Open Access Article
Open Access ArticleProjection method as a probe for multiplexing/demultiplexing of magnetically enriched biological tissues†
Mohammad Reza Zamani Kouhpanji ab and
Bethanie J. H. Stadler
ab and
Bethanie J. H. Stadler *ac
*ac
aDepartment of Electrical and Computer Engineering, University of Minnesota Twin Cities, USA. E-mail: stadler@umn.edu; Tel: +1-612-626-1628
bDepartment of Biomedical Engineering, University of Minnesota Twin Cities, USA
cDepartment of Chemical Engineering and Materials Science, University of Minnesota Twin Cities, USA
First published on 1st April 2020
Abstract
The unmet demand for cheap, accurate, and fast multiplexing of biomarkers has urged nanobiotechnology to prioritize the invention of new biomarkers that make feasible the remote detection, identification, and quantification of biological units, such as regenerative tissues. Here, we introduce a novel approach that highlights magnetic nanowires (MNWs) with such capabilities. This method employs the stable magnetization states of MNWs as a unique characteristic that can be realized by projecting the MNWs' switching field on the backward field (PHb), also known as the irreversible switching field. Experimentally, several types of MNWs were directly synthesized inside polycarbonate tissues and their PHb characteristics were measured and analyzed. Our results show that the PHb gives an excellent identification and quantification characteristic for demultiplexing MNWs embedded in these tissues. Furthermore, this method significantly improves the characterization speed by a factor of 50×–100× that makes it superior to the current state of the art that ceased the progression of magnetic nanoparticles in multiplexing/demultiplexing applications.
Introduction
Progress of biological sciences substantially depends on biomarkers because they are the backbone of essential tools, such as optical microscopy and flow cytometry techniques, in this field.1,2 However, surprisingly, none have offered the capabilities to mitigate the high-cost, high-complexity, and low-speed associated with these techniques.3–6 Furthermore, there is still a lack of biomarkers that can be excited remotely and noninvasively with a single excitation source regardless of their surrounding tissues because almost all biomarkers under study to date are optical.7–9The magnetic biomarkers, on the other hand, have potential to accelerate the progression of biological sciences. The current state of the arts in magnetic biomarkers is superparamagnetic iron oxide nanoparticles (IONs). These IONs have isotropic magnetic properties that restrict them from possessing distinct signatures that can be uniquely identified.10,11 As a result, IONs have been limited in application to the enrichment of the biological entities with conjugation to optical biomarkers, such as fluorescent molecules, for readout.12–15 Unfortunately, IONs also have weak magnetic properties that limit their use in ultralow enrichment and multiplexing applications.7
In this paper, we propose ferromagnetic nanowires (MNWs) as biomarkers for multiplexing applications. The special cylindrical geometry of MNWs gives rise to a well-defined magnetic anisotropy16 that makes them suitable for many biological applications, such as MRI contrast, magnetic enrichment, and nanowarming.11,17–21 An external field can be remotely used to physically excite MNWs, which makes them suitable for cell tracking,22–25 cells manipulation,26 cell separation,27,28 drug delivery and drug activation,29,30 magnetic hyperthermia,21 and magneto-elastic ferrogels for tissue engineering.31,32 Furthermore, the MNWs have shown a high internalization by cells in comparison to other magnetic nanoparticles, such as IONs, improving the enrichment and multiplexing yield.26,28,33 As a consequence, MNWs have recently attracted attention as enrichment agents for studying breast and lung cancers,34,35 CTCs,36 and leukemia.37
Regardless of the tremendous opportunities for MNWs, they have not been used as self-standing biomarkers for multiplexing applications up to date due to three major drawbacks. First, magnetic characterization techniques are technically inefficient because they are slow. Second, the unique magnetic characteristics cannot unambiguously be measured due to the influence of external effects, such as para/diamagnetic response of the surrounding components. Third, the magnetic signatures of MNWs can overlap, which has limited their use as multiplexed biomarkers. To suppress these substantial obstacles, we propose a magnetic measurement technique that significantly facilitates the detection of MNWs signature regardless of their surroundings.10,38,39 Along with this, we establish a technique to quantitatively demultiplex the signature of multiple types of MNWs even though the individual signatures overlap.
