Open Access Article
Open Access ArticleAn umpolung reaction of α-iminothioesters possessing a cyclopropyl group†
Makoto Shimizu *ab,
Takayoshi Morimotob,
Yusuke Yanagib,
Isao Mizotab and
Yusong Zhu
*ab,
Takayoshi Morimotob,
Yusuke Yanagib,
Isao Mizotab and
Yusong Zhu a
a
aSchool of Energy Science and Engineering, Nanjing Tech University, Nanjing 211816, Jiangsu Province, China
bDepartment of Chemistry for Materials, Graduate School of Engineering, Mie University, Tsu, Mie 514-8507, Japan. E-mail: mshimizu@chem.mie-u.ac.jp
First published on 10th March 2020
Abstract
An umpolung N-alkylation reaction of α-cyclopropyl α-iminothioesters with diethylaluminum chloride or ethylmagnesium bromide affords the corresponding N-ethylated α-aminothioesters in good yields. Subsequent oxidation and reaction of the N-ethylated product with a thiolate or a chloride anion proceed effectively to give the ring-opened products in good yields. In contrast, relatively “hard” nucleophiles did not give the ring-opened products but gave the addition products to the iminium carbon.
1 Introduction
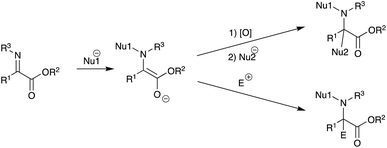
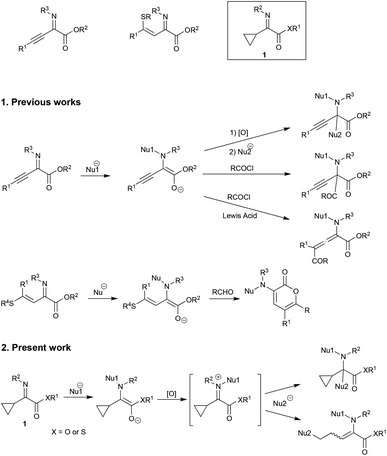
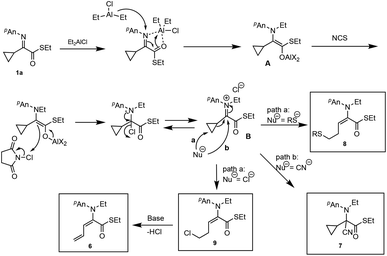
The unique properties of cyclopropyl moieties offer various useful reactions in many organic transformations.1 Among them, cyclopropane ring-opening reactions have received considerable attention as a useful carbon chain elongation reaction, since strain-release ring opening of cyclopropanes gives rise to highly functionalized products.2 On the other hand, we have been interested in the umpolung reactivity of α-iminoesters and have already disclosed several useful reactions (Scheme 1).3 Furthermore, upon the oxidation of an intermediary α-aminoester enolate derived from N-alkylation of α-iminoester, many nucleophiles can add to the resulting iminium salt, giving N,C-double addition products. When α,β-unsaturated α-iminoesters such as α-alkynyl or α-alkenyl derivatives were used as substrates, tandem N-alkylation–γ-acylation or vinylogous aldol reaction respectively proceeded in a regioselective manner.4 Along this line α-cyclopropyl α-imino(thio)esters 1 have intrigued us in particular regarding the cyclopropane ring-opening reaction of the α-cyclopropyl iminium ion intermediate (Scheme 2).We have now found that α-cyclopropyl α-iminothioesters 1 are reasonably reactive substrates for the umpolung N-alkylation and that the subsequent tandem reaction also proceeds well.
2 Results and discussion
The starting α-cyclopropyl α-iminoesters and their thioester counterparts 1 were readily prepared according to the reported procedures.5 First, the conditions for the ethylation reaction of α-cyclopropyl α-iminoester 1b and its thioester counterpart 1a, including the ethylation reagent, solvent, time, and temperature were examined. Table 1 summarizes the results.| Entry | X | Reagent | Solv. | Temp. (°C) | Time (min) | 2: yielda (%) |
|---|---|---|---|---|---|---|
| a Isolated yield.b Et2AlCl (1.5 equiv.) was used. | ||||||
| 1 | O | Et2AlCl | EtCN | −20 to rt | 240 | 53 |
| 2 | O | Et2AlCl | EtCN | −50 to rt | 120 | 41 |
| 3 | S | EtMgBr | EtCN | −50 to rt | 60 | 74 |
| 4 | S | Et2AlCl | EtCN | −50 to rt | 120 | 75 |
| 5 | S | Et2AlCl | MeCN | −40 to rt | 120 | 75 |
| 6 | S | Et2AlCl | CH2Cl2 | −50 to rt | 120 | 61 |
| 7 | S | Et2AlClb | EtCN | −50 to rt | 120 | 68 |
| 8 | S | Et2AlCl | EtCN | −50 to rt | 30 | 90 |
| 9 | S | Et2AlCl | EtCN | −50 to rt | 10 | 86 |
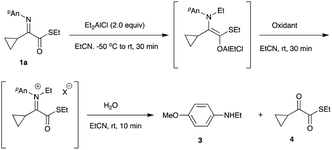
Although ethyl Grignard reagent could be used for this reaction, diethylalminum chloride recorded comparable results (entries 3 to 5). Regarding the ester moiety, normal ethyl ester gave a decreased yield of the desired N-ethylation product 2b as compared with the thioester counterpart 2a (entries 2 and 4).6 The best yield was obtained when the reaction was conducted in EtCN for 30 min, and the product 2a was formed in 90% yield (entry 8). We next examined the oxidation reaction of an intermediary aluminum enolate species to the iminium salt that could accept the second nucleophile. Table 2 summarizes the results.
