Open Access Article
Open Access ArticleRapid preparation of a Nafion/Ag NW composite film and its humidity sensing effect†
Yanjie Wang *ad,
Jiale Wanga,
Muyu Haoa,
Bo Li*b,
Zicai Zhub,
Xiaofan Gouc and
Lijie Li
*ad,
Jiale Wanga,
Muyu Haoa,
Bo Li*b,
Zicai Zhub,
Xiaofan Gouc and
Lijie Li d
d
aCollege of Mechanical and Electrical Engineering, Hohai University, Changzhou Campus, Changzhou, 213022, China. E-mail: yjwang@hhu.edu.cn
bShaanxi Key Lab for Intelligent Robots, State Key Laboratory for Manufacturing System Engineering, College of Mechanical Engineering, Xi'an Jiaotong University, Xi'an, 710049, China. E-mail: liboxjtu@xjtu.edu.cn
cCollege of Mechanics and Materials, Hohai University, Nanjing, 210098, China
dMultidisciplinary Nanotechnology Centre, College of Engineering, Swansea University, Swansea, SA1 8EN, UK
First published on 22nd July 2020
Abstract
In this study, we present a novel humidity-responsive composite film based on the integration of two silver nanowire (Ag NW) layers deposited via the physical deposition process with an ionomer (Nafion) layer sandwiched between them. As a result of the ion migration inside the ionomer, the humidity to electrical transduction displays a fast response and high sensitivity in comparison with previous research. Meanwhile, the effects of the humidity gradient on the sensing response of the Nafion/Ag NWs composite film were investigated. Specifically, a higher humidity enables a faster response rate but a slower recovery rate, as compared to a lower humidity level. In addition, breathing tests are performed, which verified the opportunities for the design and fabrication of high-performance sensors for environment and biophysical monitoring. This research also revealed the potential applications in VOC (volatile organic compound) detection.
1 Introduction
A reliable and sensitive sensor with the ability to measure and monitor humidity is indispensable in numerous industrial applications, including micromachining, semiconductor fabrication, MEMS (Microelectromechanical systems) manufacturing, and chemical processes. Consequently, tremendous efforts have been devoted to the development of low-cost and high sensitive humidity sensors for humidity detection. Many creative methods based on different mechanisms, including optical, gravimetric, capacitive, resistive, piezoresistive, and magnetoelastic, have been proposed for humidity sensing applications.1–7 During these current transduction techniques, there is a requirement of auxiliary facilities to detect and amplify the signal change in their electrical parameters, some of which are seriously affected by the environment and even need signal filtration so as to obtain more stable and accurate data. Therefore, there is a strong need to explore a passive and anti-interference humidity sensor.As one kind of ionomers, Nafion developed by DuPont exhibits multi-parameter sensitivity characteristics due to its unique ionic properties with a two-phase combination of a stable polymer matrix backbone interlaced with ion clusters. The polymer matrix structure provides the mechanical strength while the ion clusters provide the transfer channels for hydrated cations, as shown in Fig. 1a. Through the directional migration of special hydrated cations inside Nafion, Nafion-based composites with dual-ability of sensing and actuation provide potential opportunities for the development of future transduction devices, especially to provide a potential passive sensing technology. Similar to the ion channel-induced transduction mechanism inside Nafion membranes, ion channels inside the cell membrane formed by the membrane proteins allow specific ions to pass through the channel pore, realizing functions such as signal transmission and stimulus perception.8,9 Therefore, Nafion-based composites show great potential in biomimetic applications.