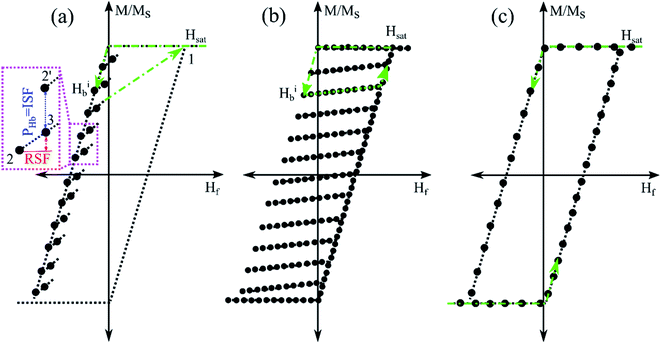
Our methodology is based on our recently developed magnetic characterization method, named “projection method” that highly accelerates detection, identification, and quantification of MNWs regardless of their surroundings. A schematic of the projection method is shown in Fig. 1a.
Briefly, the projection method relays on measuring a few points next to the upper branch hysteresis loop (UBHL), shown as the black dots in Fig. 1a. Experimentally, the projection method measures the UBHL in a 2D format as a function of two fields, forward field (Hf) and backward field (Hb). The backward field is the descending field from positive saturation (point 1 in Fig. 1a) that sets the initial magnetization state of the MNWs (point 2 in Fig. 1a).
Then, the forward field is applied from the backward field to its previous backward field (point 3 in Fig. 1a) to slightly disturb the initial magnetization state of the MNWs. At this step, the field should be returned to the saturation field (point 1 in Fig. 1a) in one step before starting another backward field. The theoretical analysis of the projection method is given in the ESI.†
According to the inset in Fig. 1a, the projection method's protocol splits the derivative of the upper branch of the hysteresis loop (vertical distance between points 2 and 2′) into two parts that eliminates the extrinsic effects (the red arrow in Fig. 1a, RSF) from the intrinsic effects (the blue arrow in Fig. 1a, PHb). The PHb is a distinct characteristic of MNWs because it indicates the magnetic stability of the magnetization related to the spin- and/or orbit-lattice coupling inducing shape and crystal anisotropies. Therefore, it provides a difference in magnetization in the same forward field after starting from two different initial states, or two different backward fields. On the other hand, the reversible switching field (RSF) indicates the instantaneous disturbance in magnetization induced by the immediate fields applied on the MNWs, such as forward field, MNWs interaction field, and para/diamagnetic response of the surroundings. This distinct feature of the project method, discrimination of the RSF and ISF, makes it an excellent method for detection, identification, and quantification of MNWs buried deeply in biological tissues, which cannot be realized by other magnetic characterization methods, such first-order reversal curve (FORC) or hysteresis loop measurements (Fig. 1b and c). Furthermore, since it only measures a few points next to the upper branch of the hysteresis loop rather than scanning the whole hysteresis loop area (as it is done in FORC measurement, Fig. 1b), it is practically very fast which makes it competitive with other detection/identification methods, such as flow cytometry or impedance cytometry methods while nondestructively measuring whole samples.
Experiments
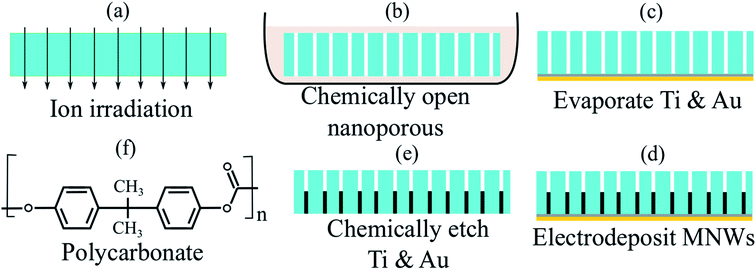
Two sets of the MNWs were synthesized inside the porous polycarbonate tissues using the electrodeposition technique, Fig. 2. More details regarding the synthesis approach are provided in the ESI.† We chose polycarbonate in this study because polycarbonate is a biocompatible polymer and has been widely used in bone/organ repairing, drug delivery, and tissue regenerative engineering.40–46 Note all these applications are in vivo, besides those, we would like to mention the polycarbonate is a polymer that has been used widely in daily applications, such as covers for cell phones, a great example of skin contact without any toxicity or side-effect. The first set was made of cobalt (Co) and another was made of nickel (Ni). The biocompatibility of the MNWs has been studied in our past studies and other researchers,22,47–50 we thus did not performed biocompatibility study here. Furthermore, here the Ni and Co MNWs are embedded inside the polycarbonate, thus they will not be in contact with biological entities, such as cells, if they are used in vivo applications. Four different types of MNWs were prepared for each set, classified according to their diameters, leading to eight different types of MNW-loaded tissues. To evaluate the accuracy of the projection method for demultiplexing, the diameters and geometrical arrangements were kept the same in each set. These parameters, diameter (inter-wire distance to diameter ratio), were 200 nm (3), 100 nm (7), 50 nm (9), and 30 nm (15).First, the magnetic characteristics of the individual MNW-loaded tissues were measured and analyzed using the projection method, see ESI.