Open Access Article
Open Access ArticleElectrophilic alkylation of arenes with 5-bromopyrimidine en route to 4-aryl-5-alkynylpyrimidines†
Stanislav S. Shcherbakov*a,
Artyom Yu. Magometova,
Viktoriia Yu. Shcherbakovaa,
Alexander V. Aksenov a,
Dmitriy A. Domenyukb,
Vladimir A. Zelenskyb and
Michael Rubin
a,
Dmitriy A. Domenyukb,
Vladimir A. Zelenskyb and
Michael Rubin *ac
*ac
aDepartment of Chemistry, North Caucasus Federal University, 1a Pushkin St., Stavropol 355009, Russian Federation. E-mail: alexaks05@rambler.ru
bDepartment of General Practice Dentistry and Child Dentistry, Stavropol State Medical University, 310 Mira Street, Stavropol 355017, Russian Federation
cDepartment of Chemistry, University of Kansas, 1567 Irving Hill Road, Lawrence, KS 66045-7582, USA. E-mail: mrubin@ku.edu; Tel: +1-785-864-5071
First published on 10th March 2020
Abstract
A new synthetic protocol for preparation of medicinally important 4-aryl-5-alkynylpyrimidines is described. The featured approach involves a sequence of chemo- and regioselective Brønsted acid-catalyzed electrophilic alkylation of arenes with 5-bromopyrimidine, followed by oxidative re-aromatization of the formed dihydropyrimidine ring. Finally, palladium-catalyzed Sonogashira cross-coupling reaction provided an end-game strategy.
Introduction
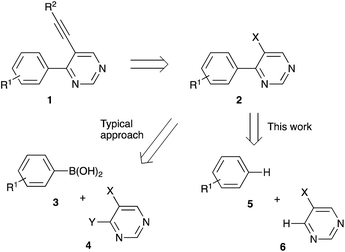
4-Arylpyrimidines 1 bearing acetylene substituents at C-5 (Scheme 1) were investigated as promising non-nucleoside-type adenosine kinase,1,2 tyrosine kinase,3,4 or lysine-specific demethylase5 inhibitors. These molecules were also tested as potential anti-proliferative agents6,7 or non-toxic selective herbicides,8 or used as a synthetic platform for further derivatization en route to medicinally relevant structures.9 Conveniently, structures of this type are accessible via Pd-catalyzed cross-coupling reaction involving terminal alkynes and hetaryl halides or sulfonates 2 (Scheme 1).10,11 Alternatively, a decarboxylative cross-coupling of alkynyl halides with hetarylcarboxylic acids can be employed.12 Although the synthetic route involving 2 as an electrophilic component in the Sonogashira reaction seems very straightforward, access to these starting materials typically relies on another transition metal-catalyzed cross-coupling step, usually chemoselective Suzuki reaction between arylboronic acid 3 and hetaryl dihalide 4 (Scheme 1).13–18 Since this conventional approach lacks any atom-economy appeal, we wondered, if an alternative synthetic strategy could be designed, utilizing a formal oxidative functionalization of two C–H bonds in arene 5 and pyrimidine 6 (Scheme 1). In perspective, this would allow for an easier assembly of 2 from more affordable precursors, while generating less chemical waste. Herein we wish to report on our synthetic studies towards this goal.Results and discussion
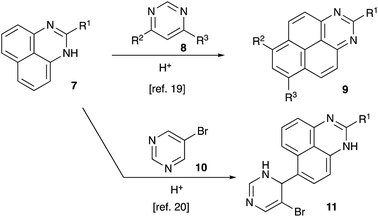
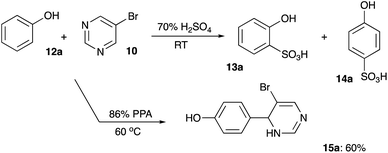
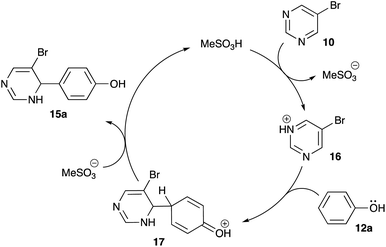
In recent years, our group was interested in designing novel acid-mediated multistep cascade transformations targeting material science and medicinal chemistry applications.19–23 In particular, an unusual annulation reaction was demonstrated, involving perimidines 7 and pyrimidines 8 and leading to the formation of 1,3-diazapyrenes 9 (Scheme 2).24 It was also shown that unlike most other pyrimidines, 5-bromopyrimidine 10 did not react according to this general scheme, forming mono-alkylation products 11 instead (Scheme 2).24,25 Apparently, this process is related to a Friedel–Crafts type SEAr alkylation reaction, in which pyrimidine in protonated form serves as an electrophile. We considered that an extension of the latter reaction to other nucleophilic arenes would help us to pursue our stated goal of installing an aryl substituent at C-4 of 5-bromopyrimidine ring.To test this idea, we carried out the reaction between phenol (12a) (as an example of electron rich nucleophilic arene) and 5-bromopyrimidine (10) in 70% aqueous sulfuric acid. Regrettably, this reaction led to the formation of a mixture of two sulfonic acids 13a and 14a, but the desired product 15a did not form under these conditions (Scheme 3). Next, we tried to carry out the same transformation in 86% polyphosphoric acid (PPA). We were pleased to find that in this case 15a was formed smoothly, and we managed to isolate it in 60% yield (Scheme 3). The same reaction carried out at room temperature in the presence of methanesulfonic acid afforded 15a in 71% yield (Scheme 4).