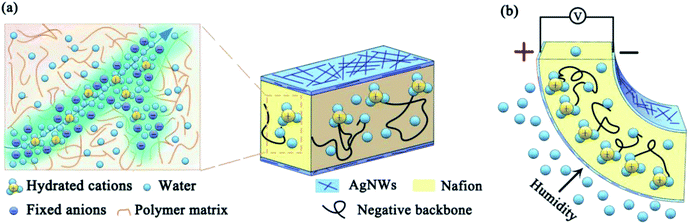 | ||
| Fig. 1 Schematic diagrams of (a) microchannel inside the Nafion/Ag NWs film; (b) humidity electrical response mechanism of the Nafion/Ag NWs film. | ||
Recently, Nafion-based composites have been widely used in various fields from energy applications to bio-inspired and biomedical applications, such as active catheter systems, heart-assistive devices, and even artificial muscles.10–12 After the conductive electrode layers are deposited on both sides of the Nafion membrane, some special phenomena occurs in the case of voltage stimulus. The movable ions together with the solvent molecules inside the membrane are forced to migrate under the presence of an electrical field, resulting in local strains along the direction of the thickness. This in turn causes macroscopic bending deformation of the composite film. In the opposite case, external physical stimulus results in a macroscopic shape or structural change, and the corresponding strain gradient is generated inside the composite film, and then the ions will flow along the gradient direction, which in turn generates the electrical signal. This composite film is often called an ionic polymer metal composite (IPMC), which is considered to be one of the popular types of artificial muscles.13–17 Due to the special solid/liquid two-phase structures inside Nafion, it has a natural affinity for moisture due to which it undergoes a large volume change when it is exposed in a humid environment, as shown in Fig. 1b. This makes Nafion an excellent humidity sensitive material.18 Recently, researchers have reported humidity sensors based on the strain, capacitance, and resistance of the Nafion composites caused by humidity change.19–21 Obviously, changes in ambient humidity can affect the anisotropic swelling characteristics of the Nafion membrane. According to the conversion mechanism of the above-mentioned IPMC, the humidity change will cause local anisotropic distribution of the ions inside the Nafion membrane, which will inevitably generate a potential difference. This signal can directly reflect the change in the ambient humidity, as shown in Fig. 1c. Kolahdouz et al. studied the IPMC-based capacitive humidity sensor and then investigated an IPMC humidity sensor that directly generated electrical signals.22,23 They used a Nafion-doped layered double hydroxide nanocomposite as the matrix and made a humidity sensor prepared by the traditional preparation process of IPMC to study the influence of the content of the layered double hydroxide on the sensing signal of the humidity sensor. However, since the dense electrode layer hinders the water molecules from entering inside Nafion, traditional IPMC is not suitable for humidity sensing. Furthermore, their research did not clarify the humidity sensing mechanism of the Nafion-based laminated structure. In previous studies, our group has carried out a series of studies on the preparation process, transduction mechanism, and actuation/sensing model of IPMC.24–28 We revealed that the presence of noble metal electrode layers and the penetrated interfacial layer of IPMC obtained by the impregnation–reduction (IR) step can seriously block the exchange between water molecules and Nafion. During the preparation process of IPMC, the pretreatment by surface roughening is very interesting. The purpose of this step is to increase the penetrated depth and bonding strength between the metal electrode layer and Nafion, which will inevitably make the junction of the surface and the interface of IPMC denser. But in the absence of this step, the surface electrode layer obtained by the IR step is still dense due to the smooth surface of the Nafion film. Both these situations prevent the penetration of water molecules into or from the IPMC. In order to solve the above-mentioned issue, we proposed a novel humidity film sensor consisting of Nafion membrane and Ag NWs, which can clearly sense the change in the ambient humidity by directly measuring its generated electrical signal. As one of the most popular ion exchange membranes, Nafion 117 exhibits high stability and ion capacity and Ag NWs as electrode materials have the advantages of high conductivity, flexibility, and gas permeability compared with ITO (indium tin oxide), metal grid, and carbon nanotubes. The rest of our work is organized as follows. In Section 2.1, we describe the list of chemicals and the preparation process of the Nafion/Ag NWs composite film. In Section 2.2, the testing methodology and the characterization of the composite film are represented. In particular, we introduce a humidity control device to achieve rapid switching of the humidity level. Later, the results and discussions are given in detail, and then the sensitivity of response of the composite film to humid gas and VOCs from the human breathing process have been simply tested and analyzed in Section 3. Finally, some highlighted conclusions are provided in Section 4.