† Next, the magnetic characteristics of combinations of MNW-loaded tissues were measured and compared to those of the individual tissues. Specifically, to analyze the reliability and reproducibility of the projection method for the quantitative description, the individual characteristics were used as calibration curves such that a volume ratio (χ) could be fit for the combinations. The fitting quality was evaluated using the root mean square (RMS) error of the difference between the “experimental data” and “calibration curve”, which is the weighted summation of the corresponding parameters of the calibration samples. This RMS error was minimized χ for each type of MNWs in the combination. More details on the measurements and statistical analysis are given in the ESI.†
Results
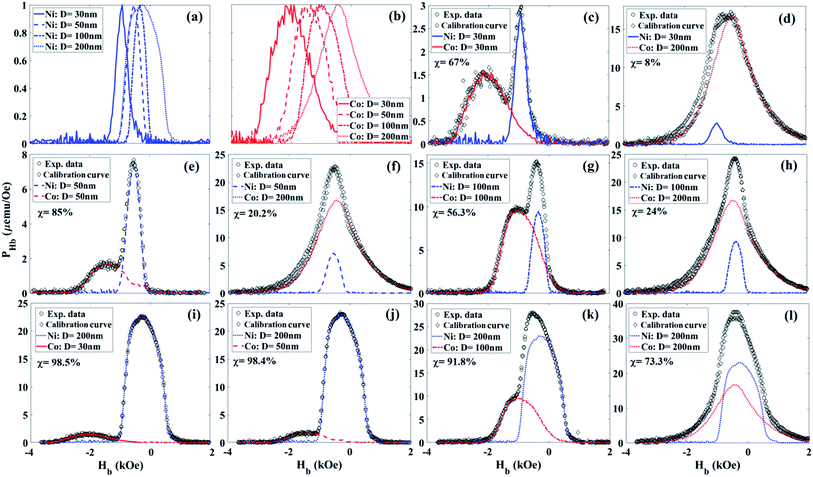
The PHb was calculated from the experimental data using a theoretical formula, given in the ESI,† and the results are plotted in Fig. 3. As can be seen in Fig. 3, the PHb maintains fairly sharp features even for very small volume ratios (χ). For example, the χ of the Co 30 nm and Ni 200 nm combination is only ∼1.5% Co (98.5% Ni). PHb solely relies on the intrinsic properties of the MNWs, such as shape and crystal anisotropies, which determine the average coercivity of each MNWs type. Note the coercivity is the location of the PHb peak, which is distinct for different types of the MNWs—the coercivities are given in Fig. 5 ESI.† For a comprehensive demultiplexing analysis, we intentionally made both MNWs sets to have moderately overlapping characteristics. The PHb of combined samples includes the feature of individual MNWs types in the combinations. The distinction of the features depends on the coercivities of the individual MNWs in each combination and on χ. As the χ increases, having less from one type compared to another, the sharpness of the features decreases too. Therefore, it is essential to have MNWs types with very distinct coercivity in the combination in order to recognize the features for a more accurate χ measurement. Since the PHb of a MNW type is independent from others, the identification and quantification can be done by comparing the experimental data from the combination with those of the individual types in the combination using a simple fitting model, see the ESI for more details.†Discussion
Fig. 4 shows the quantitative volume ratio (χ) determined using PHb. The PHb characteristics determined χ with a reasonable error. The accuracy of the PHb is directly correlated to the intrinsic properties of the MNWs. Since the projection method eliminates the extrinsic effects by separating the RSF from ISF, the projection method precisely detects, identifies, and quantifies the types and volume ratios of the components inside each combination.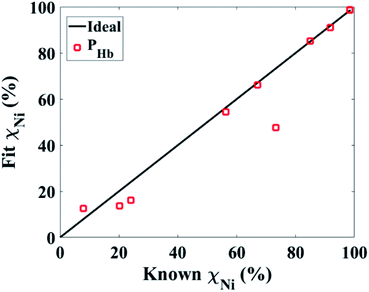 | ||
| Fig. 4 Comparison of the accuracy of the magnetic characteristics measured by the projection method for quantifying the volume ratios (χ) of the MNWs in combinations. | ||
By realizing the PHb as a reliable and accurate magnetic characteristic, now we compare this with optical techniques that employ optical nanoparticles, such as fluorophore molecules. Optical techniques measure on log scales due to large variance in measurements induced by the photo-instability or background signals. In addition, the fluorescence signals cannot overlap if they are to be demultiplexed, and they are susceptible to bleaching, blurry focusing, and other optical artifacts. Importantly, regardless of the complex and expensive components of optical techniques, there is a huge trade-off between their resolution and speed. For example, to speed up scanning a large area, one must increase the field of view and pixel size which significantly deteriorates the resolution. Interestingly, all of these limitations can be significantly mitigated using the projection method. The projection method can be done on any sample, regardless of its volume or concentration. On other words, the projection method remotely measures the whole sample at once regardless of the surroundings, and it is high-speed compared to the conventional magnetic measurements. Furthermore, the magnetic moments of the MNWs are stable so they can be remotely and noninvasively excited by a single excitation source, a magnetic field.