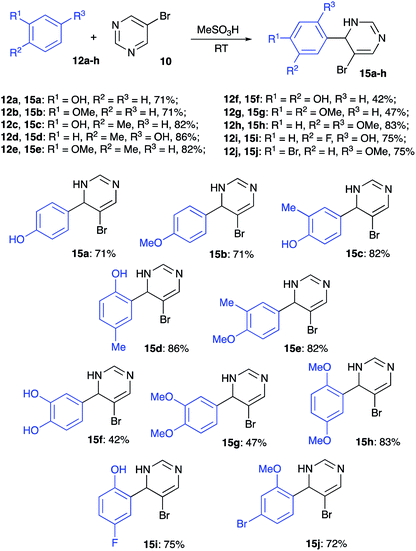
Inspired by these results, we examined the reactivity of other electron rich arenes. To our delight, anisole (12b), o- and p-cresols (12c and 12d, respectively), and o-methylanisole (12e) reacted smoothly providing the corresponding alkylated products 15b–e in high yields (Scheme 4). Reactions of catechol (12f) and veratrole (12g) also proceeded smoothly, but isolation via extraction was accompanied with notable loss of products 15f and 15g, which proved to be quite water-soluble (Scheme 4). In contrast, reaction and isolation of 1,4-dimethoxybenzene (12h) proceeded uneventfully, leading to formation of 15h in high yield (Scheme 4). Next, we tested the reactivity of halogenated phenol derivatives. Remarkably, both p-fluorophenol (12i) and m-bromoanisole (12j) afforded the corresponding coupling products 15i, j very efficiently (Scheme 4).
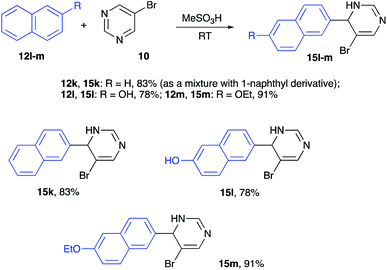
Reactions of naphthalenes with electrophilically activated 5-bromopyrimidine under the same conditions were also tested. Non-substituted naphthalene (12k) reacted very efficiently, but the regioselectivity was poor, and the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of inseparable 2-naphthyl- (15k) and 1-naphthyl-regioisomeric products was isolated in 83% combined yield (Scheme 5). Alkylation of 2-naphthol (12l) and 2-ethoxynaphthalene (12m) proceeded regioselectively and the corresponding structures 15l and 15m were obtained as sole products isolated in high yields (Scheme 5).
1 mixture of inseparable 2-naphthyl- (15k) and 1-naphthyl-regioisomeric products was isolated in 83% combined yield (Scheme 5). Alkylation of 2-naphthol (12l) and 2-ethoxynaphthalene (12m) proceeded regioselectively and the corresponding structures 15l and 15m were obtained as sole products isolated in high yields (Scheme 5).
It is believed that this reaction proceeds via SEAr mechanism. Indeed, protonation of one of the nitrogen atoms in pyrimidine 10 in the presence of strong Brønsted acid should afford highly electrophilic pyrimidinium species 16, which could serve as an electrophile in Friedel–Crafts-like reaction involving electron-rich arenes (Scheme 6). To get an additional support to this mechanistic rationale we attempted to carry out this reaction employing electron deficient substrates: nitrobenzene, acetophenone, 2-hydroxyacetophenone, 2-acetonaphthone, 1-benzonaphthone, acetanilide, or 1,8-diaminonaphthalene (the latter upon protonation is converted in poorly nucleophilic ammonium form). Expectedly, the alkylation did not proceed with either of these substrates.