| Entry | Oxidation reagenta | Equivalent | 3: yieldb (%) |
|---|---|---|---|
| a Abbreviations, BPO: benzoyl peroxide, NBS: N-bromosuccimide, NCS: N-chlorosuccinimide, DBDMH: 1,3-dibromo-5,5-dimethylhydantoin.b Isolated yield. | |||
| 1 | BPO | 1.1 | 40 |
| 2 | BPO | 1.5 | 34 |
| 3 | BPO | 2.0 | 13 |
| 4 | NBS | 1.1 | 27 |
| 5 | NBS | 2.0 | 26 |
| 6 | NCS | 1.1 | 63 |
| 7 | NCS | 2.0 | 69 |
| 8 | DBDMH | 1.1 | 27 |
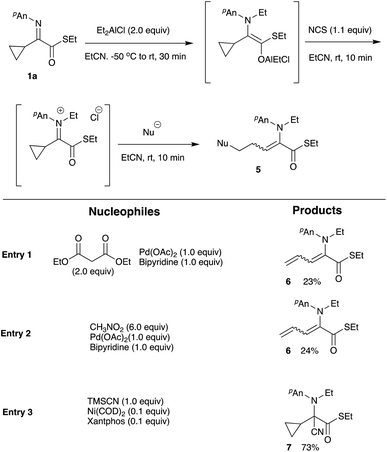
Initially, we attempted to determine the amount of the hydrolyzed cyclopropyl ketoester 4. However, this compound was transformed into other derivatives including a cyclopropane ring-opened product, a hydrate, a hydrolyzed acid, and so on under the present oxidation/hydrolysis conditions, and therefore, the yields of stable N-ethyl anisidine 3 are shown in Table 2. BPO that was effective for the tandem reaction of α-aryl α-iminoestrers was unsuitable for the present substrate, giving N-ethyl anisidine 3 in moderate yields (entries 1 to 3). NBS and DBDMH did not efficiently oxidize the present aluminum enolate either (entries 4, 5, and 8), whereas with NCS N-ethyl anisidine 3 was obtained in good yield, indicating that NCS is the oxidation reagent of choice for the present substrate (entries 6 and 7). Using NCS as an oxidation reagent for the aluminum enolate, various nucleophiles known as good reagents for conjugate addition reactions were subjected to the present tandem cyclopropane ring-opening reaction, and Scheme 3 summarizes the results.
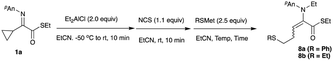
Diethyl malonate/NaH, KH or DBU, ethyl acetoacetate/NaH, KH or DBU, acetylacetone/NaH, KH or DBU, MeNO2/DBU, EtNO2/DBU, allyltrimethylsilane/TiCl4, silyl enol ether/TiCl4, ketene silyl acetal/TiCl4, indole, Bn2NLi, Et2AlCN, EtMgBr/CuI, EtMgBr/CuCN, Me2CuLi, nBu2CuLi/BF3·Et2O, and Et2CuLi/TMSCl did not give the desired addition/ring-opening product 5, whereas trimethylsilyl cyanide underwent a 1,2-addition reaction to the iminium salt to give the nitrile 7 in 73% yield (entry 3). Noteworthy is the reaction in the presence of Pd(OAc)2 and bipyridine, giving the ring-opened diene 6 in 23 and 24% yields, respectively (entries 1 and 2). This kind of diene formation is further examined, and details of this reaction are reported in the latter part of this article. One of the best nucleophiles for conjugate addition is thiolate,7 and therefore the use of thiolates was next examined. Table 3 summarizes the results.
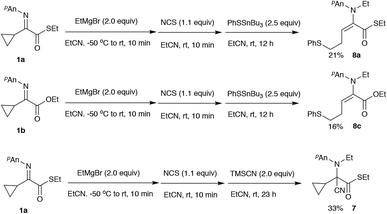
The initial ring-opening reaction was carried out with lithium benzenethiolate, and the reaction actually gave the desired ring-opened product 8a in 23% yield, indicating that thiolates could be used for the present ring-opening reaction (entry 1). Among the metal thiolates examined, sodium benzenethiolate effected the ring-opening to give the homoallylic sulfide 8a in 38% yield (entry 2). Although the reaction with phenythiotrimethylsilane8 for 2 h gave the homoallylic sulfide 8a in low yield, prolonged reaction times increased the yield to 57% (entries 3 to 5). A better result was obtained when the reaction was carried out with phenylthiotri-butylstannane9 for 12 h, and the desired product 8a was obtained in 60% yield (entry 6). Furthermore, the use of ethylthiotri-butylstannane9 recorded the best yield of 79% (entry 7).
We also examined the use of α-cyclopropyl α-iminoesters 1b for this type of ring-opening reaction, and Scheme 4 summarizes the representative results.
Although the yields of the desired ring-opened product 8c were only moderate, the use of BPO together with TMSSPh was found to be crucial for this substrate. A combined use of Et2AlCl and EtAlCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which was used for the prototype tandem reaction of α-phenyl α-iminoesters also resulted in a moderate success.3b
1), which was used for the prototype tandem reaction of α-phenyl α-iminoesters also resulted in a moderate success.3b
In terms of the availability of various alkyl and aryl derivatives, the use of Grignard reagents as the first nucleophile was next examined, and Scheme 5 summarizes the results.
Although the first N-alkylation proceeded relatively well (see, Table 1, entry 3), the subsequent oxidation/second nucleophilic addition did not give the desired products in good yields. Therefore, aluminum reagents appear to be the reagent of choice for the present tandem reaction.
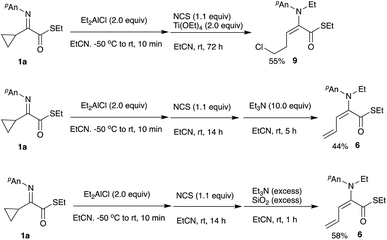
As mentioned earlier (Scheme 3, entries 1 and 2), an intriguing ring-opening reaction was observed after the oxidation of the intermediary aluminum enolate with NCS. Scheme 6 summarizes the results of ring opening–chlorination and/or diene formation.
After oxidation of the aluminum enolate with NCS the reaction mixture was allowed to stand with Ti(OEt)4 at room temperature for 72 h to give the ring-opened/chlorinated product 9 in 55% yield, indicating that the chloride anion formed during the oxidation could attack the cyclopropane ring to open it. Regarding the diene formation, we screened several conditions and found that in the presence of triethylamine or triethylamine/silica gel the elimination of hydrogen chloride proceeded to give the diene 6 in 44% or 58%, respectively.
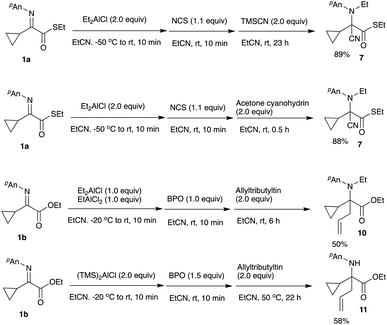
Moreover, we observed that the use of relatively “hard” nucleophiles such as metal cyanide led to the C-addition reaction of the iminium carbon instead of the ring-opening reaction (for example see, Scheme 3, entry 3 and Scheme 5). Other representative results of this type of 1,2-addition reaction are shown in Scheme 7.
As shown in Scheme 7 this iminium salt is a relatively reactive species for the nucleophiles. Both TMSCN and acetone cyanohydrin underwent a cyanation reaction to give the aminonitrile 7 in 89% and 88%, respectively. Regarding the α-cyclopropyl α-iminoester 1b, N-ethylation/C-allylation reaction proceeded to give the double addition product 10 in 50% yield. When (TMS)2AlCl10 was used in place of ethylation reagents, the C-allylation product 11 was obtained in 58% yield after removal of the TMS moiety with aqueous KF. The following Scheme 8 shows the possible reaction pathways.
