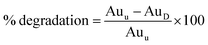Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stress stability study of simeprevir, a hepatitis C virus inhibitor, using feasible TLC-spectro-densitometry: application to pharmaceutical dosage form and human plasma
Bassam Shaaban Mohammed *a,
Amal E. Hamad
*a,
Amal E. Hamad a and
Sayed M. Derayea
a and
Sayed M. Derayea b
b
aDepartment of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Menoufia University, Shebin El-Kom, Menoufia, Egypt. E-mail: Bassam.shaaban@phrm.menofia.edu.eg
bDepartment of Analytical Chemistry, Faculty of Pharmacy, Minia University, Minia 61519, Egypt
First published on 3rd June 2020
Abstract
Simeprevir is one of the newest direct action anti-hepatitis C drugs. In the present work, a simple, highly selective and stability-indicating, high-performance thin-layer chromatography (HPTLC) method is proposed and validated for the assay of simeprevir both in pharmaceutical dosage form and spiked human plasma. The method used silica gel 60 F254 coated HPTLC aluminum plates as the stationary phase. The mobile phase system was ethyl acetate-hexane-methanol (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v). The wavelength for both detection and quantitation was set at 288 nm. This system was found to give a compact spot of simeprevir; the retardation factor (RF) value was 0.67 ± 0.02. The guidelines of the International Conference on Harmonization were followed to validate the proposed analytical method, and the results were acceptable. The calibration curve was linear over the range of 80–1000 ng per spot. The limit of detection was 19.0 ng per spot, and the limit of quantitation was 57.0 ng per spot. The drug was subjected to various stress conditions including hydrolytic, oxidative and UV-induced resulting in varying degrees of degradation. The results showed that the proposed method could efficiently separate the degradation products from the intact drug and allow its satisfactory quantitation. The proposed method was employed successfully for the accurate and reproducible analysis of the pharmaceutical preparation and human plasma containing the drug. The proposed method's precision and accuracy were statistically similar to those of a reported method.
1, v/v/v). The wavelength for both detection and quantitation was set at 288 nm. This system was found to give a compact spot of simeprevir; the retardation factor (RF) value was 0.67 ± 0.02. The guidelines of the International Conference on Harmonization were followed to validate the proposed analytical method, and the results were acceptable. The calibration curve was linear over the range of 80–1000 ng per spot. The limit of detection was 19.0 ng per spot, and the limit of quantitation was 57.0 ng per spot. The drug was subjected to various stress conditions including hydrolytic, oxidative and UV-induced resulting in varying degrees of degradation. The results showed that the proposed method could efficiently separate the degradation products from the intact drug and allow its satisfactory quantitation. The proposed method was employed successfully for the accurate and reproducible analysis of the pharmaceutical preparation and human plasma containing the drug. The proposed method's precision and accuracy were statistically similar to those of a reported method.
1. Introduction
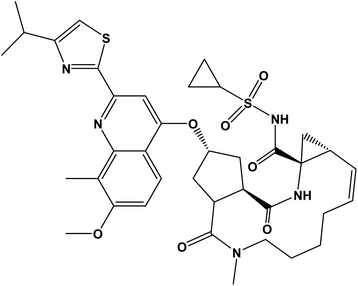
More than one hundred and fifty million people worldwide have been affected by HCV infection which represents the principal cause for hepatocellular carcinoma and liver failure.1 Many years ago, the combination of ribavirin and pegylated interferon α was the standard treatment for achieving a sustained virological response in ≥80% of patients with HCV genotypes 2 and 3 but only ∼50% in genotype 1 patients. However, it caused significant side effects.2,3 Great progress was brought about with the emergence of direct-acting antivirals which attack the viral nonstructural proteins. SMV inhibits N3S/4A protease enzyme and is orally administered once-daily for the treatment of chronic HCV genotype 1 infection in combination with other antiviral agents showing a highly sustained virological response rates in patients with HCV genotype 1 infection during phase II and III trials.4 SMV (Fig. 1) is a white powder soluble in dichloromethane, slightly soluble in acetone, very slightly soluble in ethanol; practically insoluble in water and propylene glycol.5Due to its recent market entry, not many analytical methods were found in the literature. The reported methods of analysis included; four liquid chromatographic methods for estimation of simeprevir in plasma samples using tandem mass detection,6,7 UV detection8 and diode array detection.9 Other non-chromatographic methods were also reported; synchronous spectrofluorimetric10 and spectrophotometric methods.11,12 HPTLC as a chromatographic technique shares the advantage of being able to separate and analyses complex mixtures. Although it's not as sophisticated or sensitive as HPLC, yet it possesses several strengths over HPLC such as simplicity and speed as well as great reduction in the analysis cost and time as it does not require expensive equipment nor tedious and time consuming sample pre-treatment steps. Furthermore, it uses much less mobile phase and sample. Therefore, the present work aimed to develop a new HPTLC-densitometric method for the determination of simeprevir in dosage forms and human plasma samples. The proposed method was developed also to study and evaluate degradation stability of SMV since it was able to accurately determine SMV in presence of its various degradation products without any prior extraction.