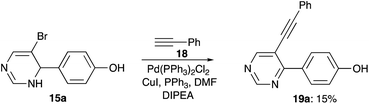
Next, we decided to investigate the possibility to use 5-bromo-3,4-dihydropyrimidin-4-yl arenes 15 in cross-coupling reaction with terminal alkynes. To this end, compound 15a was treated with phenyl acetylene 18 under standard conditions for Sonogashira reaction. Unfortunately, reaction produced a complex mixture of products, among which we only were able to isolate minute amounts of pyrimidine 19a, probably resulted from aerobic oxidation of the 3,4-dihydropyrimidine ring (Scheme 7).
Since oxidation of dihydropyrimidine ring seemed to proceed spontaneously, it was decided to perform it first to achieve cleaner Sonogashira coupling at the next step. First, oxidation with DMSO was attempted (Method C), but this method was highly inefficient. Reaction of 19a with DDQ also proceeded inefficiently, affording a mixture of three products, none of which was identified as the desired product 20a. However, we managed to obtain practical yields of aromatized products 20 in reactions with aqueous potassium ferricyanide (Method D, Scheme 8). Structure of compound 20a was unambiguously confirmed by single crystal X-ray diffraction (CCDC #1983237).
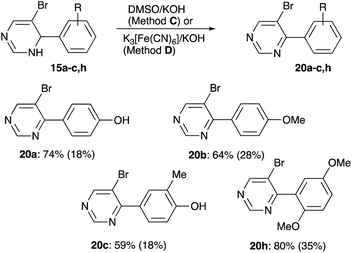 | ||
| Scheme 8 Oxidative aromatization of dihydropyrimidines 15. Yields for Method D and for Method C (in parentheses) are shown. | ||
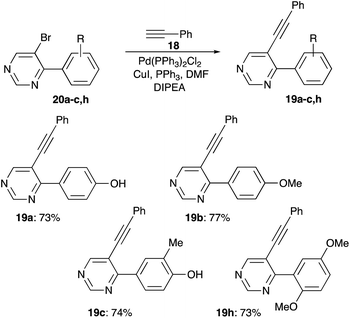
Finally, acetylene substituent was installed into pyrimidine ring via Sonogashira coupling employing microwave irradiation. This allowed to obtain the target pyrimidylacetylenes 19 in good yields (Scheme 9).
Conclusion
In conclusion, a new synthetic protocol was developed, allowing for a facile assembly of 4-aryl-5-alkynylpyrimidines involving chemo- and regioselective acid catalyzed electrophilic alkylation of arenes with 5-bromopyrimidine, followed by oxidative aromatization of the formed dihydropyrimidine moieties, and subsequent Sonogashira cross-coupling reaction.Experimental part
General information
1H and 13C NMR spectra were recorded on a Bruker Avance-III spectrometer (400 or 100 MHz, respectively) equipped with a BBO probe in CDCl3, DMSO-d6, acetone-d6 using TMS as an internal standard. High-resolution mass spectra were registered with a Bruker Maxis spectrometer (electrospray ionization, in MeCN solution, using HCO2Na–HCO2H for calibration). Melting points were measured with a Stuart smp30 apparatus. All reactions were performed in oven-dried 5 mL round-bottomed flasks equipped with reflux condensers. The reaction progress and purity of isolated compounds were controlled by TLC on Silufol UV-254 plates, with EtOAc as eluent. Polyphosphoric acids (86%) were obtained by dissolving of precise amount of P2O5 in 85% orthophosphoric acid according to the published protocols.26,27 All reagents and solvents were purchased from commercial vendors and used as received.Preparation of (5-bromo-3,4-dihydropyrimidin-4-yl)-benzenes (general procedure)
Preparation of (5-bromo-3,4-pyrimidin-4-yl)-benzenes (general procedure)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 44 mg (0.18 mmol, 18% via Method C); 138 mg (0.55 mmol, 55% via Method D). 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H, –OH), 9.13 (s, 1H, 2H 4-pyr), 9.02 (s, 1H, 6H 4-pyr), 7.73 (d, J = 8.6 Hz, 2H, 3,5H), 6.90 (d, J = 8.7 Hz, 2H, 2,6H). 13C NMR (101 MHz, DMSO-d6) δ 162.9, 160.1, 159.6, 156.8, 131.2 (2C), 127.1, 118.1, 115.0 (2C). FT IR (ZnSe, cm−1): 2923, 1701, 1558, 1436, 1390, 1150, 1028, 822. HRMS (TOF-ES): calculated for C10H8BrN2O (M + H)+ 250.9815, found 250.9816.