2 Experimental section
2.1 Material preparation
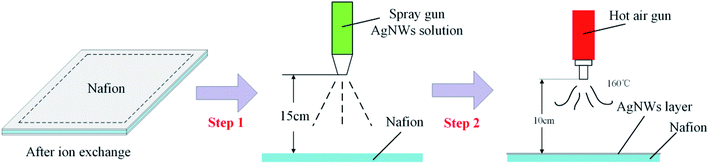
In our experiments, commercially available Nafion® 117 membrane (N117) with a typical thickness of 183 micrometers, which served as the middle layer, was selected and purchased from Dupont™. The cationic nickel(II) complexes prepared in our lab based on ref. 29 were selected as the specific cations inside the middle layer. Ag NWs (40 nm in diameter with the length range of 20–60 nm, concentration at 20 mg mL−1 using water as the solvent) were purchased from XFNANO Materials Tech Co., Ltd (Nanjing, China). The nickel(II)–chelate complexes were prepared in our lab. Auxiliary reagents such as H2O2 and HCl were obtained from J&K Chemical Inc. (Beijing, China). All the reagents were used as received without further purification. All the solutions were prepared with deionized water treated with a Hitech-kflow water purification system (Shanghai, China).To prepare the Nafion/Ag NWs composite film, a key challenge in this work is the bonding of the Ag NWs layer to the Nafion membrane. To solve this problem, we have developed a novel physical coating process, the main steps of which are shown in Fig. 2. A certain size of the Nafion membrane was cut and separately boiled in deionized water and hydrochloric acid, and then soaked in the nickel(II)–chelate complex solutions prepared in our lab (Fig. S1 and S2†). Diluted Ag NWs solution with a certain proportion of deionized water was poured into a spray gun, which can precisely control the flow rate. A small amount of the Ag NWs was sprayed evenly on the surface of the Nafion membrane and then the membrane was baked with a hot air machine for several minutes. This process was repeated 3–5 times until the surface resistance of the baked film was dropped down to 5 ohms □−1.
The detailed preparation process of the Nafion/Ag NWs composite film is as follows.
(a) Sample cutting: a Nafion film with the size of 32 mm length and 5.5 mm width was cut and rinsed with deionized water 2–3 times.
(b) Pretreatment: the Nafion film was put into hydrogen peroxide (3% wt), heated in a water bath (100 °C), and then rinsed with deionized water 2–3 times. The film was put into hydrochloric acid solution (0.2 mol L−1) and then heated in a water-bath (100 °C) for 2 h. After this, the film was taken out from the acid solution and rinsed with deionized water 2–3 times.
(c) Ion exchange: the Nafion film was put into the nickel(II)–chelate complex solutions for 48 h and then rinsed with deionized water 2–3 times, followed by drying for 2 h in an oven (80 °C).
(d) Ag NWs spraying: the treated Nafion film was placed on a glass plate, the spray gun (0.2 mm caliber, 0.15 MPa) was placed 10 cm away from the film and used to homogeneously disperse the silver nanowire solution (0.5 mg mL−1) on it.
(e) Hot air drying: the hot air gun was put 10 cm away from the film and heated at 160 °C. When the water dried, a silver nanowire electrode layer was formed. Steps (d) and (e) were repeated to form the electrode layer on the other side.
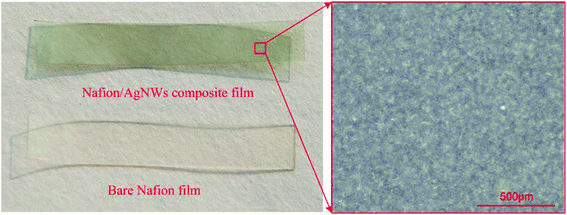
(f) Post processing: the surface resistance was adjusted by controlling the spraying time in steps (c)–(e). The film was trimmed to avoid short circuit and the Nafion/Ag NWs composite film with 30 mm length and 5 mm was obtained, as shown in Fig. 3.