2. Experimental
2.1 Instrumentation
An HPTLC system with the following specifications was used: CAMAG Linomat V sample applicator (Muttenz, Switzerland) CAMAG 100 μL sample syringe (Muttenz, Switzerland); band width, 3 mm; application rate 15 s μL−1, slit dimension, 3 × 0.45 mm; and scanning speed, 20 mm s−1. Densitometric scanning was performed using a CAMAG TLC Scanner 3, operated by win CATS evaluation software (version 1.4.4.6337).Super-mixer (GEMMY Industrial CORD, Taiwan) and Bath Sonicator (Sonicor SC-101TH) were also used. All weighing were performed on an electronic single pan balance (Precise XB 220A, 10 mg to 220 g Switzerland).
2.2 Chromatographic conditions
The sample was applied on HPTLC aluminum plates pre-coated with silica gel 60 F254. The mobile phase consisted of ethyl acetate-hexane-methanol (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v). The samples were applied as bands of 3 mm long with 5 mm spacing under nitrogen stream. The chromatogram was developed in a linear manner to a distance of 9 cm in 20–20 cm twin-trough TLC chamber (CAMAG) at room temperature. The development chamber was first saturated for 30 min with the mobile phase prior to the development. The plates were then completely dried by an air dryer before being scanned with a CAMAG TLC Scanner 3 in the absorbance mode at 288 nm.
1, v/v/v). The samples were applied as bands of 3 mm long with 5 mm spacing under nitrogen stream. The chromatogram was developed in a linear manner to a distance of 9 cm in 20–20 cm twin-trough TLC chamber (CAMAG) at room temperature. The development chamber was first saturated for 30 min with the mobile phase prior to the development. The plates were then completely dried by an air dryer before being scanned with a CAMAG TLC Scanner 3 in the absorbance mode at 288 nm.
2.3 Materials and reagents
Aluminum plate precoated with silica gel 60 F254, (20 × 10 cm) with 250 mm thickness (Merck, Darmstadt, Germany). Pooled blank plasma was collected from healthy volunteers and kept in the refrigerator until needed. Pharmaceutical dosage form: Merospevir® capsules (batch number 160117) were kindly supplied by AUG PHARMA, Giza, Egypt. Each capsule was labeled to contain 150.0 mg of simeprevir.All chemicals and reagents used throughout this work were analytical grade. SMV was kindly gifted by AUG PHARMA (6th Industrial Zone – 6 October City – Egypt). Ethyl acetate, hexane, methanol, sodium hydroxide, hydrochloric acid, and hydrogen peroxide (30 volume) were purchased from El Nasr Chemical Co. (Abo-Zaabal, Cairo, Egypt).
2.4 Standard solution and calibration graphs
The SMV stock standard solution (100 μg mL−1) was prepared by dissolving an accurately weighed amount of SMV salt powder equivalent to 10.0 mg in a 100 mL volumetric flask using ethanol.Different volumes of the stock standard solution (0.8–10 μL) were spotted on the TLC plates that gave spot concentrations in the range of 80–1000 ng per spot (Fig. 2). The calibration graph was constructed by plotting area under peak against corresponding SMV concentration.