First, ethylation at the imino nitrogen gives the aluminum enolate A, which in turn is oxidized with NCS to give the iminium salt B. This iminium salt B undergoes a ring-opening sulfenylation or chlorination reaction to give the homoallylic sulfide 8 or chloride 9, respectively. Treatment of the homoallylic chloride 9 with a certain base induces the elimination of HCl to give the diene 6.
3 Conclusions
In conclusion, α-cyclopropyl α-iminothioesters readily underwent the N-alkylation reaction with alkylaluminum reagents, giving the corresponding N-alkylated products in good yields. Subsequent oxidation/ring-opening of the N-alkylated products was conducted with NCS/nBu3SnSR to produce the homoallylic sulfides in moderate to good yields. Since homoallylic sulfides are useful compounds for further functional group interconversions including a selective C–C bond formation,11 the present procedure offers a useful addition to the existing methodologies. We also found an intriguing homoallylic chloride formation induced by the formed chloride anion during the oxidation of the intermediate aluminum enolate with NCS. Tandem N,C-addition was also found using relatively “hard” second nucleophiles.4 Experimental
General aspects
Infrared spectra were determined on a JASCO FT/IR-460 plus spectrometer. 1H NMR and 13C NMR spectra were recorded with a JEOL ECX-400P, or a JEOL A-500 spectrometer using tetramethylsilane as an internal standard. Mass spectra were recorded on a JEOL MS-700D spectrometer. Acetonitrile (MeCN) and propionitrile (EtCN) were distilled from phosphorus pentaoxide and then from calcium hydride, and stored over Molecular Sieves 4A. Diethylether (Et2O) was purified with a Glass Contour Organic Solvent Purification System of Nikko Hansen & Co., Ltd. Dichloromethane (CH2Cl2) was distilled from calcium hydride and stored over molecular sieves 4Å. S-Ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a3e,5 and ethyl 2-cyclopropyl[(4-methoxyphenyl)imino]acetate 1b3b were synthesized by the reported procedures. Purification of products was performed by column chromatography on silica gel (Kanto Silica Gel 60N) and/or preparative TLC on silica gel (Merck Kiesel Gel GF254 or Wako Gel B-5F).Synthesis of S-ethyl 2-cyclopropyl-2-[ethyl(4-methoxyphenyl)amino]ethanethioate 2a
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added Et2AlCl (0.27 mL, 0.286 mmol, 1.05 M in nhexane). After the mixture was stirred for 30 min at room temperature, the reaction was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 2a.
1) to give the title compound 2a.
Yield 90% (39.6 mg); yellow oil; Rf = 0.6 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.22–0.29 (m 1H), 0.45–0.57 (m 2H), 0.69–0.76 (m 1H), 1.14–1.25 (m 1H), 1.17 (dd, J = 7.1, 7.1 Hz, 3H), 1.21 (dd, J = 7.3, 7.3 Hz, 3H), 2.75–2.88 (m 2H), 3.22 (d, J = 9.6 Hz, 1H), 3.32–3.51 (m 2H), 3.75 (s, 3H), 6.80–6.81 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 4.2, 5.2, 12.1, 14.6, 23.0, 42.8, 55.5, 114.4, 118.2, 142.1, 153.1, 203.4; IR (neat) 2970, 2931, 2833, 1684, 1511, 1244, 1040, 830, 814, 728, 514 cm−1; HRMS (EI) calcd for C16H23NO2S (M)+ 293.1450, found 293.1459.
1); 1H NMR (400 MHz, CDCl3) δ 0.22–0.29 (m 1H), 0.45–0.57 (m 2H), 0.69–0.76 (m 1H), 1.14–1.25 (m 1H), 1.17 (dd, J = 7.1, 7.1 Hz, 3H), 1.21 (dd, J = 7.3, 7.3 Hz, 3H), 2.75–2.88 (m 2H), 3.22 (d, J = 9.6 Hz, 1H), 3.32–3.51 (m 2H), 3.75 (s, 3H), 6.80–6.81 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 4.2, 5.2, 12.1, 14.6, 23.0, 42.8, 55.5, 114.4, 118.2, 142.1, 153.1, 203.4; IR (neat) 2970, 2931, 2833, 1684, 1511, 1244, 1040, 830, 814, 728, 514 cm−1; HRMS (EI) calcd for C16H23NO2S (M)+ 293.1450, found 293.1459.
Synthesis of ethyl 2-cyclopropyl-2-[ethyl(4-methoxyphenyl)amino]acetate 2b
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed ethyl 2-cyclopropyl[(4-methoxyphenyl)imino]acetate 1b (37.1 mg, 0.150 mmol) in propionitrile (1.5 mL) at −20 °C. To it was added Et2AlCl (0.15 mL, 0.165 mmol, 1.07 M in nhexane). After the mixture was stirred for 4 h at room temperature, the reaction was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 2b.
1) to give the title compound 2b.
Yield 53% (22.0 mg); yellow oil; Rf = 0.5 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.32–0.38 (m, 1H), 0.44–0.50 (m, 1H), 0.58–0.71 (m, 2H), 1.14 (dd, 3H, J = 7.1, 7.1 Hz), 1.21 (t, 3H, J = 7.1 Hz), 1.23–1.30 (m, 1H), 3.21 (d, J = 9.6 Hz, 1H), 3.38–3.57 (m, 2H), 3.74 (s, 3H), 4.13 (q, 2H, J = 7.1 Hz), 6.76–6.81 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 4.6, 4.8, 12.4, 14.1, 14.2, 42.6, 55.6, 60.4, 68.9, 114.4, 117.6, 142.7, 152.8, 173.1; IR (neat) 2979, 1732, 1512, 1464, 1370, 1244, 1180, 1038, 815, 535 cm−1; HRMS (EI) calcd for C16H23NO2S (M)+ 293.1449, found 293.1450.
1); 1H NMR (400 MHz, CDCl3) δ 0.32–0.38 (m, 1H), 0.44–0.50 (m, 1H), 0.58–0.71 (m, 2H), 1.14 (dd, 3H, J = 7.1, 7.1 Hz), 1.21 (t, 3H, J = 7.1 Hz), 1.23–1.30 (m, 1H), 3.21 (d, J = 9.6 Hz, 1H), 3.38–3.57 (m, 2H), 3.74 (s, 3H), 4.13 (q, 2H, J = 7.1 Hz), 6.76–6.81 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 4.6, 4.8, 12.4, 14.1, 14.2, 42.6, 55.6, 60.4, 68.9, 114.4, 117.6, 142.7, 152.8, 173.1; IR (neat) 2979, 1732, 1512, 1464, 1370, 1244, 1180, 1038, 815, 535 cm−1; HRMS (EI) calcd for C16H23NO2S (M)+ 293.1449, found 293.1450.
Oxidation of the intermediary aluminum enolate to produce N-ethylanisidine 3
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added Et2AlCl (0.27 mL, 0.286 mmol, 1.05 M in nhexane). After the mixture was stirred for 30 min at room temperature, a solution of NCS (39.9 mg, 0.30 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 30 min at room temperature and then quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give N-ethylanisidine 3.