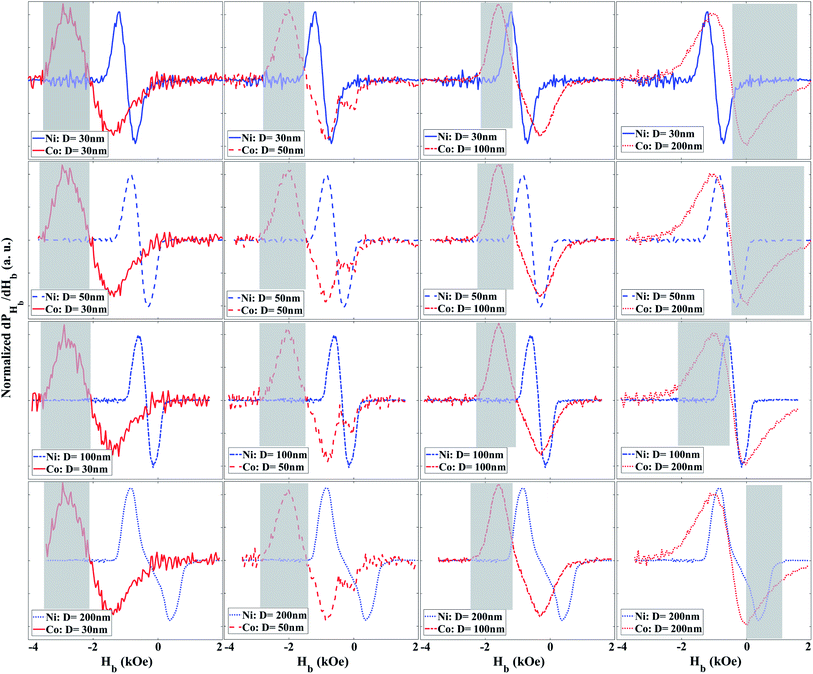
As shown in the ESI,† the PHb indeed is the derivative of the magnetization with respect to the Hb. The peak of PHb determines the average coercivity of the MNWs. Furthermore, its width and overall shape are correlated to the standard deviation of the coercivity and the interaction fields among the MNWs. Therefore, we define the specificity of the MNWs as the derivative of the PHb with respect to Hb (dPHb/dHb), shown in Fig. 4. That is because the dPHb/dHb determines the deformation or variation of the PHb with respect to the Hb. In other words, the dPHb/dHb is the curvature of the magnetization that is a unique function of the coercivity distribution and the interaction fields, which are solely depend on the MNWs type, size, and arrangement.
According to Fig. 5, the derivative has two peaks that are symmetric with respect to the coercivity, which is the location of the PHb's peak, see Fig. 5 ESI.† The symmetry of the peaks significantly improves the ability of the projection method to determine the component type and χ inside a combination, because it increases the opportunity to have distinct signatures. The intermediate between the peaks, where the dPHb/dHb is zero, determines the average coercivity. Moreover, the separation of the peaks is related to the standard deviation of the coercivity and the interaction fields. Since the MNWs are synthesized in the similar polycarbonate templates, it is expected to have similar standard deviation for the coercivity. However, since the Co MNWs have larger crystal anisotropy and magnetic moment compared to the Ni MNWs for the same dimensions, the location of the intermediate point and the distribution of the peaks are different between the Co and Ni MNWs leading to distinguishable signatures for demultiplexing them. Furthermore, for the same material, the coercivity increases as the diameter decreases. For example, changing the diameter from 30 nm to 200 nm moves the intermediate points to smaller Hb values. Therefore, the dPHb/dHb can be further engineered by changing the composition and diameters of the magnetic nanowires leading in generating diverse signatures for multiplexing/demultiplexing of the magnetically enriched biological tissues.