2.2 Method and characterization
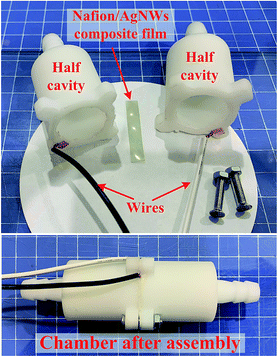
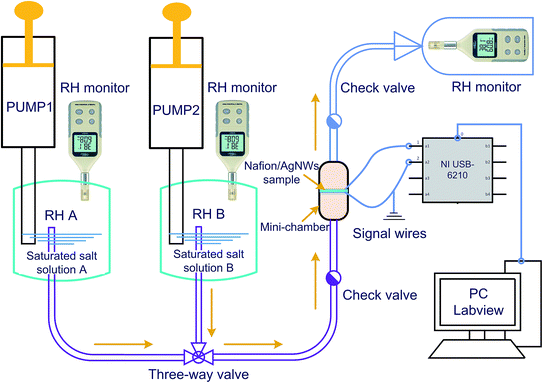
To date, it is still a convenient and low-cost method to obtain a constant humidity environment by using a fixed saturated salt solution. To implement the RH sensing test and to achieve a rapid switching of the humidity level, we designed and fabricated a mini-chamber of 6 cm length and 3 cm diameter by 3D printing technology, as shown in Fig. 4. Due to this small volume inside the mini-chamber, it can not only hold a certain amount of humidity gas for exchanging but can also easily achieve fast switching from one humidity level to another.The total set-up is shown in Fig. 5. Both the tanks RH A and RH B with fixed humidity were obtained for the specific saturated salt solutions by exchange for 24 h, connected into two ports of the three-way valve, and the remaining port was connected to the mini-chamber. We obtained different humidity levels by replacing the tank with another one. In our experiments, we put excess color changing silica gel into the tank. If most of the silica gel always stayed blue, we considered that the humidity level in the tank was close to 0% RH, which is approximately defined as 0% RH. Two check valves were set near the inlet and the outlet of the chamber. The film sample was fixed inside the mini-chamber and the generated electrical signal due to the change in the humidity level was transmitted to the PC through an NI acquisition card. In the measurement process, the side of the Nafion/Ag NW composite film that first contacts the humid gas was grounded (Fig. 5). It is important to keep the humidity constant in the mini-chamber during the test, which was achieved by monitoring the humidity level in the tanks and the outlet of the chamber. Meanwhile, in order to eliminate the interference of the electrical signal generated by the vibration of the sample, we fixed the sample on a stiff frame to avoid macroscopic deformation brought due to the gas flow.
In order to explore the electrical properties of the Nafion/Ag NWs composite film's response to humidity, the room humidity of approximately 64% RH in our laboratory was set as the reference (room temperature was 20 °C). The humidity levels, including 0% RH, 23% RH, 43% RH, 84% RH, and 100% RH, were approximately obtained, as shown in Table 1. Then, the gas with a fixed relative humidity was filled into the mini-chamber. The humidity change will cause local anisotropic distribution of the cations inside the mid-layer so as to generate an electrical signal change in the voltage (ΔV) on both sides of the composite film, which were recorded on the PC.
| Approximate relative humidity (RH/20 °C) | 0% | 23% | 43% | 64% | 84% | 100% |
| Methods | Color changing silica gel (mostly remained blue) | Saturated salt solution (CH3COOK) | Saturated salt solution (K2CO3) | Room humidity | Saturated salt solution (KCl) | Excess DI water |
| Exchange time | More than 24 h | |||||
3 Results and discussions
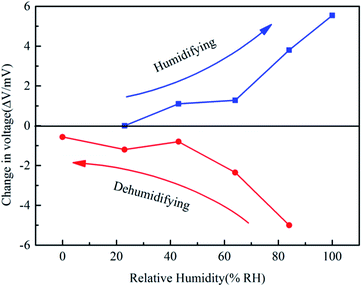
As shown in Fig. 6, all the response trends corresponding to humidity change displayed a fast response time of less than 500 ms in the range of 23–100% RH. All the results show that the change in the voltage (ΔV) of the composite film exhibits the relaxed characteristics of a slow recovery after a quick rise, which is very similar to the actuation and sensing process of IPMC.30,31 When the Nafion/Ag NWs composite film is in a certain humidity state (64% RH, for example) for a long time (over 24 h), the water molecules inside the composite film and the moisture of the ambient humidity will exchange until a dynamic equilibrium is reached. After the high-humidity or low-humidity gas is charged, the equilibrium state will be broken. Then, the abundant water molecules in the environment will be exchanged into the composite film or the water molecules inside the composite film will go through the Ag NWs layer into the surroundings, finally reaching a balance again, which causes the anisotropic swelling of the composite film and in turn forces the water molecules to drag and move the ions to migrate along the direction of the strain gradient. Along with this process, a potential difference is generated. We extracted the maximum amplitude (ΔV) of each curve in Fig. 6 and the time (Δt) required to reach the maximum amplitude, and then calculated the response rate (ΔV/Δt) in each case in the experiments, as shown in Fig. 7. Both the maxima and the response rates show an increasing trend with an increase in the humidity. We fitted the data of electrical response maxima by using the