1) to give N-ethylanisidine 3.
Yield 69% (15.6 mg); yellow oil; Rf = 0.3 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.24 (t, J = 7.3 Hz, 3H), 3.11 (q, J = 7.3 Hz, 2H), 3.74 (s, 3H), 6.59 (d, J = 8.9 Hz, 2H), 6.78 (d, J = 8.9 Hz, 2H), The N–H proton could not be detected; 13C NMR (100 MHz, CDCl3) δ 15.0, 39.5, 55.8, 114.1, 114.9, 142.7, 152.1; IR (neat) 2880, 1530, 1260, 1050, 830 cm−1; HRMS (EI) calcd for C9H13NO (M+) 151.0997, found: 151.0981.
1); 1H NMR (400 MHz, CDCl3) δ 1.24 (t, J = 7.3 Hz, 3H), 3.11 (q, J = 7.3 Hz, 2H), 3.74 (s, 3H), 6.59 (d, J = 8.9 Hz, 2H), 6.78 (d, J = 8.9 Hz, 2H), The N–H proton could not be detected; 13C NMR (100 MHz, CDCl3) δ 15.0, 39.5, 55.8, 114.1, 114.9, 142.7, 152.1; IR (neat) 2880, 1530, 1260, 1050, 830 cm−1; HRMS (EI) calcd for C9H13NO (M+) 151.0997, found: 151.0981.
Synthesis of S-ethyl (E)-2-(ethyl(4-methoxyphenyl)amino)penta-2,4-dienethioate 6
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (26.8 mg, 0.10 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added Et2AlCl (0.19 mL, 0.20 mmol, 1.05 M in nhexane). After the mixture was stirred for 10 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 14 h at room temperature and then quenched with sat. aq. NaHCO3 (10 mL). The whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 10% Et3N) to give the title compound 9.
1 + 10% Et3N) to give the title compound 9.
Yield 58% (25.3 mg); reddish oil; Rf = 0.45 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.21 (t, J = 7.3 Hz, 3H), 1.24 (t, J = 7.0 Hz, 3H), 2.88 (q, J = 7.3 Hz, 2H), 3.53 (q, J = 7.0 Hz, 2H), 3.74 (s, 3H), 5.43 (dd, J = 10.0, 1.0 Hz, 1H), 5.64 (dd, J = 17.2, 1.0 Hz, 1H), 6.35 (ddd, J = 17.0, 11.0, 10.0 Hz, 1H), 6.63–6.67 (m, 2H), 6.78–6.82 (m, 2H) 7.13 (d, J = 11.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 13.0, 14.4, 23.4, 46.0, 55.6, 114.2, 114.7, 126.6, 131.5, 133.8, 140.1, 141.2, 152.2, 194.4; HRMS (EI) calcd for C16H21NO2S (M)+ 291.1293, found 291.1299.
1); 1H NMR (400 MHz, CDCl3) δ 1.21 (t, J = 7.3 Hz, 3H), 1.24 (t, J = 7.0 Hz, 3H), 2.88 (q, J = 7.3 Hz, 2H), 3.53 (q, J = 7.0 Hz, 2H), 3.74 (s, 3H), 5.43 (dd, J = 10.0, 1.0 Hz, 1H), 5.64 (dd, J = 17.2, 1.0 Hz, 1H), 6.35 (ddd, J = 17.0, 11.0, 10.0 Hz, 1H), 6.63–6.67 (m, 2H), 6.78–6.82 (m, 2H) 7.13 (d, J = 11.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 13.0, 14.4, 23.4, 46.0, 55.6, 114.2, 114.7, 126.6, 131.5, 133.8, 140.1, 141.2, 152.2, 194.4; HRMS (EI) calcd for C16H21NO2S (M)+ 291.1293, found 291.1299.
Synthesis of S-ethyl 2-cyano-2-cyclopropyl-2-[ethyl(4-methoxyphenyl)amino]ethanethioate 7
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added Et2AlCl (0.27 mL, 0.286 mmol, 1.05 M in nhexane). After the mixture was stirred for 10 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 10 min at room temperature, and TMSCN (0.04 mL 0.30 mmol) was added. After the mixture was stirred for 23 h at room temperature, it was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 7.
1) to give the title compound 7.
Yield 89% (42.5 mg); yellow oil; Rf = 0.5 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.06–0.13 (m, 1H), 0.26–0.33 (m, 1H), 0.39–0.46 (m, 1H), 0.52–0.59 (m, 1H), 0.94 (t, J = 7.1 Hz, 3H), 1.04–1.11 (m, 1H), 1.28 (t, J = 7.6 Hz, 3H), 2.77–2.98 (m, 3H), 3.19–3.27 (m, 1H), 3.80 (s, 3H), 6.83–6.87 (m, 2H), 7.24–7.28 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 1.7, 5.6, 13.0, 14.4, 18.7, 23.5, 48.3, 55.4, 79.42, 113.9, 129.8, 136.5, 158.4, 198.8; IR (neat) 2973, 2364, 2332, 1690, 1510, 1244, 1167, 1082, 1038, 879, 835, 547, 502 cm−1; HRMS (EI) calcd for C17H22N2O2S (M)+ 318.1402, found 318.1402.
1); 1H NMR (400 MHz, CDCl3) δ 0.06–0.13 (m, 1H), 0.26–0.33 (m, 1H), 0.39–0.46 (m, 1H), 0.52–0.59 (m, 1H), 0.94 (t, J = 7.1 Hz, 3H), 1.04–1.11 (m, 1H), 1.28 (t, J = 7.6 Hz, 3H), 2.77–2.98 (m, 3H), 3.19–3.27 (m, 1H), 3.80 (s, 3H), 6.83–6.87 (m, 2H), 7.24–7.28 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 1.7, 5.6, 13.0, 14.4, 18.7, 23.5, 48.3, 55.4, 79.42, 113.9, 129.8, 136.5, 158.4, 198.8; IR (neat) 2973, 2364, 2332, 1690, 1510, 1244, 1167, 1082, 1038, 879, 835, 547, 502 cm−1; HRMS (EI) calcd for C17H22N2O2S (M)+ 318.1402, found 318.1402.
Synthesis of S-ethyl (E)-2-[ethyl(4-methoxyphenyl)amino]-5-(phenylthio)pent-2-enethioate 8a
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added Et2AlCl (0.27 mL, 0.286 mmol, 1.05 M in nhexane). After the mixture was stirred for 10 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 10 min at room temperature, and a solution of nBu3SnSPh (119.7 mg, 0.30 mmol) in propionitrile (1.0 mL) was added. After the mixture was stirred for 64 h at room temperature, it was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 8a.
1) to give the title compound 8a.