The grey boxes in Fig. 5 highlight the range of Hb that can be used to detect one of the MNW biomarker types as distinct from the other, which we could call primary. If one of the secondary MNW peaks is detected (specificity), the other peak can be replicated due to symmetry, such that χ can be quantified even with an overlapping primary peak. Importantly, distinct peaks can be engineered by careful design of MNW coercivity, which is a function of the magnetic component, size, and aspect ratio (diameter to length). For example, in these combinations, the 200 nm Co tissues did not enable combinations that were demultiplexed with as high confidence as the other combinations. This still means that 12 combinations of 2 tissue types were successful. Further “barcodes” will be possible as this technique is expanded to new MNW-loaded tissues as components for new combinations.
Most biomarker signatures, such as the emission spectra of optical biomarkers, have only one peak and that peak must be distinct from the others. We believe the unique symmetric-peak property of the MNWs, correlated with the projection method and our straightforward analysis, opens a venue towards more accurate and fast multiplexing/demultiplexing of the large volume of biological entities that will fuel the progress of molecular biology, nanomedicine, and medical therapies.
Conclusion
Here, we proposed a novel magnetic measurement, called the projection method, for accurate and fast quantitative demultiplexing of the MNWs. We showed that the projection method can rapidly and precisely illustrate the unique magnetic characteristics of the MNWs by suppressing the influence of extrinsic effects on intrinsic properties by scanning the vicinity of the upper branch of the MNWs hysteresis loop. Our experimental observations prove that the projection of the switching response on the backward field (PHb), also known as irreversible switching, is a reliable and accurate characteristic for multiplexing/demultiplexing applications. More interesting, its derivative has two peaks that provide more flexibility for generating biomarkers with distinct characteristics compared to optical biomarkers. The PHb and its derivative have a strong dependence on the intrinsic properties of the MNWs, such as spin-lattice coupling, with minimal extrinsic effects. This finding along with our high-speed measurement protocol for the projection method, 50×–100× faster than the conventional magnetic measurements, makes the projection method competitive to the expensive and complex current state of the art of multiplexing techniques.Conflicts of interest
There are not conflicts to declare.Acknowledgements
This work is based upon work supported primarily by the National Science Foundation under grant no. CMMI-1762884. Portions of this work were conducted in the Minnesota Nano Center, which is supported by the National Science Foundation through the National Nano Coordinated Infrastructure Network (NNCI) under Award Number ECCS-1542202. Part of this work was performed at the Institute for Rock Magnetism (IRM) at the University of Minnesota. The IRM is a US National Multi-user Facility supported through the Instrumentation and Facilities program of the National Science Foundation, Earth Sciences Division (NSF/EAR 1642268), and by funding from the University of Minnesota. Parts of this work were also carried out in the Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRSEC program.References
- Z. A. Nima, M. Mahmood, Y. Xu, T. Mustafa, F. Watanabe, D. A. Nedosekin, M. A. Juratli, T. Fahmi, E. I. Galanzha, J. P. Nolan, A. G. Basnakian, V. P. Zharov and A. S. Biris, Sci. Rep., 2015, 4, 4752 CrossRef PubMed.
- L. Zhang, D. Lv, W. Su, Y. Liu, Y. Chen and R. Xiang, Am. J. Biochem. Biotechnol., 2013, 9, 71–89 CrossRef CAS.
- E. Martz, Introduction to Flow Cytometry, http://www.bio.umass.edu/micro/immunology/facs542/facsprin.htm Search PubMed.
- J. P. Robinson, Encycl. Biomater. Biomed. Eng., 2004, 922–931 Search PubMed.