linear equation y = a + bx step by step. There is an obvious linear relationship from 64% RH to 0% RH and 64% RH to 100% RH, which shows a slope of 0.237 mV/% RH during the humidifying process and 0.0567 mV/% RH during the dehumidifying process. According to this equation, the electrical response of the composite film from 64% RH to other humidity levels can be predicted. Meanwhile, it can be seen from the curve of the response rates that the greater the humidity gradient, the faster the humidity response rate, and accordingly, the stronger the electrical response. It should be noted that the gas of specific humidity is filled into the chamber so quickly that the contact time between the sample and the gas could be neglected due to the minor influence during the contact process.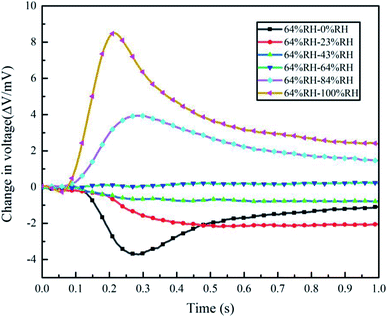 | ||
| Fig. 6 Humidity electrical response curves of the Nafion/Ag NWs composite film from 64% RH to other humidity levels. | ||
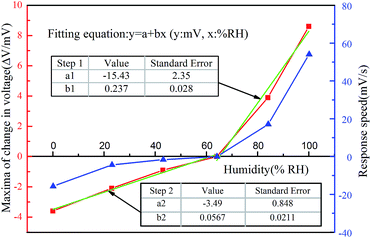 | ||
| Fig. 7 The maximum values and the response rate of the Nafion/Ag NWs composite film. The red line with the square symbols displays the trend of maximum values extracted from Fig. 6. The blue line with triangle symbols represents the trend of the response speed values calculated by ΔV/Δt. The green piecewise straight line is obtained by fitting the response speed data. | ||
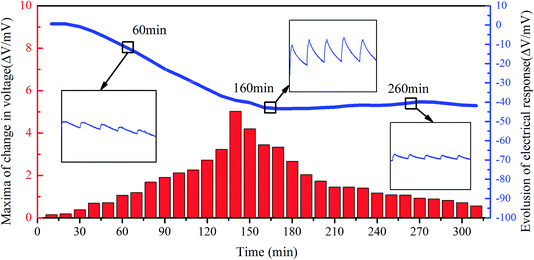
There is an obvious linear relationship for the case of 64% RH to 0% RH but no obvious change in the voltage was observed when the gas of 64% RH was charged into the chamber after the sample exchanged for over 24 h at 0% RH. In order to further confirm the sensing performance of the sample after complete exchange with the 0% RH environment, the gas with 64% RH was filled into the chamber every 25 seconds (each filling time lasts 5 seconds) and the continuous loading time was over 320 minutes. The changes in the voltage (ΔV) of the sample are shown in Fig. 8. It can be seen that the total electrical response curve is composed of two parts: a strain-drift electrical response (seen in the general trend in the curves) and an electrical response stimulated by the humidity pulses (zoomed-in regions). The total electrical response curve can be considered to be the strain-drift electrical response modulated by the humidity electrical responses. From the maxima histograms in Fig. 8, almost no voltage signal is observed in the beginning and the signal gradually increases with the continuous injection of the 64% RH gas. After about 140 minutes, the change in the voltage responding to humidity changes reaches a maximum value of 5.03 mV and then gradually decreases to a steady-state change in the voltage, which is about 0.5 mV at 315 min. When the sample was maintained in 0% RH for a long time, most of the internal water molecules, including free water, loosely bound water, and partial strongly bound water molecules, inside the composite film were exchanged into the external environment.32–34 The cationic complexes were bound to the charged sulfonic groups of Nafion due to the lack of water. At the initial stage of evolution, water molecules from the charged gas with a specific humidity were first absorbed to bond with the cationic complexes and during this process, almost no electrical response was detected. As the water molecules were continuously replenished, the electrical response was gradually generated and increased. This can be attributed to the excess of water molecules that provide the medium and the strain gradient for the migration of cationic complexes. At the final stage of evolution, when the water molecules inside the composite film were in equilibrium with the external humid environment, the composite film no longer swelled and the electrical response gradually weakened to almost zero. In addition, the total trend of the humidity electrical response of the composite film presents a large drift from 0 mV to −41 mV, which is probably attributed to the strain charge accumulation of the composite film. According to the transduction mechanism of the composite film, the humidity change will cause local anisotropic distribution of the hydrated cations inside the Nafion membrane, which will inevitably generate a potential difference. The distribution of the hydrated cations is closely related to the strain gradient. Before the next humidity loading begins, the strain gradient is not recovered yet. Thus, the strain gradient gradually strengthens as the humidity loading continues and the strain charge gradually accumulates, thereby generating a large strain-drift electrical response.