1). Yield 44 mg (0.18 mmol, 18% via Method C); 138 mg (0.55 mmol, 55% via Method D). 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H, –OH), 9.13 (s, 1H, 2H 4-pyr), 9.02 (s, 1H, 6H 4-pyr), 7.73 (d, J = 8.6 Hz, 2H, 3,5H), 6.90 (d, J = 8.7 Hz, 2H, 2,6H). 13C NMR (101 MHz, DMSO-d6) δ 162.9, 160.1, 159.6, 156.8, 131.2 (2C), 127.1, 118.1, 115.0 (2C). FT IR (ZnSe, cm−1): 2923, 1701, 1558, 1436, 1390, 1150, 1028, 822. HRMS (TOF-ES): calculated for C10H8BrN2O (M + H)+ 250.9815, found 250.9816.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 73 mg (0.28 mmol, 28% via Method C); 169 mg (0.64 mmol, 64% via Method D). 1H NMR (400 MHz, CDCl3) δ 9.11 (s, 1H, 2H), 8.87 (s, 1H, 6H), 7.87 (d, J = 8.8 Hz, 2H, 2,6H 4-(4-methoxyphenyl)), 7.01 (d, J = 8.8 Hz, 2H, 3,5H 4-(4-methoxyphenyl)), 3.88 (s, 3H, OMe). 13C NMR (101 MHz, CDCl3) δ 163.7, 161.5, 160.3, 156.9, 131.3 (2C), 129.1, 118.8, 113.8 (2C), 55.6. FT IR (ZnSe, cm−1): 2955, 2325, 1895, 1726, 1607, 1554, 1431, 1259, 1154, 1023, 825, 776. HRMS (TOF-ES): calculated for C11H10BrN2O (M + H)+ 264.9971, found 264.9974
1). Yield 73 mg (0.28 mmol, 28% via Method C); 169 mg (0.64 mmol, 64% via Method D). 1H NMR (400 MHz, CDCl3) δ 9.11 (s, 1H, 2H), 8.87 (s, 1H, 6H), 7.87 (d, J = 8.8 Hz, 2H, 2,6H 4-(4-methoxyphenyl)), 7.01 (d, J = 8.8 Hz, 2H, 3,5H 4-(4-methoxyphenyl)), 3.88 (s, 3H, OMe). 13C NMR (101 MHz, CDCl3) δ 163.7, 161.5, 160.3, 156.9, 131.3 (2C), 129.1, 118.8, 113.8 (2C), 55.6. FT IR (ZnSe, cm−1): 2955, 2325, 1895, 1726, 1607, 1554, 1431, 1259, 1154, 1023, 825, 776. HRMS (TOF-ES): calculated for C11H10BrN2O (M + H)+ 264.9971, found 264.9974![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 46 mg (0.18 mmol, 18% via Method C); 155 mg (0.59 mmol, 59% via Method D). 1H NMR (400 MHz, acetone-d6) δ 9.07 (s, 1H, 2H 4-pyr), 8.92 (s, 1H, 6H 4-pyr), 8.88 (s, 1H, –OH), 7.70 (s, 1H, 5H), 7.65 (d, J = 8.3 Hz, 1H, 3H), 6.96 (d, J = 8.3 Hz, 1H, 2H), 2.28 (s, 3H, –Me). 13C NMR (101 MHz, acetone-d6) δ 164.3, 160.9, 158.4, 157.6, 133.2, 129.6, 128.9, 119.1, 115.0, 16.2. FT IR (ZnSe, cm−1): 3049, 2358, 2217, 1565, 1485, 1401, 1279, 1116, 917, 788. HRMS (TOF-ES): calculated for C11H10BrN2O (M + H)+ 264.9971, found 264.9968.
1). Yield 46 mg (0.18 mmol, 18% via Method C); 155 mg (0.59 mmol, 59% via Method D). 1H NMR (400 MHz, acetone-d6) δ 9.07 (s, 1H, 2H 4-pyr), 8.92 (s, 1H, 6H 4-pyr), 8.88 (s, 1H, –OH), 7.70 (s, 1H, 5H), 7.65 (d, J = 8.3 Hz, 1H, 3H), 6.96 (d, J = 8.3 Hz, 1H, 2H), 2.28 (s, 3H, –Me). 13C NMR (101 MHz, acetone-d6) δ 164.3, 160.9, 158.4, 157.6, 133.2, 129.6, 128.9, 119.1, 115.0, 16.2. FT IR (ZnSe, cm−1): 3049, 2358, 2217, 1565, 1485, 1401, 1279, 1116, 917, 788. HRMS (TOF-ES): calculated for C11H10BrN2O (M + H)+ 264.9971, found 264.9968.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 102 mg (0.35 mmol, 35% via Method C); 236 mg (0.80 mmol, 80% via Method D). 1H NMR (400 MHz, CDCl3) δ 9.17 (s, 1H, 2H), 8.89 (s, 1H, 6H), 7.01 (d, J = 9.0 Hz, 1H, 3H 4-(2,5-dimethoxyphenyl)), 6.94 (d, J = 9.0 Hz, 1H, 4H 4-(2,5-dimethoxyphenyl)), 6.86 (s, 1H, 6H 4-(2,5-dimethoxyphenyl)), 3.80 (s, 3H, 2-MeO–), 3.78 (s, 3H, 5-MeO–). 13C NMR (101 MHz, CDCl3) δ 166.8, 164.8, 158.7, 156.3, 153.7, 150.8, 126.9, 117.1, 115.1 (2C), 112.6, 56.2, 56.0. FT IR (ZnSe, cm−1): 2947, 2322, 1723, 1554, 1506, 1435, 1221, 1019, 810. HRMS (TOF-ES): calculated for C12H14BrN2O2 (M + H)+ 297.0233, found 297.0237.