2.5 Preparation of plasma samples
The authors got the permission for using plasma sample of human volunteers from Minia Hospital according to the declaration of Helsinki, and International Conference on Harmonization (ICH) guidelines. All experiments were performed in compliance with the guidelines and approved by “The Commission on the Ethics of Scientific Research”, located in Faculty of Pharmacy, Minia University. An informed written consent was obtained from each participant. Drug-free human blood samples were obtained from healthy volunteers. 5 mL of which were centrifuged in a heparinized tube at 4000 rpm for 30 min. 1.0 mL aliquots of the supernatant were pipetted into 10.0 mL volumetric flasks and spiked with 1.0 mL of SMV solution containing 0.8–10 μg mL−1. Acetonitrile (2 mL) was used to precipitate protein. The mixture was diluted to the mark with ethanol giving a final SMV concentration of 80–1000 ng mL−1. The solution was centrifuged at 4000 rpm for about 20 min, and the clear colorless solution was subjected to HPTLC analysis. A blank was prepared by treating the drug-free blood sample in the same manner without adding any SMV.2.6 Analysis of pharmaceutical dosage forms
The contents of ten capsules Merospevir® capsules were evacuated and their contents were accurately weighed and mixed well. An amount equivalent to 10.0 mg of SMV was accurately weighted and transferred into a 100 mL volumetric flask, where they were dissolved in about 50 mL of ethanol by sonication for 5 min, and completed to the mark with ethanol and mixed well. The flask contents were filtered, rejecting the first portion of the filtrate. Different volumes of the obtained filtrate were spotted on the TLC plates, giving a final concentration of SMV between 80–1000 ng per spot.2.7 Procedures for forced degradation study
After degradation (procedures detailed in the following subsections), aliquots of the final solutions were applied to the HPTLC plate in triplicates of SMV concentrations within the linear range of the calibration and the chromatograms were developed as described under (Section 2.2). The degradation procedures were as follows.3. Results and discussion
The current study's aim was to develop and validate an efficient, fast and cost-effective method of analysis for the determination and stability testing of simeprevir with low impact on the environment. HPTLC densitometric technique delivered on the desired method objectives since it was able to separate multiple analyte simultaneously with little solvent consumption and very simple procedure for sample preparation. To attain good resolution with sharp symmetric beaks, and with acceptable RF values, several chromatographic conditions had to be optimized;3.1 Method optimization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) were examined, but the bands were not satisfactorily resolved. Ternary mobile phase systems were then tried including ethyl acetate–hexane–acetonitrile (8
2, v/v) were examined, but the bands were not satisfactorily resolved. Ternary mobile phase systems were then tried including ethyl acetate–hexane–acetonitrile (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and ethyl acetate–hexane–methanol (7.0
1, v/v) and ethyl acetate–hexane–methanol (7.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0
2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, by volume) were investigated. Still, poor resolutions, band broadening and tailing were observed. By changing the ratio of ethyl acetate–hexane–methanol, the resolution was improved but with tailed bands to some extent until using the ratio of (5
1.0, by volume) were investigated. Still, poor resolutions, band broadening and tailing were observed. By changing the ratio of ethyl acetate–hexane–methanol, the resolution was improved but with tailed bands to some extent until using the ratio of (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) which gave optimum resolution. Quantitative determination SMV was performed by scanning the bands at 288 nm, which gave RF value of 0.67 ± 0.02.
1 v/v) which gave optimum resolution. Quantitative determination SMV was performed by scanning the bands at 288 nm, which gave RF value of 0.67 ± 0.02.After optimization, the selected chromatographic conditions were applied for developing the plate spotted with the drug and scanning at 288 nm. It was found that the band of the drug was compact and symmetric with a RF value of 0.67 ± 0.02.
In addition, using the same chromatographic conditions proved successful in the separation of degraded SMV samples components with high resolution and sharp, compact, and symmetric peaks for the intact drug as well as all its degradation products. Consequently, it was not necessary to change the mobile phase or other parameters for the purpose of studying the forced stability of the drug.