According to the above results, we measured the humidity electrical response of the Nafion/Ag NWs composite film from 0% RH to 100% RH (humidifying process) and from 100% RH to 0% RH (dehumidifying process), respectively. After the sample was fully exchanged in the initial humidity (e.g., for 24 h at 0% RH) environment, the gas with the next higher humidity (23% RH) was charged into the chamber so as to exchange with the sample and then record the change in the voltage (ΔV). The results are shown in Fig. 9. During the humidifying process, the change in the voltage presents a continuous increase up to 5.54 mV at 100% RH. It is still a special electrical response point for the sample from 0% RH to 23% RH since the sample did not absorb enough water molecules to make the cationic complexes moveable. But for the sample from 23% RH to 43% RH, there is an obvious electrical response up to 1.1 mV, which indicates that the critical point of the cationic complexes that absorbed strongly bound water is below 23% RH. During the dehumidifying process, the absolute value of the electrical response presents a continuous decrease from 5 mV at 100% RH to 0.565 mV at 0% RH. The electrical response of the dehumidifying sample is higher than that of the humidifying sample at the same reversible humidity level, similar to that depicted in Fig. 6. It is noteworthy that the values of electrical response did not show any hysteresis characteristics although dehumidification and humidification are reversible, which is different from other sensing mechanisms.
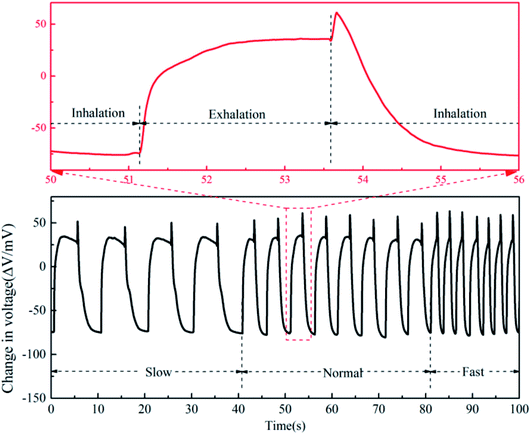
Since the Nafion/Ag NWs composite film exhibits fast response and relatively high sensitivity to high humidity, it can be expected to be used in humidity monitoring. To this end, we explored its application in monitoring the humidity levels of a human's breathing process. We used a healthy adult male as a volunteer to test his breathing process by blowing into the chamber, in which the composite film was placed. Before the breathing test, the sample was first equilibrated at the room humidity environment (about 64% RH). As shown in Fig. 10, the breathing zoom-in curve shows a rapid increase, then slowly decreases, and finally, there is a quick return after a sharp rise, which corresponds to the exhalation, pause, and inhalation of a human's breath in the breathing cycle. The maximum electrical response is over 120 mV, which is attributed to the numerous droplets in the breathing process rather than the gas form of the water molecules. Considering that a healthy adult's breathing rate is about 16–20 beats per minute, we further tested the electrical response of the composite film at a slow, normal, and fast respiration rate, respectively, which can also be clearly perceived from the breathing process. It can be seen from Fig. 10 that there is an obviously slow relaxation during the exhalation process. Also, when the inhalation process began, a sharp peak appeared in each breathing process, which could be attributed to rapid gas impact from inhalation. It is known that Nafion in a composite film that not only absorbs water molecules but also has good absorption for other organic solvents. We further tested the electrical response of the composite film towards ethanol and Chinese wine (53% ethanol content) for drunken detection. The test was still based on the breathing process, and the results are shown in Fig. S3 and S4,† respectively. Compared with the normal breathing test, the two groups have similar trends but have obvious signal characteristics with differences in the curve profiles. This shows that the composite film can not only detect the change in humidity but can also distinguish other volatile organic solvents (VOC), such as ethanol and its mixtures.