Yield 60% (36.3 mg); yellow oil; Rf = 0.4 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.16 (t, J = 7.1 Hz, 3H), 1.21 (t, J = 7.5 Hz, 3H), 2.32 (dt, J = 7.3, 7.3 Hz, 2H), 2.83 (q, J = 7.5 Hz, 2H), 2.91 (t, J = 7.3 Hz, 2H), 3.41 (q, J = 7.1 Hz, 2H), 3.75 (s, 3H), 6.59–6.63 (m, 2H), 6.74–6.81 (m, 3H), 7.13–7.24 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 12.9, 14.3, 23.4, 27.9, 31.8, 45.5, 55.6, 114.2, 114.7, 126.1, 128.9, 129.4, 134.6, 135.5, 140.8, 142.1, 152.2, 193.9; IR (neat) 2932, 1715, 1649, 1510, 1247, 1029, 834, 740, 692, 530, 516 cm−1; HRMS (EI) calcd for C22H27NO2S (M)+ 401.1483, found 401.1502.
1); 1H NMR (400 MHz, CDCl3) δ 1.16 (t, J = 7.1 Hz, 3H), 1.21 (t, J = 7.5 Hz, 3H), 2.32 (dt, J = 7.3, 7.3 Hz, 2H), 2.83 (q, J = 7.5 Hz, 2H), 2.91 (t, J = 7.3 Hz, 2H), 3.41 (q, J = 7.1 Hz, 2H), 3.75 (s, 3H), 6.59–6.63 (m, 2H), 6.74–6.81 (m, 3H), 7.13–7.24 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 12.9, 14.3, 23.4, 27.9, 31.8, 45.5, 55.6, 114.2, 114.7, 126.1, 128.9, 129.4, 134.6, 135.5, 140.8, 142.1, 152.2, 193.9; IR (neat) 2932, 1715, 1649, 1510, 1247, 1029, 834, 740, 692, 530, 516 cm−1; HRMS (EI) calcd for C22H27NO2S (M)+ 401.1483, found 401.1502.
Synthesis of S-ethyl (E)-2-[ethyl(4-methoxyphenyl)amino]-5-(ethylthio)pent-2-enethioate 8b
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added Et2AlCl (0.27 mL, 0.286 mmol, 1.05 M in nhexane). After the mixture was stirred for 10 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 10 min at room temperature, and a solution of nBu3SnSEt (131.7 mg, 0.38 mmol) in propionitrile (1.0 mL) was added. After the mixture was stirred for 13 h at room temperature, it was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 8b.
1) to give the title compound 8b.
Yield 79% (41.7 mg); yellow oil; Rf = 0.4 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.16 (t, J = 7.5 Hz, 3H), 1.20 (t, J = 7.3 Hz, 3H), 1.23 (t, J = 7.2 Hz, 3H), 2.27 (q, J = 7.3 Hz, 2H), 2.40 (dt, J = 7.5, 7.5 Hz, 2H), 2.53 (t, J = 7.5 Hz, 2H), 2.82 (q, J = 7.5 Hz, 2H), 3.48 (q, J = 7.2 Hz, 2H), 3.73 (s, 3H), 6.62–6.67 (m, 2H), 6.73 (t, J = 7.5 Hz, 1H), 6.77–6.81 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 11.7, 14.3, 14.7, 24.0, 26.5, 28.0, 31.4, 46.8, 55.5, 114.4, 118.6, 129.5, 142.1, 145.6, 153.5, 194.2; IR (neat) 2930, 1712, 1645, 1510, 1247, 1029, 833, 741, 691, 536 cm−1; HRMS (EI) calcd for C18H27NO2S2 (M)+ 353.1483, found 353.1479.
1); 1H NMR (400 MHz, CDCl3) δ 1.16 (t, J = 7.5 Hz, 3H), 1.20 (t, J = 7.3 Hz, 3H), 1.23 (t, J = 7.2 Hz, 3H), 2.27 (q, J = 7.3 Hz, 2H), 2.40 (dt, J = 7.5, 7.5 Hz, 2H), 2.53 (t, J = 7.5 Hz, 2H), 2.82 (q, J = 7.5 Hz, 2H), 3.48 (q, J = 7.2 Hz, 2H), 3.73 (s, 3H), 6.62–6.67 (m, 2H), 6.73 (t, J = 7.5 Hz, 1H), 6.77–6.81 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 11.7, 14.3, 14.7, 24.0, 26.5, 28.0, 31.4, 46.8, 55.5, 114.4, 118.6, 129.5, 142.1, 145.6, 153.5, 194.2; IR (neat) 2930, 1712, 1645, 1510, 1247, 1029, 833, 741, 691, 536 cm−1; HRMS (EI) calcd for C18H27NO2S2 (M)+ 353.1483, found 353.1479.
Synthesis of ethyl (E)-2-[ethyl(4-methoxyphenyl)amino]-5-(phenylthio)pent-2-enethioate 8c
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon were placed ethyl 2-cyclopropyl[(4-methoxyphenyl)imino]acetate 1b (37.1 mg, 0.150 mmol) and BPO (0.150 mmol, 36.3 mg) in propionitrile (5.0 mL) at −50 °C. To it was added EtAlCl2 (0.14 mL, 0.150 mmol, 1.07 M in nhexane), Et2AlCl (0.14 mL, 0.150 mmol, 1.07 M in nhexane), and TMSSPh (0.029 mL, 0.15 mmol). After the mixture was stirred for 22 h at room temperature, it was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 8c.
1) to give the title compound 8c.
Yield 53% (30.8 mg); yellow oil; Rf = 0.4 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.13 (t, 3H, J = 7.3 Hz), 1.17 (t, 3H, J = 7.1 Hz), 2.51 (q, 2H, J = 7.3 Hz), 2.96 (t, 2H, J = 7.3 Hz), 3.38 (dt, 2H, J = 7.1, 7.3 Hz), 3.73 (s, 3H), 4.12 (q, 2H, J = 7.1 Hz), 6.56–6.60 (m, 2H), 6.74–6.79 (m, 2H), 6.87 (t, 1H, J = 7.1 Hz), 7.13–7.18 (m, 1H), 7.21–7.29 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 13.2, 14.1, 27.8, 32.1, 45.3, 55.6, 60.8, 114.3, 114.6, 126.1, 128.9, 129.0, 129.3, 136.2, 139.0, 141.7, 152.0, 165.8; IR (neat) 2975, 2360, 1715, 1509, 1241, 1180, 1040, 818, 739, 690, 535, 510 cm−1; HRMS (EI) calcd for C22H27NO3S (M)+ 385.1712, found 385.1719.