3.2 Validation of the proposed analytical method
The planned analytical technique was validated according to the International Conference on Harmonization (ICH) guidelines regarding linear range, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, robustness, and selectivity.15| Parameters | Value |
|---|---|
| Concentration range (ng per spot) | 80–1000 |
| Correlation coefficient (r) | 0.9997 |
| Determination coefficient (r2) | 0.9994 |
| Slope (b) | 7.9134 |
| Standard deviation of slope | 0.076 |
| Intercept (a) | 1477.8 |
| Standard deviation of intercept | 45.103 |
| Limit of detection (LOD), ng per spot | 19.0 |
| Limit of quantitation (LOQ), ng per spot | 57.0 |
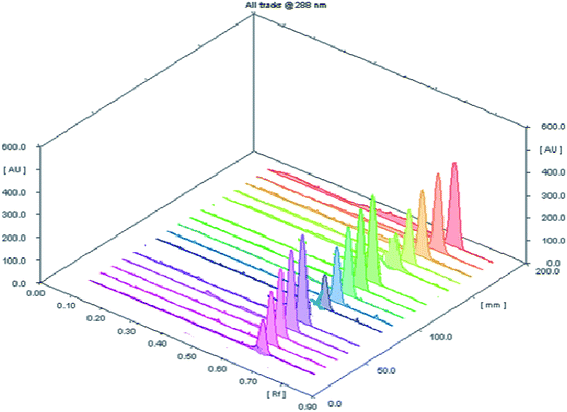 | ||
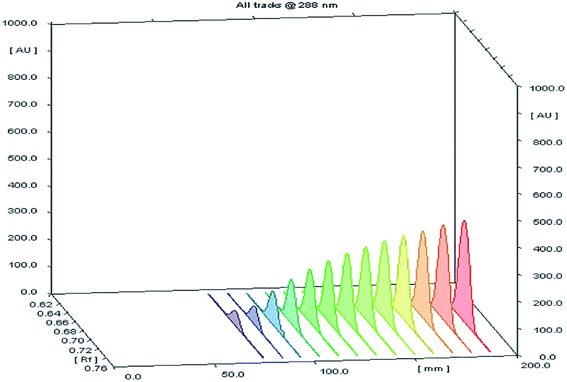
| Fig. 3 A 3D chromatogram for evaluation of the accuracy of investigated analytical procedure for determination of SMV at five concentration levels with three replicate measurements. | ||
| Sample number | Taken conc. (ng per spot) | Found conc.a (ng per spot) | % recovery ± SD |
|---|---|---|---|
| a Mean of three replicate measurements, (SD) standard deviation and (RSD) relative standard deviation. | |||
| 1 | 100 | 100.63 | 100.64 ± 2.91 |
| 2 | 200 | 206.22 | 103.11 ± 0.36 |
| 3 | 400 | 389.51 | 97.37 ± 0.18 |
| 4 | 600 | 584.51 | 97.41 ± 1.51 |
| 5 | 800 | 790.20 | 98.77 ± 0.11 |
| Parameters | Factor | % recovery ± RSD |
|---|---|---|
| Mobile phase composition | 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (v/v/v) 1.0 (v/v/v) |
Optimized |
5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.1 4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 (v/v/v) 1.1 (v/v/v) |
99.92 ± 1.54 | |
4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.9 3.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 (v/v/v) 0.9 (v/v/v) |
100.05 ± 1.06 | |
| Saturation time | 20 min | Optimized |
| 25 min | 99.88 ± 2.39 | |
| 15 min | 100.01 ± 0.27 | |
| Standing time after spotting | 20 min | Optimized |
| 10 min | 100.09 ± 1.90 | |
| 30 min | 100.10 ± 2.19 | |
| Time from development to scanning | 15 min | Optimized |
| 10 min | 99.91 ± 1.82 | |
| 30 min | 99.97 ± 0.56 |
3.3 Analysis of the pharmaceutical sample
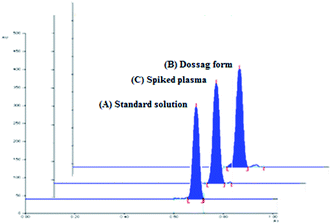
The densitometric responses (peak area) from the standard and dosage form sample solutions were used to quantify the amounts of SMV in Merospevir® capsules. The results of the proposed technique were then statistically compared to that of the reference method.10 No significant difference was found at 95% confidence level (Table 5), this clearly showed that the proposed method could be reliably used for assay of SMV in its dosage form without interference from excipients two-dimensional TLC-densitograms of SMV in dosage form are presented in (Fig. 4B).| Sample number | Taken conc. (ng per spot) | % recoverya | |
|---|---|---|---|
| Proposed method | Reference method [10] | ||
| a Average of five separate determinations ± standard deviation.b Theoretical values at 95% confidence limit: t = 2.31, F = 6.39. | |||
| 1 | 300.00 | 100.15 | 98.43 |
| 2 | 400.00 | 99.88 | 97.89 |
| 3 | 500.00 | 102.18 | 101.34 |
| 4 | 600.00 | 102.59 | 102.49 |
| 5 | 700.00 | 98.82 | 98.55 |
| Mean ± SD | 100.72 ± 1.60 | 99.76 ± 1.82 | |
| F-Valueb | 0.77 | ||
| t-Valueb | 0.94 | ||
3.4 Application to biological fluid (spiked human plasma)