4 Conclusions
In conclusion, a novel responsive humidity sensor was proposed based on a composite film consisting of Nafion membranes and Ag NWs prepared by the physical deposition process, which not only shows good electrical response towards gas-containing water molecules but also to other organic solvents. During all the cases of electrical response, the change in the humidity level from 100% RH to 64% RH generated an electrical response of over 8 mV, even more than 120 mV that is generated during the human breathing process without any amplification. In particular, the electrical response of the composite film presented a positive and negative voltage during the humidifying and dehumidifying process, respectively. We also confirmed that this composite film had a very slow response in 0% RH, which means that it is difficult to detect humidity changes in relatively low humidity environments. In addition, in this work, we built a fast humidity switch device to test the dynamic electrical response. In order to demonstrate the humidity response capability, we performed a preliminary attempt to monitor the human breathing process and drunken driving detection. The proposed humidity sensor has potential for applications in areas such as humidity sensing and VOA detection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Mr Jiahui Wang for illustration drawing. This research was supported by the financial support from the National Natural Science Foundation of China (Grant No. 51975184 and 91748124), the Fundamental Research Funds for the Central Universities (2019B21514), National Key R&D Program of China (2019YFB1311600), Shaanxi Key R&D Program (2020ZDLGY06-11), the National Natural Science Foundation of China (91748124), the Foundation of Jiangsu Key Laboratory of Special Robot Technology (2017B21114). The authors gratefully acknowledge the supports.References
- F. Beigi, et al., Doped Nafion-layered double hydroxide nanoparticles as a modified ionic polymer metal composite sheet for a high-responsive humidity sensor, Appl. Clay Sci., 2018, 166, 131–136 CrossRef CAS.
- T. Blank, L. Eksperiandova and K. Belikov, Recent trends of ceramic humidity sensors development: a review, Sens. Actuators, B, 2016, 228, 416–442 CrossRef CAS.
- E. Esmaeli, et al., Humidity sensor based on the ionic polymer metal composite, Sens. Actuators, B, 2017, 247, 498–504 CrossRef CAS.
- X. Gan, et al., High performance graphene oxide-based humidity sensor integrated on a photonic crystal cavity, Appl. Phys. Lett., 2017, 110(15), 151107 CrossRef.
- S. Ghosh, et al., Enhanced proton conductivity of graphene oxide/Nafion composite material in humidity sensing application, IEEE Trans. Nanotechnol., 2016, 15(5), 782–790 CAS.
- M. Natali, et al., Engineering of keratin functionality for the realization of bendable all-biopolymeric micro-electrode array as humidity sensor, Biosens. Bioelectron., 2019, 111480 CrossRef CAS PubMed.
- Y. Pang, et al., Wearable humidity sensor based on porous graphene network for respiration monitoring, Biosens. Bioelectron., 2018, 116, 123–129 CrossRef CAS PubMed.
- G. Harsányi, Polymer films in sensor applications: a review of present uses and future possibilities, Sens. Rev., 2000, 20(2), 98–105 CrossRef.
- D. W. Hofmann, et al., Investigation of water structure in Nafion membranes by infrared spectroscopy and molecular dynamics simulation, J. Phys. Chem. B, 2008, 113(3), 632–639 CrossRef PubMed.
- H. Hosokawa, Y. Funasako and T. Mochida, Colorimetric Solvent Indicators Based on Nafion Membranes Incorporating Nickel(II)–Chelate Complexes, Chem.–Eur. J., 2014, 20(46), 15014–15020 CrossRef CAS PubMed.
- B. Jiang, et al., Insights into the impact of the Nafion membrane pretreatment process on vanadium flow battery performance, ACS Appl. Mater. Interfaces, 2016, 8(19), 12228–12238 CrossRef CAS PubMed.
- C. Jo, et al., Recent advances in ionic polymer–metal composite actuators and their modeling and applications, Prog. Polym. Sci., 2013, 38(7), 1037–1066 CrossRef CAS.
- S. Kano, K. Kim and M. Fujii, Fast-response and flexible nanocrystal-based humidity sensor for monitoring human respiration and water evaporation on skin, ACS Sens., 2017, 2(6), 828–833 CrossRef CAS PubMed.
- Y. Han, et al., Sulfonic SiO2 nanocolloid doped perfluorosulfonic acid films with enhanced water uptake and inner channel for IPMC actuators, RSC Adv., 2019, 9(72), 42450–42458 RSC.
- A. Khan, K. A. Alamry and R. K. Jain, Polypyrrole nanoparticles-based soft actuator for artificial muscle applications, RSC Adv., 2019, 9(68), 39721–39734 RSC.
- J. Ru, et al., Tunable actuation behavior of ionic polymer metal composite utilizing carboxylated carbon nanotube-doped Nafion matrix, RSC Adv., 2018, 8(6), 3090–3094 RSC.
- C. Oh, et al., Effects of membrane thickness on the performance of ionic polymer–metal composite actuators, RSC Adv., 2019, 9(26), 14621–14626 RSC.
- X. Leng, et al., Modified graphene oxide/Nafion composite humidity sensor and its linear response to the relative humidity, Sens. Actuators, B, 2018, 257, 372–381 CrossRef CAS.
- Y. Liu, et al., Rough interface in IPMC: modeling and its influence analysis, Smart Mater. Struct., 2018, 27(7), 075055 CrossRef.
- Z. Lu, et al., State of water in perfluorosulfonic ionomer (Nafion 117) proton exchange membranes, J. Electrochem. Soc., 2008, 155(2), B163–B171 CrossRef CAS.
- I. A. Pašti, et al., Resistive gas sensors based on the composites of nanostructured carbonized polyaniline and Nafion, J. Solid State Electrochem., 2016, 20(11), 3061–3069 CrossRef.
- K. Saotome, et al., Structure of the mechanically activated ion channel Piezo1, Nature, 2018, 554(7693), 481 CrossRef CAS PubMed.
- S. Sikarwar and B. Yadav, Opto-electronic humidity sensor: a review, Sens. Actuators, A, 2015, 233, 54–70 CrossRef CAS.
- Y. Wang, et al., Aided manufacturing techniques and applications in optics and manipulation for ionic polymer-metal composites as soft sensors and actuators, J. Polym. Eng., 2015, 35(7), 611–626 Search PubMed.
- Y. Wang, et al., Effect of dehydration on the mechanical and physicochemical properties of gold-and palladium-ionomeric polymer-metal composite (IPMC) actuators, Electrochim. Acta, 2014, 129, 450–458 CrossRef CAS.
- Y. Wang, et al., Effects of preparation steps on the physical parameters and electromechanical properties of IPMC actuators, Smart Mater. Struct., 2014, 23(12), 125015 CrossRef.
- Y. Wang, et al., Effects of surface roughening of Nafion 117 on the mechanical and physicochemical properties of ionic polymer–metal composite (IPMC) actuators, Smart Mater. Struct., 2016, 25(8), 085012 CrossRef.
- Y. Yin, et al., Structure of the cold-and menthol-sensing ion channel TRPM8, Science, 2018, 359(6372), 237–241 CrossRef CAS PubMed.
- X. Yu, J. Joseph and A. Manthiram, Polymer lithium–sulfur batteries with a Nafion membrane and an advanced sulfur electrode, J. Mater. Chem. A, 2015, 3(30), 15683–15691 RSC.
- K.-L. Zhang, et al., Highly sensitive humidity sensor based on graphene oxide foam, Appl. Phys. Lett., 2017, 111(15), 153101 CrossRef.
- Z. Zhu, H. Chen, and Y. Wang, Sensing Properties and Physical Model of Ionic Polymer, in Soft Actuators 2019, Springer. pp. 503–545 Search PubMed.
- Z. Zhu, et al., NMR study on mechanisms of ionic polymer-metal composites deformation with water content, Europhys. Lett., 2011, 96(2), 27005 CrossRef.
- Z. Zhu, et al., Influence of Ambient Humidity on the Voltage Response of Ionic Polymer–Metal Composite Sensor, J. Phys. Chem. B, 2016, 120(12), 3215–3225 CrossRef CAS PubMed.
- Z. Zhu, et al., Application-oriented simplification of actuation mechanism and physical model for ionic polymer-metal composites, J. Appl. Phys., 2016, 120(3), 034901 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01650k |
| This journal is © The Royal Society of Chemistry 2020 |