1); 1H NMR (400 MHz, CDCl3) δ 1.13 (t, 3H, J = 7.3 Hz), 1.17 (t, 3H, J = 7.1 Hz), 2.51 (q, 2H, J = 7.3 Hz), 2.96 (t, 2H, J = 7.3 Hz), 3.38 (dt, 2H, J = 7.1, 7.3 Hz), 3.73 (s, 3H), 4.12 (q, 2H, J = 7.1 Hz), 6.56–6.60 (m, 2H), 6.74–6.79 (m, 2H), 6.87 (t, 1H, J = 7.1 Hz), 7.13–7.18 (m, 1H), 7.21–7.29 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 13.2, 14.1, 27.8, 32.1, 45.3, 55.6, 60.8, 114.3, 114.6, 126.1, 128.9, 129.0, 129.3, 136.2, 139.0, 141.7, 152.0, 165.8; IR (neat) 2975, 2360, 1715, 1509, 1241, 1180, 1040, 818, 739, 690, 535, 510 cm−1; HRMS (EI) calcd for C22H27NO3S (M)+ 385.1712, found 385.1719.
Synthesis of S-ethyl (E)-2-[ethyl(4-methoxyphenyl)amino]-5-(phenylthio)pent-2-enethioate 8a using ethyl Grignard reagent
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added EtMgBr (0.27 mL, 0.300 mmol, 1.10 M in THF). After the mixture was stirred for 10 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 10 min at room temperature, and a solution of nBu3SnSPh (119.7 mg, 0.30 mmol) in propionitrile (1.0 mL) was added. After the mixture was stirred for 12 h at room temperature, it was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 8a (12.7 mg, 21% yield).
1) to give the title compound 8a (12.7 mg, 21% yield).
Synthesis of ethyl (E)-2-[ethyl(4-methoxyphenyl)amino]-5-(phenylthio)pent-2-enethioate 8c using ethyl Grignard reagent
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed ethyl 2-cyclopropyl[(4-methoxyphenyl)imino]acetate 1b (37.1 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added EtMgBr (0.27 mL, 0.300 mmol, 1.10 M in THF). After the mixture was stirred for 10 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 10 min at room temperature, and a solution of nBu3SnSPh (119.7 mg, 0.30 mmol) in propionitrile (1.0 mL) was added. After the mixture was stirred for 12 h at room temperature, it was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 8c (9.3 mg, 16% yield).
1) to give the title compound 8c (9.3 mg, 16% yield).
Synthesis of S-ethyl 2-cyano-2-cyclopropyl-2-[ethyl(4-methoxyphenyl)amino]ethanethioate 7 using ethyl Grignard reagent
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added EtMgBr (0.27 mL, 0.300 mmol, 1.10 M in THF). After the mixture was stirred for 10 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) was added. The mixture was stirred for 10 min at room temperature, and TMSCN (0.04 mL 0.30 mmol) was added. After the mixture was stirred for 23 h at room temperature, it was quenched with sat. aq. NaHCO3 (10 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 7 (15.8 mg. 33% yield).
1) to give the title compound 7 (15.8 mg. 33% yield).
Synthesis of S-ethyl (E)-5-chloro-2-[ethyl(4-methoxyphenyl)amino]pent-2-enethioate 9
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed S-ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]ethanethioate 1a (39.5 mg, 0.150 mmol) in propionitrile (0.5 mL) at −50 °C. To it was added Et2AlCl (0.27 mL, 0.286 mmol, 1.05 M in nhexane). After the mixture was stirred for 30 min at room temperature, a solution of NCS (22.0 mg, 0.15 mmol) in propionitrile (0.75 mL) and Ti(OEt)4 (0.063 mL 0.30 mmol) were added successively. The mixture was stirred for 72 h at room temperature and then quenched with sat. aq. NaHCO3 (10 mL). The whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 9.
1) to give the title compound 9.
Yield 55% (27.0 mg); yellow oil; Rf = 0.4 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.22 (t, J = 7.3 Hz, 3H), 1.24 (t, J = 7.1 Hz, 3H), 2.46 (dt, J = 6.9, 6.9 Hz, 2H), 2.84 (q, J = 7.3 Hz, 2H), 3.48 (q, J = 7.1 Hz, 2H), 3.52 (t, J = 6.9 Hz, 2H), 3.75 (s, 3H), 6.63–6.67 (m, 2H), 6.72 (t, J = 6.9 Hz, 1H), 6.78–6.82 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.0, 14.3, 23.8, 31.1, 42.4, 45.5, 55.6, 114.3, 114.8, 132.4, 140.6, 143.0, 152.3, 193.8; IR (neat) 2967, 2932, 2470, 1673, 1512, 1456, 1250, 1031, 835, 753, 560, 513 cm−1; HRMS (EI) calcd for C16H22ClNO2S (M)+ 327.1060, found 327.1056.
1); 1H NMR (400 MHz, CDCl3) δ 1.22 (t, J = 7.3 Hz, 3H), 1.24 (t, J = 7.1 Hz, 3H), 2.46 (dt, J = 6.9, 6.9 Hz, 2H), 2.84 (q, J = 7.3 Hz, 2H), 3.48 (q, J = 7.1 Hz, 2H), 3.52 (t, J = 6.9 Hz, 2H), 3.75 (s, 3H), 6.63–6.67 (m, 2H), 6.72 (t, J = 6.9 Hz, 1H), 6.78–6.82 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.0, 14.3, 23.8, 31.1, 42.4, 45.5, 55.6, 114.3, 114.8, 132.4, 140.6, 143.0, 152.3, 193.8; IR (neat) 2967, 2932, 2470, 1673, 1512, 1456, 1250, 1031, 835, 753, 560, 513 cm−1; HRMS (EI) calcd for C16H22ClNO2S (M)+ 327.1060, found 327.1056.
Ethyl 2-cyclopropyl-2-[ethyl(4-methoxyphenyl)amino]-4-pentenoate 10
Under an argon atmosphere, to a propionitrile (0.5 mL) solution of ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)imino]acetate 1b (41.6 mg, 0.150 mmol) and benzoyl peroxide (36.3 mg, 0.150 mmol) were added diethylaluminum chloride (0.158 mL, 0.150 mmol, 0.95 M in nhexane), ethylaluminum dichloride (0.167 mL, 0.150 mmol, 0.90 M in nhexane) and allyltributyltin (0.094 mL, 0.300 mmol) at −20 °C. The reaction mixture was allowed to warm to ambient temperature with stirring for 8 h. After the addition of 10% aq. Na2SO3 (5 mL) and subsequently sat. aq. KF (5 mL), the whole mixture was extracted with ethyl acetate (10 mL × 3). The combined extracts were washed with brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by preparative TLC on silica gel to give the title compound 10.Yield 50% (15.9 mg); yellow oil; Rf = 0.3 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.27–0.40 (m, 3H), 0.43–0.49 (m, 1H), 0.85 (t, 3H, J = 7.0 Hz), 1.07–1.13 (m, 1H), 1.31 (t, 3H, J = 7.3 Hz), 2.35 (dd, 1H, J = 7.9, 14.0 Hz), 2.43 (dd, 1H, J = 6.1, 14.0 Hz), 3.04 (dq, 1H, J = 7.0, 13.8 Hz), 3.20 (dq, 1H, J = 7.0, 13.8 Hz), 3.78 (s, 3H), 4.18 (q, 2H, J = 7.3 Hz), 5.02–5.05 (m, 2H), 5.91–5.99 (m, 1H), 6.76–6.79 (m, 2H), 7.14–7.17 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 2.8, 3.3, 14.4, 14.8, 17.1, 41.6, 45.0, 55.3, 60.3, 69.8, 113.1, 117.2, 130.4, 134.9, 140.0, 156.6, 174.1; IR (neat) 2950, 1735, 1515, 1220, 1045, 920, 840 cm−1; HRMS (EI) calcd for C19H27NO3 (M)+ 317.1991, found 317.1999.
1); 1H NMR (400 MHz, CDCl3) δ 0.27–0.40 (m, 3H), 0.43–0.49 (m, 1H), 0.85 (t, 3H, J = 7.0 Hz), 1.07–1.13 (m, 1H), 1.31 (t, 3H, J = 7.3 Hz), 2.35 (dd, 1H, J = 7.9, 14.0 Hz), 2.43 (dd, 1H, J = 6.1, 14.0 Hz), 3.04 (dq, 1H, J = 7.0, 13.8 Hz), 3.20 (dq, 1H, J = 7.0, 13.8 Hz), 3.78 (s, 3H), 4.18 (q, 2H, J = 7.3 Hz), 5.02–5.05 (m, 2H), 5.91–5.99 (m, 1H), 6.76–6.79 (m, 2H), 7.14–7.17 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 2.8, 3.3, 14.4, 14.8, 17.1, 41.6, 45.0, 55.3, 60.3, 69.8, 113.1, 117.2, 130.4, 134.9, 140.0, 156.6, 174.1; IR (neat) 2950, 1735, 1515, 1220, 1045, 920, 840 cm−1; HRMS (EI) calcd for C19H27NO3 (M)+ 317.1991, found 317.1999.
Ethyl 2-cyclopropyl-2-[(4-methoxyphenyl)amino]-4-pentenoate 11
Bis(trimethylsilyl)aluminum chloride was prepared by mixing aluminum chloride (13.4 mg, 0.100 mmol) and tris(trimethylsilyl)aluminum (0.385 mL, 0.200 mmol) from 0 °C to ambient temperature under an argon atmosphere. Under an argon atmosphere, to a propionitrile (0.5 mL) solution of ethyl 2-cycloprpyl-2-[(4-methoxyphenyl)imino]acetate 1b (39.5 mg, 0.150 mmol) and benzoyl peroxide (54.5 mg, 0.225 mmol) were added bis(trimethylsilyl)aluminum chloride (0.300 mmol) prepared as above and allyltributyltin (0.094 mL, 0.300 mmol) at −20 °C. The reaction mixture was allowed to warm to ambient temperature and heated at 50 °C with stirring for 23 h. After the addition of 10% aq. Na2SO3 (5 mL) and subsequently sat. aq. KF (5 mL), the whole mixture was extracted with ethyl acetate (10 mL × 3). The combined extracts were washed with brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by preparative TLC on silica gel to give the title compound 11.Yield 58% (25.2 mg); yellow oil; Rf = 0.25 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.36–0.60 (m, 4H), 1.20 (t, 3H, J = 7.3 Hz), 1.24–1.34 (m, 1H), 2.69 (dd, 1H, J = 7.3, 14.2 Hz), 2.82 (dd, 1H, J = 7.6, 14.0 Hz), 3.73 (s, 3H), 4.08 (br, 1H), 4.15 (q, 2H, J = 7.3 Hz), 5.00–5.07 (m, 2H), 5.65–5.80 (m, 1H), 6.64–6.74 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 2.9, 3.1, 14.2, 18.0, 40.2, 55.6, 61.2, 64.1, 114.2, 117.9, 118.6, 133.1, 139.4, 152.7, 174.2; IR (neat) 3380, 2980, 1740, 1520, 1245, 1190, 1050, 925, 830 cm−1; HRMS (EI) calcd for C17H23NO3 (M)+ 289.1678, found 289.1691.
1); 1H NMR (400 MHz, CDCl3) δ 0.36–0.60 (m, 4H), 1.20 (t, 3H, J = 7.3 Hz), 1.24–1.34 (m, 1H), 2.69 (dd, 1H, J = 7.3, 14.2 Hz), 2.82 (dd, 1H, J = 7.6, 14.0 Hz), 3.73 (s, 3H), 4.08 (br, 1H), 4.15 (q, 2H, J = 7.3 Hz), 5.00–5.07 (m, 2H), 5.65–5.80 (m, 1H), 6.64–6.74 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 2.9, 3.1, 14.2, 18.0, 40.2, 55.6, 61.2, 64.1, 114.2, 117.9, 118.6, 133.1, 139.4, 152.7, 174.2; IR (neat) 3380, 2980, 1740, 1520, 1245, 1190, 1050, 925, 830 cm−1; HRMS (EI) calcd for C17H23NO3 (M)+ 289.1678, found 289.1691.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B) and on Innovative Areas “Organic Synthesis Based on Reaction Integration. Development of New Methods and Creation of New Substances” from JSPS and MEXT.Notes and references
- For selected reviews, see: (a) L. A. Paquette, Chem. Rev., 1986, 86, 733–750 CrossRef CAS; (b) J. Salaun, Chem. Rev., 1989, 89, 1247–1270 CrossRef CAS; (c) H. N. C. Wong, M. Y. Hon, C. W. Tse, Y. C. Yip, J. Tanko and T. Hudlicky, Chem. Rev., 1989, 89, 165–198 CrossRef CAS; (d) O. G. Kulinkovich, Chem. Rev., 2003, 103, 2597–2632 CrossRef CAS PubMed; (e) H. Lebel, J.-F. Marcoux, C. Molinaro and A. B. Charette, Chem. Rev., 2003, 103, 977–1050 CrossRef CAS PubMed; (f) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117–3179 CrossRef CAS PubMed; (g) H. Pellissier, Tetrahedron, 2008, 64, 7041–7095 CrossRef CAS; (h) C. A. Carson and M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051–3060 RSC; (i) D. Y. K. Chen, R. H. Pouwer and J.-A. Richard, Chem. Soc. Rev., 2012, 41, 4631–4642 RSC; (j) P. Tang and Y. Qin, Synthesis, 2012, 44, 2969–2984 CrossRef CAS; (k) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804–818 RSC; (l) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655–671 RSC; (m) C. Ebner and E. M. Carreira, Chem. Rev., 2017, 117, 11651–11679 CrossRef CAS PubMed; (n) W. Wu, Z. Lin and H. Jiang, Org. Biomol. Chem., 2018, 16, 7315–7329 RSC; (o) A. J. Craig and B. C. Hawkins, Synthesis, 2020, 52, 27–39 CrossRef CAS.
- (a) E. P. Kohler and J. B. Conant, J. Am. Chem. Soc., 1917, 39, 1404–1420 CrossRef CAS; (b) G. Daviaud and P. Miginiac, Tetrahedron Lett., 1972, 13, 997–1000 CrossRef; (c) D. Tunemoto, N. Araki and K. Kondo, Tetrahedron Lett., 1977, 18, 109–112 CrossRef; (d) I. Böhm, R. Schulz and H.-U. Reissig, Tetrahedron Lett., 1982, 23, 2013–2016 CrossRef; (e) H. H. Wasserman and R. P. Dion, Tetrahedron Lett., 1982, 23, 1413–1416 CrossRef CAS; (f) S. Ogoshi, M. Nagata and H. Kurosawa, J. Am. Chem. Soc., 2006, 128, 5350–5351 CrossRef CAS PubMed; (g) L. Liu and J. Montgomery, J. Am. Chem. Soc., 2006, 128, 5348–5349 CrossRef CAS PubMed; (h) M. S. Anstey, C. M. Yung, J. Du and R. G. Bergman, J. Am. Chem. Soc., 2007, 129, 776–777 CrossRef CAS PubMed; (i) T. Ok, A. Jeon, J. Lee, J. H. Lim, C. S. Hong and H. S. Lee, J. Org. Chem., 2007, 72, 7390–7393 CrossRef CAS PubMed; (j) K. Wang, D. Xiang, J. Liu, W. Pan and D. Dong, Org. Lett., 2008, 10, 1691–1694 CrossRef CAS PubMed; (k) Y. Sumida, H. Yorimithu and K. Oshima, J. Org. Chem., 2009, 74, 3196–3198 CrossRef CAS PubMed; (l) Q. Li, G. Jing, L. Jiao and Z. Yu, Org. Lett., 2010, 12, 1332–1335 CrossRef CAS PubMed; (m) C. Sparr and R. Gilmour, Angew. Chem., Int. Ed., 2011, 50, 8391–8395 CrossRef CAS PubMed; (n) H. K. Grover, T. P. Lebold and M. A. Kerr, Org. Lett., 2011, 13, 220–223 CrossRef CAS PubMed; (o) W. Qi, P. Wang, L. Fan and S. Zhang, J. Org. Chem., 2013, 78, 5918–5924 CrossRef CAS PubMed; (p) M. Li, S. Lin, Z. Dong, X. Zhang, F. Liang and J. Zhang, Org. Lett., 2013, 15, 3978–3981 CrossRef CAS PubMed; (q) P. Kumar, R. Dey and P. Banerjee, Org. Lett., 2018, 20, 5163–5166 CrossRef CAS PubMed.
- For N-alkylation to α-iminoesters found in our laboratories, see: (a) Y. Niwa, K. Takayama and M. Shimizu, Bull. Chem. Soc. Jpn., 2002, 75, 1819–1825 CrossRef CAS; (b) Y. Niwa and M. Shimizu, J. Am. Chem. Soc., 2003, 125, 3720–3721 CrossRef CAS PubMed; (c) M. Shimizu, H. Itou and M. Miura, J. Am. Chem. Soc., 2005, 127, 3296–3297 CrossRef CAS PubMed; (d) I. Mizota and M. Shimizu, Chem. Rec., 2016, 16, 688–702 CrossRef CAS PubMed; (e) I. Mizota, C. Ueda, Y. Tesong, Y. Tsujimoto and M. Shimizu, Org. Lett., 2018, 20, 2291–2296 CrossRef CAS PubMed; (f) I. Mizota, Y. Tadano, Y. Nakamura, T. Haramiishi, M. Hotta and M. Shimizu, Org. Lett., 2019, 21, 2663–2667 CrossRef CAS PubMed; (g) M. Shimizu, H. Imazato, I. Mizota and Y. Zhu, RSC Adv., 2019, 9, 17341–17346 RSC; (h) M. Shimizu, M. Mushika, I. Mizota and Y. Zhu, RSC Adv., 2019, 9, 23400–23407 RSC; (i) M. Shimizu, Y. Furukawa, I. Mizota and Y. Zhu, New J. Chem., 2020, 44, 152–161 RSC.
- (a) I. Mizota, Y. Matsuda, S. Kamimura, H. Tanaka and M. Shimizu, Org. Lett., 2013, 15, 4206–4209 CrossRef CAS PubMed; (b) H. Tanaka, I. Mizota and M. Shimizu, Org. Lett., 2014, 16, 2276–2279 CrossRef CAS PubMed; (c) K. Nakahama, M. Suzuki, M. Ozako, I. Mizota and M. Shimizu, Asian J. Org. Chem., 2018, 7, 910–913 CrossRef CAS.
- I. Mizota, Y. Nakajima, A. Higashino and M. Shimizu, Arabian J. Sci. Eng., 2017, 42, 4249–4261 CrossRef CAS . Also see, ref. 3b and 3e.
- To clarify the exact origin of the reactivity of thioesters, computational study was carried out using the Gaussian 03 program. After the structural optimization, we found that the LUMO energy of the thioester was lower than that of the normal ester. We also found that the frontier electron density of the thioester was higher than that of the normal ester, leading to the increased reactivity of the nitrogen atom of the thioester. These computational analyses support the experimental results. Details of these studies have already been communicated.3e.
- P. Perlmutter, Conjugated Addition Reactions in Organic Synthesis, Pergamon, Oxford, 1992 Search PubMed.
- W. C. Groutas, L. B. Krasnova and A. K. Yudin, Phenylthiotrimethylsilane, in e-EROS Encyclopedia of Reagents for Organic Synthesis, 2008, DOI:10.1002/047084289x.rp132.pub2.
- C. G. Gutierrez and L. R. Summerhays, J. Org. Chem., 1984, 49, 5206–5213 CrossRef CAS.
- (a) L. Rçsch, G. Altnau and W. H. Otto, Angew. Chem., Int. Ed., 1981, 20, 581–582 CrossRef; (b) M. A. Avery, W. K. M. Chong and C. Jennings-White, J. Am. Chem. Soc., 1992, 114, 974–979 CrossRef CAS.
- (a) R. C. Hartley, I. C. Richards and S. Warren, J. Chem. Soc., Perkin Trans. 1, 1995, 359–376 Search PubMed; (b) A. N. Matukonis, J. D. Musselman and T. A. Tiley, Tetrahedron Lett., 2002, 43, 7031–7034 CrossRef; (c) M. M. Coulter, K. G. M. Kou, B. Galligan and V. M. Dong, J. Am. Chem. Soc., 2010, 132, 16330–16333 CrossRef CAS PubMed; (d) X.-H. Yang, R. T. Davison, S.-Z. Nie, F. A. Cruz, T. M. McGinnis and V. M. Dong, J. Am. Chem. Soc., 2019, 141, 3006–3013 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01152e |
| This journal is © The Royal Society of Chemistry 2020 |