The pharmacokinetic parameters of SMV explaining the maximum plasma concentration (Cmax) of SMV after multiple 150 mg once daily oral doses, Cmax was approximately 2.588 μg mL−1 at tmax (4–9 h).4 The high sensitivity of the proposed technique permitted the estimation of SMV in human plasma spiked with different concentrations of SMV within the specified range. According to (ICH) guidelines of bioanalytical method,16 the recovery percentages ranged from 88.44% to 101.69%. The results showed in (Table 6) explain the suitability of the proposed method for the analysis of human plasma containing SMV without any significant interference from plasma components. 2D TLC densitograms are presented in (Fig. 4C).| Precision level | Conc. (ng per spot) | % recoverya ± RSD |
|---|---|---|
| a The value is the mean of three determinations. | ||
| Intra-day precision | 300 | 88.44 ± 2.46 |
| 400 | 90.23 ± 0.98 | |
| 500 | 90.83 ± 4.00 | |
| 600 | 94.32 ± 0.62 | |
| 700 | 99.41 ± 4.68 | |
| Inter-day precision | 300 | 89.75 ± 1.32 |
| 400 | 89.70 ± 0.52 | |
| 500 | 92.67 ± 3.43 | |
| 600 | 94.67 ± 0.20 | |
| 700 | 101.69 ± 3.56 | |
3.5 Forced degradation studies of SMV
Hydrolytic, oxidative and photo forced degradation were studied. In all cases, an appreciable SMV degradation was observed. Hydrolytic degradation was performed in the dark to cancel any photo degradation. The percentage of degradation of SMV was calculated according to the following equation:where; Auu is the area under the peak of untreated stock drug solution and AuD is the area under the peak of the drug in the degraded sample.
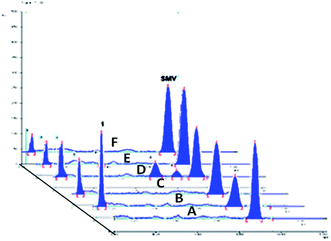
The drug contains several amide bonds and therefore, the drug is very highly liable for hydrolytic conditions. About 71.2% degradation of SMV was observed after its exposure to 1.0 M HCl at 60 °C for 2 h. In the case of alkaline degradation, the percentage of degradation was 39.8% using 1.0 M NaOH at 60 °C for 2 h. In both case, only one degradation product was observed at RF = 0.03 which indicates the high polarity of the degradation product. Oxidative degradation by using 30% v/v H2O2 at 60 °C for 2 hours induced 51.3% degradation. A previously reported study11 claimed that only one oxidation product was formed. However, in the present study, three degradation products were observed on the chromatogram of the sample subjected to oxidative degradation. The same peak of the hydrolytic degradation was also observed in addition to two products appeared at RF of 0.47 and 0.57. Although the extent of photochemical and neutral hydrolytic degradation are lower than the acidic hydrolytic, alkaline hydrolytic and oxidative routs, it still high. The percentages of degradation were 26.6 and 19.3% for photochemical degradation (UV irradiation at 254 nm for 48 h) and neutral hydrolysis (at 80 °C for 6 h), respectively. Again the peak at RF = 0.03 was observed in both case with a minor peak at 0.29 for photochemical degradation. Typical densitograms obtained for SMV under different stress conditions are shown in (Fig. 5) and a summery for the results are presented in (Table 7). Based on this study, SMV should be protected from moisture, light and elevated temperature due to the high liability of the drug to all the studied stress degradation condition. Possible pathways for the degradation of SMV are presented in (Fig. 6).10