1). Yield 102 mg (0.35 mmol, 35% via Method C); 236 mg (0.80 mmol, 80% via Method D). 1H NMR (400 MHz, CDCl3) δ 9.17 (s, 1H, 2H), 8.89 (s, 1H, 6H), 7.01 (d, J = 9.0 Hz, 1H, 3H 4-(2,5-dimethoxyphenyl)), 6.94 (d, J = 9.0 Hz, 1H, 4H 4-(2,5-dimethoxyphenyl)), 6.86 (s, 1H, 6H 4-(2,5-dimethoxyphenyl)), 3.80 (s, 3H, 2-MeO–), 3.78 (s, 3H, 5-MeO–). 13C NMR (101 MHz, CDCl3) δ 166.8, 164.8, 158.7, 156.3, 153.7, 150.8, 126.9, 117.1, 115.1 (2C), 112.6, 56.2, 56.0. FT IR (ZnSe, cm−1): 2947, 2322, 1723, 1554, 1506, 1435, 1221, 1019, 810. HRMS (TOF-ES): calculated for C12H14BrN2O2 (M + H)+ 297.0233, found 297.0237.Preparation of 4-(5-(phenylethynyl)pyrimidin-4-yl)benzenes via Sonogashira coupling (general procedure)
Pyrex microwave vessel was charged with phenylacetylene (102 mg, 1.00 mmol) and the corresponding substituted 4-(5-bromopyrimidin-4-yl)-2-benzene (1.00 mmol) in dry DMF (0.5 mL). Dry nitrogen was passed to the vessel for 50 min, then anhydrous DIPEA (1.5 mL, 1.11 g, 8.6 mmol), dry triphenylphosphine (23 mg, 0.087 mmol) were introduced, followed by bis(triphenylphosphine)palladium chloride (Pd(PPh3)Cl2, 15 mg, 0.021 mmol) and copper(I) iodide (CuI, 3.8 mg, 0.020 mmol). The vessel was sealed and microwaved at 115 °C for 25 min. Then the mixture was cooled down and extracted with ethyl acetate (2 × 50 mL). Combined organic extracts were consecutively washed with aqueous ammonium chloride solution (2 × 25 mL) and water (2 × 25 mL), and dried with sodium sulfate. Pure product was crystallized from concentrated solution obtained after evaporation of most solvent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 199 mg (0.73 mmol, 73%). 1H NMR (400 MHz, acetone-d6) δ 9.09 (s, 1H, 2H 4-pyr), 8.92 (s, 1H, 6H 4-pyr), 8.27 (d, J = 8.8 Hz, 2H, 2,6H), 7.63–7.53 (m, 2H, 2,6H 4-(5-(phenylethynyl)pyr)), 7.46 (m, 3H, 3-5H 4-(5-(phenylethynyl)pyr)), 7.04 (d, J = 8.8 Hz, 2H, 3,5H). 13C NMR (101 MHz, acetone-d6) δ 164.7, 161.9, 161.0, 157.6, 132.3 (2C), 132.1 (2C), 130.2, 129.6 (2C), 129.1, 123.2, 116.1 (2C), 115.7, 97.9, 85.7. FT IR (ZnSe, cm−1): 3057, 2362, 2217, 1565, 1504, 1432, 1287, 1165, 845. HRMS (TOF-ES): calculated for C18H13N2O (M + H)+ 273.1022, found 273.1025.
1). Yield 199 mg (0.73 mmol, 73%). 1H NMR (400 MHz, acetone-d6) δ 9.09 (s, 1H, 2H 4-pyr), 8.92 (s, 1H, 6H 4-pyr), 8.27 (d, J = 8.8 Hz, 2H, 2,6H), 7.63–7.53 (m, 2H, 2,6H 4-(5-(phenylethynyl)pyr)), 7.46 (m, 3H, 3-5H 4-(5-(phenylethynyl)pyr)), 7.04 (d, J = 8.8 Hz, 2H, 3,5H). 13C NMR (101 MHz, acetone-d6) δ 164.7, 161.9, 161.0, 157.6, 132.3 (2C), 132.1 (2C), 130.2, 129.6 (2C), 129.1, 123.2, 116.1 (2C), 115.7, 97.9, 85.7. FT IR (ZnSe, cm−1): 3057, 2362, 2217, 1565, 1504, 1432, 1287, 1165, 845. HRMS (TOF-ES): calculated for C18H13N2O (M + H)+ 273.1022, found 273.1025.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 220 mg (0.77 mmol, 77%). 1H NMR (400 MHz, CDCl3) δ 9.14 (s, 1H, 2H), 8.89 (s, 1H, 6H), 8.31 (d, J = 8.8 Hz, 2H, 3,5H 4-(4-methoxyphenyl)), 7.53–7.49 (m, 2H, 2,6H 5-(phenylethynyl)), 7.41–7.36 (m, 3H, 3-5H 5-(phenylethynyl)), 7.05 (d, J = 8.9 Hz, 2H, 2,6H 4-(4-methoxyphenyl)), 3.90 (s, 3H 4-MeO). 13C NMR (101 MHz, CDCl3) δ 164.9, 162.5, 159.7, 155.2, 131.7 (2C), 131.5 (2C), 129.5, 128.8, 128.7 (2C), 122.1, 115.8, 114.0 (2C), 98.5, 84.3, 55.6. FT IR (ZnSe, cm−1): 2929, 2221, 1726, 1607, 1509, 1435, 1259, 1173, 1027, 783. HRMS (TOF-ES): calculated for C19H15N2O (M + H)+ 287.1179, found 287.1180.
1). Yield 220 mg (0.77 mmol, 77%). 1H NMR (400 MHz, CDCl3) δ 9.14 (s, 1H, 2H), 8.89 (s, 1H, 6H), 8.31 (d, J = 8.8 Hz, 2H, 3,5H 4-(4-methoxyphenyl)), 7.53–7.49 (m, 2H, 2,6H 5-(phenylethynyl)), 7.41–7.36 (m, 3H, 3-5H 5-(phenylethynyl)), 7.05 (d, J = 8.9 Hz, 2H, 2,6H 4-(4-methoxyphenyl)), 3.90 (s, 3H 4-MeO). 13C NMR (101 MHz, CDCl3) δ 164.9, 162.5, 159.7, 155.2, 131.7 (2C), 131.5 (2C), 129.5, 128.8, 128.7 (2C), 122.1, 115.8, 114.0 (2C), 98.5, 84.3, 55.6. FT IR (ZnSe, cm−1): 2929, 2221, 1726, 1607, 1509, 1435, 1259, 1173, 1027, 783. HRMS (TOF-ES): calculated for C19H15N2O (M + H)+ 287.1179, found 287.1180.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 210 mg (0.74 mmol, 74%). 1H NMR (400 MHz, CDCl3) δ 9.13 (s, 1H, 2H 4-pyr), 8.87 (s, 1H, 6H 4-pyr)), 8.12 (s, 1H, 5H), 8.07 (d, J = 8.4 Hz, 1H, 3H), 7.55–7.45 (m, 2H, 2,6H 4-(5-(phenylethynyl)pyr)), 7.41–7.35 (m, 3H, 3-5H 4-(5-(phenylethynyl)pyr), 6.94 (d, J = 8.4 Hz, 1H, 6H), 2.34 (s, 3H, –Me). 13C NMR (101 MHz, CDCl3) δ 164.7, 160.6, 157.2, 156.1, 132.6, 131.7 (2C), 129.4, 129.0, 128.7 (2C), 124.3, 122.4 (2C), 115.8, 114.9, 98.0, 84.7, 16.2. FT IR (ZnSe, cm−1): 3049, 2358, 2217, 1565, 1485, 1401, 1279, 1116, 917, 788. HRMS (TOF-ES): calculated for C19H15N2O (M + H)+ 287.1179, found 287.1175.
1). Yield 210 mg (0.74 mmol, 74%). 1H NMR (400 MHz, CDCl3) δ 9.13 (s, 1H, 2H 4-pyr), 8.87 (s, 1H, 6H 4-pyr)), 8.12 (s, 1H, 5H), 8.07 (d, J = 8.4 Hz, 1H, 3H), 7.55–7.45 (m, 2H, 2,6H 4-(5-(phenylethynyl)pyr)), 7.41–7.35 (m, 3H, 3-5H 4-(5-(phenylethynyl)pyr), 6.94 (d, J = 8.4 Hz, 1H, 6H), 2.34 (s, 3H, –Me). 13C NMR (101 MHz, CDCl3) δ 164.7, 160.6, 157.2, 156.1, 132.6, 131.7 (2C), 129.4, 129.0, 128.7 (2C), 124.3, 122.4 (2C), 115.8, 114.9, 98.0, 84.7, 16.2. FT IR (ZnSe, cm−1): 3049, 2358, 2217, 1565, 1485, 1401, 1279, 1116, 917, 788. HRMS (TOF-ES): calculated for C19H15N2O (M + H)+ 287.1179, found 287.1175.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 220 mg (0.73 mmol, 73%). 1H NMR (400 MHz, CDCl3) δ 9.21 (s, 1H, 2H), 8.90 (s, 1H, 6H), 7.34–7.29 (m, 5H), 7.08–7.01 (m, 2H, 3,6H 4-(2,5-dimethoxyphenyl)), 6.97 (d, J = 8.6 Hz, 1H, 4H 4-(2,5-dimethoxyphenyl)), 3.82 (s, 3H, 2-MeO), 3.78 (s, 3H 4-MeO). 13C NMR (101 MHz, CDCl3) δ 166.3, 158.2, 154.1, 153.6, 151.7, 131.8 (2C), 129.6, 128.7 (2C), 125.6, 122.0, 120.3, 118.4, 115.6, 112.7, 98.8, 83.1, 56.3, 56.1. FT IR (ZnSe, cm−1): 2943, 2831, 2217, 1726, 1498, 1431, 1397, 1274, 1221, 1034, 810. HRMS (TOF-ES): calculated for C20H17N2 (M + H)+ 317.1285, found 317.1286.
1). Yield 220 mg (0.73 mmol, 73%). 1H NMR (400 MHz, CDCl3) δ 9.21 (s, 1H, 2H), 8.90 (s, 1H, 6H), 7.34–7.29 (m, 5H), 7.08–7.01 (m, 2H, 3,6H 4-(2,5-dimethoxyphenyl)), 6.97 (d, J = 8.6 Hz, 1H, 4H 4-(2,5-dimethoxyphenyl)), 3.82 (s, 3H, 2-MeO), 3.78 (s, 3H 4-MeO). 13C NMR (101 MHz, CDCl3) δ 166.3, 158.2, 154.1, 153.6, 151.7, 131.8 (2C), 129.6, 128.7 (2C), 125.6, 122.0, 120.3, 118.4, 115.6, 112.7, 98.8, 83.1, 56.3, 56.1. FT IR (ZnSe, cm−1): 2943, 2831, 2217, 1726, 1498, 1431, 1397, 1274, 1221, 1034, 810. HRMS (TOF-ES): calculated for C20H17N2 (M + H)+ 317.1285, found 317.1286.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Russian Foundation for Basic Research (grant #18-33-00849 mol_a) and by the Ministry of Education and Science of the Russian Federation (grant #0795-2020).Notes and references
- M. A. Matulenko, E. S. Paight, R. R. Frey, A. Gomtsyan, S. DiDomenico, M. Jiang, C.-H. Lee, A. O. Stewart, H. Yu, K. L. Kohlhaas, K. M. Alexander, S. McGaraughty, J. Mikusa, K. C. Marsh, S. W. Muchmore, C. L. Jakob, E. A. Kowaluk, M. F. Jarvis and S. S. Bhagwat, Bioorg. Med. Chem., 2007, 15, 1586–1605 CrossRef PubMed.
- S. Schneider, D. Kessler, L. Van der Veen and T. Wunberg, WO2011092198A1, 2011.
- M. Takasaki, T. Tsujino, S. Tanabe, M. Ookubo, K. Sato, A. Hirai, D. Terada, S. Inuki and S. Mizumoto, WO2013157540A1, 2013.
- Y. K. Chen, T. Kanouni, S. W. Kaldor, J. A. Stafford and J. M. Veal, WO2015089192A1, 2015.
- Y. K. Chen, T. Kanouni, J. A. Stafford and J. M. Veal, WO2016037005A1, 2016.
- T. Wunberg, R. Brueckner, D. Kessler, O. Kraemer, D. McConnell, S. Schneider and L. Van der Veen, WO2010012740A1, 2010.
- T. Wunberg, L. Van der Veen and O. Kraemer, WO2012101184A1, 2012.
- T. Kuragano and H. Ikeda, JP2004115431A, 2004.
- M. Beesu, A. C. D. Salyer, K. L. Trautman, J. K. Hill and S. A. David, J. Med. Chem., 2016, 59, 8082–8093 CrossRef PubMed.
- T. Lechel, S. Moehl and H.-U. Reissig, Synlett, 2009, 1059–1062 Search PubMed.
- T. Lechel and H.-U. Reissig, Eur. J. Org. Chem., 2010, 2555–2564 CrossRef.