- A. Cossarizza, H. D. Chang, A. Radbruch, M. Akdis, I. Andrä, F. Annunziato, P. Bacher, V. Barnaba, L. Battistini, W. M. Bauer, S. Baumgart, B. Becher, W. Beisker, C. Berek, A. Blanco, G. Borsellino, P. E. Boulais, R. R. Brinkman, M. Büscher, D. H. Busch, T. P. Bushnell, X. Cao, A. Cavani, P. K. Chattopadhyay, Q. Cheng, S. Chow, M. Clerici, A. Cooke, A. Cosma, L. Cosmi, A. Cumano, V. D. Dang, D. Davies, S. De Biasi, G. Del Zotto, S. Della Bella, P. Dellabona, G. Deniz, M. Dessing, A. Diefenbach, J. Di Santo, F. Dieli, A. Dolf, V. S. Donnenberg, T. Dörner, G. R. A. Ehrhardt, E. Endl, P. Engel, B. Engelhardt, C. Esser, B. Everts, A. Dreher, C. S. Falk, T. A. Fehniger, A. Filby, S. Fillatreau, M. Follo, I. Förster, J. Foster, G. A. Foulds, P. S. Frenette, D. Galbraith, N. Garbi, M. D. García-Godoy, J. Geginat, K. Ghoreschi, L. Gibellini, C. Goettlinger, C. S. Goodyear, A. Gori, J. Grogan, M. Gross, A. Grützkau, D. Grummitt, J. Hahn, Q. Hammer, A. E. Hauser, D. L. Haviland, D. Hedley, G. Herrera, M. Herrmann, F. Hiepe, T. Holland, P. Hombrink, J. P. Houston, B. F. Hoyer, B. Huang, C. A. Hunter, A. Iannone, H. M. Jäck, B. Jávega, S. Jonjic, K. Juelke, S. Jung, T. Kaiser, T. Kalina, B. Keller, S. Khan, D. Kienhöfer, T. Kroneis, D. Kunkel, C. Kurts, P. Kvistborg, J. Lannigan, O. Lantz, A. Larbi, S. LeibundGut-Landmann, M. D. Leipold, M. K. Levings, V. Litwin, Y. Liu, M. Lohoff, G. Lombardi, L. Lopez, A. Lovett-Racke, E. Lubberts, B. Ludewig, E. Lugli, H. T. Maecker, G. Martrus, G. Matarese, C. Maueröder, M. McGrath, I. McInnes, H. E. Mei, F. Melchers, S. Melzer, D. Mielenz, K. Mills, D. Mirrer, J. Mjösberg, J. Moore, B. Moran, A. Moretta, L. Moretta, T. R. Mosmann, S. Müller, W. Müller, C. Münz, G. Multhoff, L. E. Munoz, K. M. Murphy, T. Nakayama, M. Nasi, C. Neudörfl, J. Nolan, S. Nourshargh, J. E. O'Connor, W. Ouyang, A. Oxenius, R. Palankar, I. Panse, P. Peterson, C. Peth, J. Petriz, D. Philips, W. Pickl, S. Piconese, M. Pinti, A. G. Pockley, M. J. Podolska, C. Pucillo, S. A. Quataert, T. R. D. J. Radstake, B. Rajwa, J. A. Rebhahn, D. Recktenwald, E. B. M. Remmerswaal, K. Rezvani, L. G. Rico, J. P. Robinson, C. Romagnani, A. Rubartelli, B. Ruckert, J. Ruland, S. Sakaguchi, F. Sala-de-Oyanguren, Y. Samstag, S. Sanderson, B. Sawitzki, A. Scheffold, M. Schiemann, F. Schildberg, E. Schimisky, S. A. Schmid, S. Schmitt, K. Schober, T. Schüler, A. R. Schulz, T. Schumacher, C. Scotta, T. V. Shankey, A. Shemer, A. K. Simon, J. Spidlen, A. M. Stall, R. Stark, C. Stehle, M. Stein, T. Steinmetz, H. Stockinger, Y. Takahama, A. Tarnok, Z. G. Tian, G. Toldi, J. Tornack, E. Traggiai, J. Trotter, H. Ulrich, M. van der Braber, R. A. W. van Lier, M. Veldhoen, S. Vento-Asturias, P. Vieira, D. Voehringer, H. D. Volk, K. von Volkmann, A. Waisman, R. Walker, M. D. Ward, K. Warnatz, S. Warth, J. V. Watson, C. Watzl, L. Wegener, A. Wiedemann, J. Wienands, G. Willimsky, J. Wing, P. Wurst, L. Yu, A. Yue, Q. Zhang, Y. Zhao, S. Ziegler and J. Zimmermann, Eur. J. Immunol., 2017, 47, 1584–1797 CrossRef CAS PubMed.
- H. KassassirK. SiewieraT. Przygodzki, M. Labieniec-Watala and C. Watala, in Flow Cytometry – Select Topics, ed. K.Siewiera, InTech, Rijeka, 2016, ch. 3 Search PubMed.
- S. Bhana, Y. Wang and X. Huang, Micro Nanosyst., 2015, 7, 1973–1990 Search PubMed.
- M. V. DaCosta, S. Doughan, Y. Han and U. J. Krull, Anal. Chim. Acta, 2014, 832, 1–33 CrossRef CAS PubMed.
- X. Xie, N. Gao, R. Deng, Q. Sun, Q. H. Xu and X. Liu, J. Am. Chem. Soc., 2013, 135, 12608–12611 CrossRef CAS PubMed.
- M. R. Z. Kouhpanji and B. J. H. Stadler, 2019, 1–9, arXiv:1911.12480.
- M. R. Zamani Kouhpanji, J. Um and B. J. H. Stadler, ACS Appl. Nano Mater., 2020, 3(3), 3080–3087 CrossRef CAS.
- D. Shi, M. E. Sadat, A. W. Dunn and D. B. Mast, Nanoscale, 2015, 7, 8209–8232 RSC.
- M. Worden, L. Bergquist and T. Hegmann, RSC Adv., 2015, 5, 100384–100389 RSC.
- C. Iriarte-Mesa, Y. C. López, Y. Matos-Peralta, K. de la Vega-Hernández and M. Antuch, Top. Curr. Chem., 2020, 378, 12 CrossRef CAS PubMed.
- R. C. Popescu, E. Andronescu and B. S. Vasile, Nanomaterials, 2019, 9, 1–31 CrossRef PubMed.
- J. Um, M. R. Zamani Kouhpanji, S. Liu, Z. Nemati, J. Kosel and B. Stadler, IEEE Trans. Magn., 2020, 56(2), 1–6 Search PubMed.
- D. Shore, S. L. Pailloux, J. Zhang, T. Gage, D. J. Flannigan, M. Garwood, V. C. Pierre and B. J. H. Stadler, Chem. Commun., 2016, 52, 12634–12637 RSC.
- Y. S. Jeon, H. M. Shin, Y. J. Kim, D. Y. Nam, B. C. Park, E. Yoo, H.-R. Kim and Y. K. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 23901–23908 CrossRef CAS PubMed.
- J. A. Fernandez-Roldan, D. Serantes, R. P. del Real, M. Vazquez and O. Chubykalo-Fesenko, Appl. Phys. Lett., 2018, 112, 212402 CrossRef.
- D. Shore, A. Ghemes, O. Dragos-Pinzaru, Z. Gao, Q. Shao, A. Sharma, J. Um, I. Tabakovic, J. C. Bischof and B. J. H. Stadler, Nanoscale, 2019, 11, 14607–14615 RSC.
- J. Alonso, H. Khurshid, V. Sankar, Z. Nemati, M. H. Phan, E. Garayo, J. A. García and H. Srikanth, J. Appl. Phys., 2015, 117, 17D113 CrossRef.
- Z. Nemati, J. Um, M. R. Zamani Kouhpanji, F. Zhou, T. Gage, D. Shore, K. Makielski, A. Donnelly and J. Alonso, ACS Appl. Nano Mater., 2020, 3, 2058–2069 CrossRef CAS.
- M. Hoehn, D. Wiedermann, C. Justicia, P. Ramos-cabrer, K. Kruttwig, T. Farr and U. Himmelreich, J. Physiol, 2007, 584, 25–30 CrossRef CAS PubMed.
- J. W. M. Bulte, T. Douglas, B. Witwer, S. C. Zhang, E. Strable, B. K. Lewis, H. Zywicke, B. Miller, P. Van Gelderen, B. M. Moskowitz, I. D. Duncan and J. A. Frank, Nat. Biotechnol., 2001, 19, 1141–1147 CrossRef CAS PubMed.
- M. Safi, M. Yan, M. A. Guedeau-Boudeville, H. Conjeaud, V. Garnier-Thibaud, N. Boggetto, A. Baeza-Squiban, F. Niedergang, D. Averbeck and J. F. Berret, ACS Nano, 2011, 5, 5354–5364 CrossRef CAS PubMed.
- A. Hultgren, M. Tanase, C. S. Chen, G. J. Meyer and D. H. Reich, J. Appl. Phys., 2003, 93, 7554–7556 CrossRef CAS.
- D. E. Shore, T. Dileepan, J. F. Modiano, M. K. Jenkins and B. J. H. Stadler, Sci. Rep., 2018, 8, 15696 CrossRef PubMed.
- A. Hultgren, M. Tanase, C. S. Chen and D. H. Reich, IEEE Trans. Magn., 2004, 40, 2988–2990 CrossRef.
- E. Kaniukov, A. Shumskaya, D. Yakimchuk, A. Kozlovskiy, I. Korolkov, M. Ibragimova, M. Zdorovets, K. Kadyrzhanov, V. Rusakov, M. Fadeev, E. Lobko, K. Saunina and L. Nikolaevich, Appl. Nanosci., 2019, 9, 835–844 CrossRef CAS.
- S. Jafari, L. O. Mair, I. N. Weinberg, J. Baker-McKee, O. Hale, J. Watson-Daniels, B. English, P. Y. Stepanov, C. Ropp, O. F. Atoyebi and D. Sun, J. Magn. Magn. Mater., 2019, 469, 302–305 CrossRef CAS.
- A. P. Safronov, B. J. H. Stadler, J. Um, M. R. Zamani Kouhpanji, J. Alonso Masa, A. G. Galyas and G. V Kurlyandskaya, Materials, 2019, 12, 2582 CrossRef CAS PubMed.
- A. Sharma, M. D. DiVito, D. E. Shore, A. D. Block, K. Pollock, P. Solheid, J. M. Feinberg, J. Modiano, C. H. Lam, A. Hubel and B. J. H. Stadler, J. Magn. Magn. Mater., 2018, 459, 176–181 CrossRef CAS.
- W. Hong, S. Lee, H. Jin, E. Sook and Y. Cho, Biomaterials, 2016, 106, 78–86 CrossRef CAS PubMed.
- H. Lee, M. Choi, J. Lim, M. Jo, J. Han, T. M. Kim and Y. Cho, Theranostics, 2018, 8, 505–517 CrossRef CAS PubMed.
- N. A. Alsharif, A. Martiinez-Banderas, J. Merzaban, T. Ravasi and J. Kosel, IEEE Trans. Magn., 2019, 55, 1–5 Search PubMed.
- M. Reyes, D. Vickers, K. Billman, T. Eisenhaure, P. Hoover, E. P. Browne, D. A. Rao, N. Hacohen and P. C. Blainey, Sci. Adv., 2019, 5, 1–10 Search PubMed.
- N. Cummings, R. King, A. Rickers, A. Kaspi, S. Lunke, I. Haviv and J. B. M. Jowett, BMC Genomics, 2010, 11, 641 CrossRef PubMed.
- M. R. Z. Kouhpanji and B. J. H. Stadler, arXiv:2003.06911, 2020, 1–9, http://https://arxiv.org/abs/2003.06911.
- M. R. Z. Kouhpanji, P. B. Visscher and B. J. H. Stadler, arXiv:2002.07742, 2020, 1–7http://https://arxiv.org/abs/2002.07742.
- S. B. Song, J. W. So, H. S. Ryu and B. S. Kim, US Pat., 2013, 133, 1179–1185.
- J. Kim, M. H. R. Magno, H. Waters, B. A. Doll, S. McBride, P. Alvarez, A. Darr, A. Vasanji, J. Kohn and J. O. Hollinger, Tissue Eng., Part A, 2012, 18, 1132–1139 CrossRef CAS PubMed.
- R. Glaum, M. Wiedmann-Al-Ahmad, U. Huebner and R. Schmelzeisen, J. Biomed. Mater. Res., Part A, 2010, 93, 704–715 CAS.
- M. N. Ganivada, P. Kumar, P. Kanjilal, H. Dinda, J. Das Sarma and R. Shunmugam, Polym. Chem., 2016, 7, 4237–4245 RSC.
- C. M. Agrawal and R. B. Ray, J. Biomed. Mater. Res., 2001, 55, 141–150 CrossRef CAS PubMed.
- J. Liao, L. Zhang, Y. Zuo, H. Wang, J. Li, Q. Zou and Y. Li, J. Biomater. Appl., 2009, 24, 31–45 CrossRef CAS PubMed.
- A. R. Shrivats, M. C. McDermott and J. O. Hollinger, Drug Discovery Today, 2014, 19, 781–786 CrossRef CAS PubMed.
- A. Sharma, Y. Zhu, S. Thor, F. Zhou, B. Stadler and A. Hubel, IEEE Trans. Magn., 2013, 49, 453–456 Search PubMed.
- A. Sharma, G. M. Orlowski, Y. Zhu, D. Shore, S. Y. Kim, M. D. DiVito, A. Hubel and B. J. H. Stadler, Nanotechnology, 2015, 26(13), 1–12 CrossRef PubMed.
- P. C. Pinheiro, C. T. Sousa, J. P. Araújo, A. J. Guiomar and T. Trindade, J. Colloid Interface Sci., 2013, 410, 21–26 CrossRef CAS PubMed.
- S. Schrittwieser, D. Reichinger and J. Schotter, Materials, 2018, 11(1), 45 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01574a |
| This journal is © The Royal Society of Chemistry 2020 |