. B. Hu, K. Wen and H. Yang, Chem. Eng. J., 2019, 372, 399–407 CrossRef CAS.
- J. S. Wang, J. Liu, B. Zhang, F. Cheng, Y. J. Ruan, X. Ji, K. Xu, C. Chen, L. Miao and J. J. Jiang, Nano Energy, 2018, 53, 144–151 CrossRef CAS.
- K. Ding, B. Chen, Z. Fang, Y. Zhang and Z. Chen, Phys. Chem. Chem. Phys., 2014, 16, 13465–13476 RSC.
- Y. Wang, F. Li, X. Zhou, F. Yu, J. Du, L. Bai and L. Sun, Angew. Chem., Int. Ed., 2017, 56, 6911–6915 CrossRef CAS PubMed.
- F. Wang, Z. B. Liu, K. X. Wang, X. D. Zhu, X. H. Fan, J. Gao, Y. J. Feng, K. N. Sun and Y. T. Liu, Chem. Commun., 2018, 54, 5138–5141 RSC.
- Q. Shi, X. Song, H. Wang and Z. Bian, J. Electrochem. Soc., 2018, 165, H3018–H3027 CrossRef CAS.
- B. Zhang, L. Wang, Y. Zhang, Y. Ding and Y. Bi, Angew. Chem., Int. Ed., 2018, 57, 2248–2252 CrossRef CAS PubMed.
- G. Wu, N. Li, D. R. Zhou, K. Mitsuo and B. Q. Xu, J. Electrochem. Soc., 2004, 177, 3682–3692 CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01961e |
| This journal is © The Royal Society of Chemistry 2020 |