- N. S. Le Pham, J. Lee, H. Shin and J.-H. Sohn, Tetrahedron, 2018, 74, 3843–3851 CrossRef.
- J. Liu, A. E. Fitzgerald and N. S. Mani, J. Org. Chem., 2008, 73, 2951–2954 CrossRef PubMed.
- A. Mollard, S. L. Warner, L. T. Call, M. L. Wade, J. J. Bearss, A. Verma, S. Sharma, H. Vankayalapati and D. J. Bearss, ACS Med. Chem. Lett., 2011, 2, 907–912 CrossRef PubMed.
- A. Steffen, M. I. Sladek, T. Braun, B. Neumann and H.-G. Stammler, Organometallics, 2005, 24, 4057–4064 CrossRef.
- L. M. Toledo-Sherman, M. E. Prime, L. Mrzljak, M. G. Beconi, A. Beresford, F. A. Brookfield, C. J. Brown, I. Cardaun, S. M. Courtney, U. Dijkman, E. Hamelin-Flegg, P. D. Johnson, V. Kempf, K. Lyons, K. Matthews, W. L. Mitchell, C. O'Connell, P. Pena, K. Powell, A. Rassoulpour, L. Reed, W. Reindl, S. Selvaratnam, W. W. Friley, D. A. Weddell, N. E. Went, P. Wheelan, C. Winkler, D. Winkler, J. Wityak, C. J. Yarnold, D. Yates, I. Munoz-Sanjuan and C. Dominguez, J. Med. Chem., 2015, 58, 1159–1183 CrossRef PubMed.
- A. D. William, A. C. H. Lee, S. Blanchard, A. Poulsen, E. L. Teo, H. Nagaraj, E. Tan, D. Chen, M. Williams, E. T. Sun, K. C. Goh, W. C. Ong, S. K. Goh, S. Hart, R. Jayaraman, M. K. Pasha, K. Ethirajulu, J. M. Wood and B. W. Dymock, J. Med. Chem., 2011, 54, 4638–4658 CrossRef PubMed.
- J. Xiao, J. J. Marugan, W. Zheng, S. Titus, N. Southall, J. J. Cherry, M. Evans, E. J. Androphy and C. P. Austin, J. Med. Chem., 2011, 54, 6215–6233 CrossRef PubMed.
- A. V. Aksenov, A. N. Smirnov, N. A. Aksenov, I. V. Aksenova, L. V. Frolova, A. Kornienko, I. V. Magedov and M. Rubin, Chem. Commun., 2013, 49, 9305–9307 RSC.
- A. V. Aksenov, A. N. Smirnov, N. A. Aksenov, I. V. Aksenova, J. P. Matheny and M. Rubin, RSC Adv., 2015, 5, 8647–8656 RSC.
- A. V. Aksenov, A. N. Smirnov, N. A. Aksenov, A. S. Bijieva, I. V. Aksenova and M. Rubin, Org. Biomol. Chem., 2015, 13, 4289–4295 RSC.
- A. V. Aksenov, A. N. Smirnov, I. V. Magedov, M. R. Reisenauer, N. A. Aksenov, I. V. Aksenova, A. L. Pendleton, G. Nguyen, R. K. Johnston, M. Rubin, A. De Carvalho, R. Kiss, V. Mathieu, F. Lefranc, J. Correa, D. A. Cavazos, A. J. Brenner, B. A. Bryan, S. Rogelj, A. Kornienko and L. V. Frolova, J. Med. Chem., 2015, 58, 2206–2220 CrossRef PubMed.
- N. A. Aksenov, A. V. Aksenov, O. N. Nadein, D. A. Aksenov, A. N. Smirnov and M. Rubin, RSC Adv., 2015, 5, 71620–71626 RSC.
- A. V. Aksenov, S. V. Shcherbakov, I. V. Lobach, I. V. Aksenova and M. Rubin, Eur. J. Org. Chem., 2017, 2017, 1666–1673 CrossRef.
- A. V. Aksenov, S. V. Shcherbakov, I. V. Lobach, L. G. Voskressensky and M. Rubin, Eur. J. Org. Chem., 2018, 2018, 4121–4127 CrossRef.
- F. Uhlig, Angew. Chem., 1954, 66, 435–436 CrossRef.
- A. L. Huhti and P. A. Gartaganis, Can. J. Chem., 1956, 34, 785–797 CrossRef.
- D. Xue, Z.-H. Jia, C.-J. Zhao, Y.-Y. Zhang, C. Wang and J. Xiao, Chem.–Eur. J., 2014, 20, 2960–2965 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data. CCDC 1983237. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra01335h |
| This journal is © The Royal Society of Chemistry 2020 |