| Degradation type | Condition | Number of degradation products (RF) | % degradation |
|---|---|---|---|
| Acidic | 1.0 M HCl at 60 °C for 2 h | 1 (0.03) | 71.2% |
| Basic | 1.0 M NaOH 60 °C for 2 h | 1 (0.04) | 39.8% |
| Oxidative | 30% v/v H2O2 at 60 °C for 2 h | 3 (0.03, 0.47, 0.57) | 51.3% |
| UV light | UV irradiation at 254 nm for 48 h | 2 (0.03, 0.29) | 26.6% |
| Neutral | At 90 °C for 6 hours | 1 (0.03) | 19.3% |
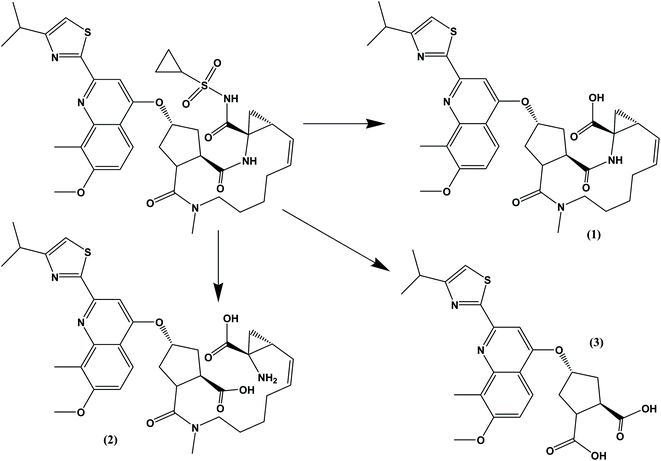 | ||
| Fig. 6 Possible pathways for the hydrolytic and oxidative degradations of SMV. Compound (1) was previously suggested as the oxidative product. | ||
4. Conclusion
The developed HPTLC technique is specific, direct, fast and sensitive for determination of simeprevir in pharmaceutical formulations and spiked human plasma. The planned methodology exhibited smart advantage, such as the ability of direct sample application with none any derivatization, short analysis time, batch analysis and minimal solvent usage. Moreover, it does not involve the extensive cleanup or sample pretreatment which is routinely used in the liquid chromatographic methods. The proposed method clearly showed stability-indicating potential being able to satisfactorily quantify SMV simultaneously with its degradation products.Conflicts of interest
There are no conflicts to declare.References
- G. M. Lauer and B. D. Walker, N. Engl. J. Med., 2001, 345, 41–52 CrossRef CAS.
- M. W. Fried, M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Gonçales Jr, D. Häussinger, M. Diago, G. Carosi and D. Dhumeaux, N. Engl. J. Med., 2002, 347, 975–982 CrossRef CAS PubMed.
- M. P. Manns, J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M.-H. Ling and J. K. Albrecht, Lancet, 2001, 358, 958–965 CrossRef CAS.
- S. Ouwerkerk-Mahadevan, M. Beumont-Mauviel, S. Mortier, M. Peeters, R. Verloes, C. Truyers, G. Mannens, I. Wynant and A. Simion, Drugs R&D, 2015, 15, 261–270 CrossRef CAS PubMed.
- PubChem Database, Simeprevir, 2019 Search PubMed.
- A. Ariaudo, F. Favata, A. De Nicolò, M. Simiele, L. Paglietti, L. Boglione, C. S. Cardellino, C. Carcieri, G. Di Perri and A. D'avolio, J. Pharm. Biomed. Anal., 2016, 125, 369–375 CrossRef CAS.
- I. Vanwelkenhuysen, R. De Vries, P. Timmerman and T. Verhaeghe, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 958, 43–47 CrossRef CAS.
- G. Nannetti, S. Pagni, S. G. Parisi, A. Alberti, A. Loregian and G. Palù, J. Pharm. Biomed. Anal., 2016, 121, 197–203 CrossRef CAS PubMed.
- K. A. Attia, N. M. El-Abasawi, A. El-Olemy and A. Serag, Anal. Chem. Lett., 2017, 7, 43–51 CrossRef CAS.
- F. M. Salama, K. A. Attia, A. A. Abouserie, A. El-Olemy and E. Abolmagd, International Journal of Science, 2018, 5, 16–21 Search PubMed.
- K. A. Attia, N. M. El-Abasawi, A. El-Olemy and A. Serag, Spectrochim. Acta, Part A, 2018, 190, 1–9 CrossRef CAS PubMed.
- S. H. Mohamed, Y. M. Issa and A. I. Salim, Spectrochim. Acta, Part A, 2019, 210, 290–297 CrossRef CAS PubMed.
- B. Fried and J. Sherma, Thin-layer chromatography: techniques and applications, 1982 Search PubMed.
- A.-M. I. Mohamed, M. A. Omar, S. M. Derayea, M. A. Hammad and A. A. Mohamed, Talanta, 2018, 176, 318–328 CrossRef CAS PubMed.
- I. H. T. Guideline, 2005.
- C. Nehls, M. Buonarati, S. Cape, R. Islam, C. Satterwhite, C. Briscoe, R. Hayes, A. Dinan, K. Sales and S. Anderson, Bioanalysis, 2019, 11, 1–228 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |