Open Access Article
Open Access ArticleThe construction of fluorophoric thiazolo-[2,3-b]quinazolinone derivatives: a multicomponent domino synthetic approach†
Prajna Parimita Mohanta,
Hari Narayan Pati and
Ajaya Kumar Behera *
*
School of Chemistry, Sambalpur University, Jyoti Vihar, Burla-768019, India. E-mail: ajaykumar.behera@yahoo.com; ajaykumar.behera@suniv.ac.in
First published on 17th April 2020
Abstract
Acid-mediated one-pot domino reactions of substituted 2-amino thiazoles, substituted benzaldehydes and cyclic diketones have been developed for the synthesis of novel and architecturally unique thiazolo[2,3-b]quinazolinone derivatives under microwave irradiation. In this protocol, a series of thiazolo[2,3-b]quinazolinone derivatives have been synthesized and the excellent fluorescence behaviors of some of the molecules have been reported based on the incorporation of different electron-donating and electron-withdrawing substituents on the aryl moieties of the target molecules.
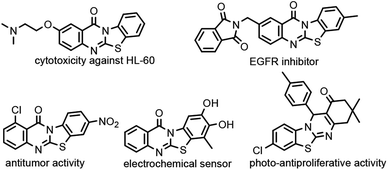
A fusion of biodynamic heterosystems for designing new molecular frameworks has pivotal importance in the process of drug discovery.1 Thiazole serves as a privileged scaffold in many natural and synthetic medicinal compounds.2 Conversely, quinazolinones have ubiquitous and interesting structural architectures, which contribute towards the development of medicinally relevant products3 with fluorescence properties.4 An assembly of such vital nuclei results in an intriguing scaffold not only in pharmacology, but also in photochemistry. Notably, benzothiazolo[2,3-b]quinazolinone scaffolds exhibit assorted biological and pharmaceutical properties such as cytotoxicity against HL-60 cells, inhibitory activities towards EGFR, anti-bacterial, antiviral and antitumor activities, electrochemical sensing for glutathione, amoxicillin or L-cysteine, and photo-antiproliferative activity (Fig. 1).5–7
To the best of our knowledge, to date, no examples of fluorescent thiazoloquinazolinones have been explored. The functions of quinazoline-based pharmacophores are usually analyzed by using external fluorescent labels within the cells. However, the properties of the analyzed compounds and drugs can be influenced or altered by the fluorescent markers. Therefore, the drug candidates should ideally display their own fluorescence to monitor the cellular uptake and visualize their interactions with biological partners. The motivation and desire to address these gaps encouraged us to pursue research in this field.
The more efficient and economical methods towards the synthesis of heterocycles are domino processes.8 In terms of efficiency and sustainability, one-pot multi-component domino reactions (MDRs) grant remarkable advantages over conventional bimolecular reactions owing to their convergence, atom-economy, operational simplicity, structural diversity and short synthetic pathway.9 MDRs have gained a new dimension in the field of designing methods to produce elaborate libraries of biologically active compounds.10 Similarly, microwave-assisted organic synthesis (MAOS) has been recognized as one of the most powerful and sustainable tools in synthetic chemistry because it can reduce reaction times, improve yields and improve the purity of the final compounds by regulating the temperature, pressure and power of irradiation.11 Consequently, the combination of both MDR and MAOS approaches provides a rapid and cost-efficient synthetic strategy to prepare a diverse range of products of great interest towards drug discovery endeavours. Some polyazaheterocycles have been synthesized from functionalized imidazoles12 and benzimidazoles13 by employing microwave-mediated MDRs.
In view of the importance of polyheterocyclic compounds, several allied protocols have been quoted from the literature (Scheme 1). Kidwai et al.14 have explored an environmentally benign approach for the synthesis of benzothiazolo-[2,3-b]-quinazolinones using Amberlyst-15. Diverse synthetic approaches towards the synthesis of benzothiazolo-[2,3-b]-quinazolinones have also been reported by Sangshetti et al.15 and Arya et al.7 employing water and an ionic liquid as green solvents, respectively. Shujiang et al.16 have reported microwave synthetic annulation towards the formation of naptho[2,3-f]quinoline derivatives in an acidic medium. Similarly, Georgescu et al.17 have explored the one-pot multicomponent synthesis of diverse pyrrolo[1,2-a]benzimidazole and pyrrolo[1,2-a]quinoxaline derivatives. In all these synthetic protocols, the target molecules contain no –OH group in the angular position. Conversely, Chebanov et al.18 have developed an efficient base-catalyzed synthetic strategy for pyrazolo[4,3-c]quinolizinones with an angular –OH group under microwave irradiation. Herein, we have reported the synthesis of an unprecedented and skeletally diverse thiazolo-[2,3-b]-quinazolinone scaffold having an –OH group at the angular position by an efficient acid-catalyzed one-pot domino reaction under microwave irradiation. The presence of all the heteroatoms on one side of the molecule might open up new avenues for its wide applications. The unique structural arrangement and interesting fluorescence properties of the thiazoloquinazolinone moiety gave us an inducement to synthesize a library of such types of molecules.
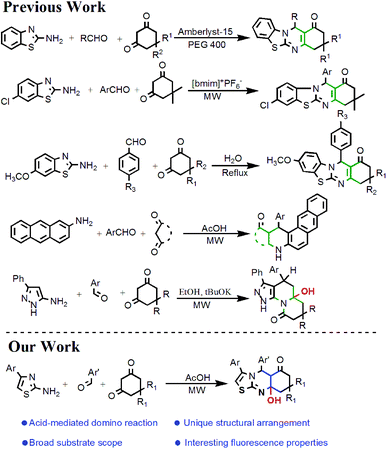 | ||
| Scheme 1 A brief description of strategies for the synthesis of various condensed heterocycles from cyclic amino compounds, aromatic aldehydes, and cyclic 1,3-diketones. | ||
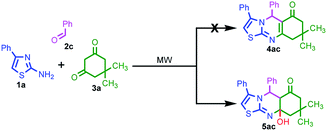
Simple and efficient synthetic protocols for fused ring heterocycles containing nitrogen and sulfur are important in all facets of chemistry. Consequently, we have reported a mild process for the synthesis of unexpected thiazoloquinazolinones by microwave irradiation. This synthetic protocol involved a three component condensation of 2-amino-4-phenyl thiazole 1a, benzaldehyde 2c and dimedone 3a under microwave irradiation for the synthesis of the thiazoloquinazolinone derivative 4ac (Table 1). The substrate 2-amino-4-phenyl thiazole 1a was prepared according to a previously reported procedure.19 Initially, the reaction was performed in ethanol without any catalyst, which did not provide any desired product (Table 1, entry 1). Next, catalytic amounts of different bases such as KOH, TBAB and TEA were added to trigger the reaction (Table 1, entries 2–4). The desired product was not detected in this case also and the starting materials were recovered. However, in the presence of catalytic amounts of acids such as acetic acid and oxalic acid, a trace amount of the product was obtained (Table 1, entries 5 and 6).
| Entry | Solvent | Temp. (°C) | Catalyst | Condition | Time | Yield (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 0.001 mol (1a), 0.001 mol (2c) and 0.001 mol (3a) in 3 ml acetic acid were heated via microwave as well as conventional heating. The mentioned yields are isolated yields. | ||||||
| 1 | EtOH | 100 | MW | 15 min | NR | |
| 2 | EtOH | 100 | KOH | MW | 15 min | NR |
| 3 | EtOH | 100 | TBAB | MW | 15 min | NR |
| 4 | EtOH | 100 | TEA | MW | 15 min | NR |
| 5 | EtOH | 100 | Oxalic acid | MW | 15 min | Trace |
| 6 | EtOH | 100 | AcOH | MW | 15 min | Trace |
| 7 | AcOH | 120 | — | MW | 15 min | 31 |
| 8 | DMF | 150 | — | MW | 15 min | 29 |
| 9 | Ethylene glycol | 150 | — | MW | 15 min | 21 |
| 10 | AcOH | 130 | — | MW | 15 min | 36 |
| 11 | AcOH | 140 | — | MW | 15 min | 41 |
| 12 | AcOH | 150 | — | MW | 15 min | 62 |
| 13 | AcOH | 150 | — | Reflux | 8 h | 43 |
Interestingly, the isolated product was an unexpected compound 5ac rather than the usual compound 4ac. The molecular structure was confirmed by NMR and LC-mass techniques (Fig. S9–S11; ESI†).
Encouraged by this result, the model reaction was carried out in polar solvents such as acetic acid, DMF and ethylene glycol (Table 1, entries 7–9). Low yields were obtained when the reaction was carried out in these solvents, which indicated the importance of solvents for this reaction. We further optimized the reaction conditions by conducting the reaction in acetic acid at different temperatures ranging from 120 to 150 °C (Table 1, entries 7 and 10–12). Increasing the temperature resulted in a significant increase in the yield. This MDR effectively amended the unprecedented formation of thiazoloquinazolinone 5ac by employing a higher temperature (150 °C) in acetic acid (Table 1, entry 12). Thus, the best result was achieved in glacial acetic acid. The mass spectra of the synthesized compounds played a vital role in establishing the structural diversity of the thiazoloquinazolinone derivatives 5. The experiment was also performed at 150 °C under conventional oil bath heating (Table 1, entry 13). A similar target compound 5ac was obtained here. Despite the presence of three non-equivalent nucleophilic reaction centers in the aminothiazole scaffold (N, S, and NH2), the reaction under microwave irradiation was more selective with a higher yield of the target molecule 5ac. Rapid microwave irradiation promotes molecular mixing, which increases the intimate contact between the reactant molecules. The microwave heating process accelerates the reaction to afford a higher yield and tune the selectivity in organic synthesis as compared to conventional heating.
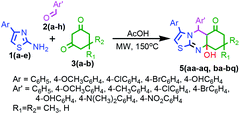
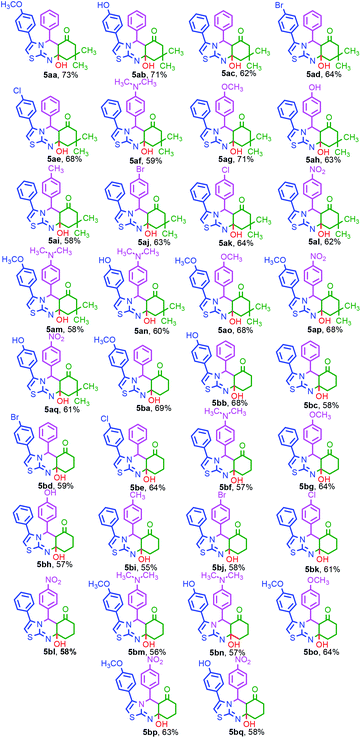
Under the aforesaid optimized reaction conditions, the scope of the microwave-mediated MDR was extended to the synthesis of other thirty-three thiazolo[2,3-b]quinazolin-6-one derivatives 5(aa–aq, ba–bq). The results are summarized in Table 2. We scrutinized several reactions involving substituted 2-amino-4-aryl thiazole 1(a–e), substituted aryl aldehydes 2(a–h) and dimedone 3a to afford the unexpected compounds 5aa–5aq in moderate to good yields. Another series of compounds 5ba–5bq could also be obtained from the MDR of the same substituted aminothiazole 1(a–e), substituted arylaldehyde 2(a–h) and 1,3-cyclohexanedione 3b in moderate to good yields.
Encouraged by the previous results (Scheme 1), we also attempted another MDR involving 2-amino benzothiazole 6 in place of 2-amino-4-aryl thiazole 1, substituted arylaldehyde 2 and cyclic diketones 3, and we expected the unusual target molecule 8. Controlled experiments were carried out under the same reaction conditions using microwave as well as conventional heating (Scheme 2). However, the usual reported product 7 was obtained completely without affording the exceptional product 8, suggesting the crucial role of 2-amino-4-aryl thiazole 1 in the above-mentioned MDR. The reason for this experimental finding might be that the tetracyclic ring in 8 obtained from benzothiazole 6 is structurally more rigid as compared to the aryl-flanked tricyclic ring in 5 obtained from 2-amino-4-aryl thiazole 1. The structural rigidity of the compound 8 possibly results in dehydration in order to maintain planarity.
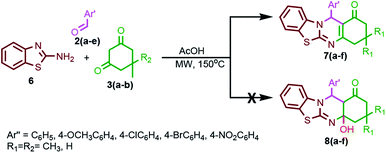 | ||
| Scheme 2 Controlled experiments towards the reaction of 2-amino benzothiazole 6, cyclic 1,3-diketones 3(a–b) and aromatic aldehyde 2(a–e). | ||
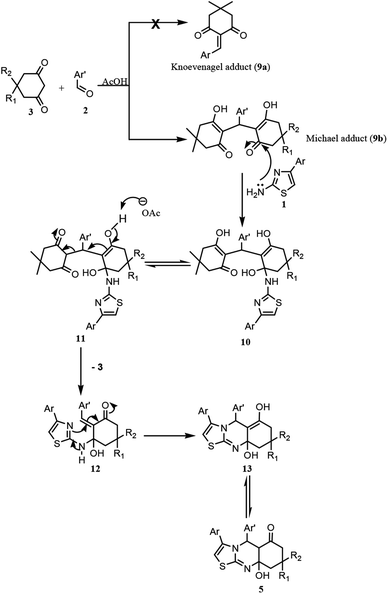
According to the results of the MDR for synthesizing thiazolo[2,3-b]-quinazolin-6-one 5ac (Scheme 1), a plausible mechanism has been suggested in Scheme 3. Initially, the acid-catalyzed condensation of dimedone and aryl aldehyde resulted in the formation of the Michael adduct20 9b instead of the Knoevenagel adduct 9a. This assumption was supported by LC-MS analysis by identifying the intermediate in the product mixture. Subsequently, the intermediate 9b reacted with 2-amino-4-aryl thiazole 1 to afford the amino thiazole derivative 10, which tautomerized to its keto form 11. The deprotonation of 11 followed by the elimination of one molecule of dimedone 3 resulted in the formation of the thiazole derivative 12. The target thiazoloquinazoline derivative 5 could be obtained by the intramolecular cyclisation of 12 through Michael addition, followed by the keto–enol tautomerism of 13 (Scheme 3).
The utility of the synthesized compounds 5aa–aq and 5ba–bq was demonstrated by measuring their emission spectra (Fig. 2) at 10 μM concentration in MeOH. The solutions of these compounds were excited separately at their respective absorption maxima (from 272 to 408 nm) in MeOH under identical conditions. The emission spectra were measured in the wavelength region from 354 to 524 nm keeping the slit width fixed at 2.5 nm for both the excitation and emission paths. Depending on the type of the substituents on the aryl moieties of the thiazole and pyrimidine rings of the thiazoloquinazolinone derivatives, we have divided these compounds in three different series. The first series consists of compounds 5aa–ae and 5ba–5be containing electron-donating substituents on the aryl moiety of the thiazole ring of the thiazoloquinazolinone derivatives (Fig. 2a and b). Interestingly, all of them were found to be strongly emissive and a gradual increase in fluorescence intensities was observed on increasing the strength of the electron-donating substituents.
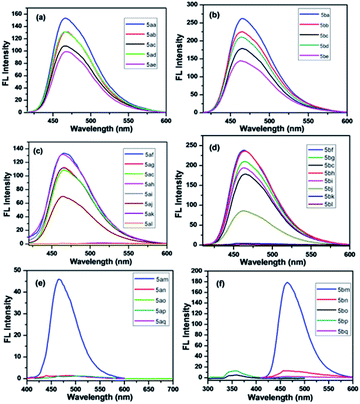 | ||
| Fig. 2 Emission intensities at 10 μM conc. in MeOH of compounds 5aa–5ae (a), 5ba–5be (b), compounds 5af–5al (c), 5bf–5bl (d), 5am–5aq (e) and 5bm–5bq (f) (λex = 354 to 524). | ||
The second series consists of compounds 5af–al and 5bf–5bl containing both electron-donating and electron-withdrawing substituents on the aryl moiety of the pyrimidine ring of the thiazoloquinazolinone derivatives (Fig. 2c and d). A similar trend in the fluorescence behavior of the compounds 5ag–5ak and 5bg–5bk having electron-donating groups was observed, as found in the case of 5aa–ae and 5ba–5be. In contrast, 5af and 5bf in this series having the strong electron-donating substituent, i.e., N(CH3)2 did not give satisfactory emission intensities in comparison to the above-mentioned compounds. The unexpected loss in the fluorescence intensity of these compounds could be attributed to the inhibition of electron delocalization. The lone pair of electrons on the nitrogen atom of the N,N-dimethyl amino substituent cannot delocalize through π-conjugation probably due to the structural complexity and bulkiness of the group. On the other hand, 5al and 5bl of this series containing the electron-withdrawing –NO2 group are non-emissive in nature.
The last series comprises compounds 5am–aq and 5bm–5bq containing both electron-donating and electron-withdrawing substituents on the aryl moiety of the respective thiazole and pyrimidine rings of the thiazoloquinazolinone derivatives (Fig. 2e and f). In this series, 5ao and 5bo are strongly emissive due to the attachment of strong electron-donating –OCH3 groups on both the rings. The compounds 5am, 5an, 5bm and 5bn are found to be weakly emissive due to the presence of –N(CH3)2 on the aryl-pyrimidine ring of the thiazoloquinazoline derivatives despite the presence of strong electron-donating groups –OCH3 and –OH on the aryl-thiazole ring. The compounds 5ap, 5aq, 5bp and 5bq of this series containing the electron-withdrawing nitro group are found to be non-emissive. From the above-mentioned results, it is evident that molecules having electron-donating groups attached to the thiazole ring are found to be superior luminophores as compared to other molecules. Thus, the synthesis of such molecules and their applications in the field of fluorescence imaging for clinical diagnostics and biomedical research are highly desirable.
Conclusions
In summary, we have synthesized a huge library of unique fluorophoric thiazolo[2,3-b]-quinazolin-6-one derivatives selectively by a simple and easily accessible domino procedure. These unprecedented findings open up an immense possibility in molecular imaging for drug development and mechanistic studies, avoiding the requirement of linking external fluorescent markers. Further detailed investigations towards the application of these novel compounds are in progress in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research work was supported by DST-DRS, UGC, New Delhi, India. We are grateful to NIT-Rourkela, IIT-Chennai and IISC-Bangalore for providing HPLC, NMR and mass spectra. We thank Dr S. N. Sahu and Dr H. Chakraborty of our department for their valuable suggestion for the interpretation of fluorescence spectra. P. P. M. thanks UGC, New Delhi, India for providing BSR fellowship.Notes and references
- (a) N. D. McClenaghan, R. Passalacqua, F. Loiseau, S. Campagna, B. Verheyde, A. Hameurlaine and W. Dehaen, J. Am. Chem. Soc., 2003, 125, 5356–5365 CrossRef PubMed; (b) H. Jian and J. M. Tour, J. Org. Chem., 2003, 68, 5091–5103 CrossRef CAS PubMed; (c) S. C. Cherng, W. H. Huang, C. Y. Shiau, A. R. Lee and T. C. Chou, Eur. J. Pharmacol., 2006, 532, 32–37 CrossRef CAS PubMed.
- M. T. Chhabria, S. Patel, P. Modi and P. S. Brahmkshatriya, Curr. Top. Med. Chem., 2016, 16(26), 2841–2862 CrossRef CAS PubMed.
- D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef CAS PubMed.
- F. E. Held, A. A. Guryev, T. Fröhlich, F. Hampel, A. Kahnt, C. Hutterer, M. Steingruber, H. Bahsi, C. Bojničić-Kninski, D. S. Mattes, T. C. Foertsch, A. Nesterov-Mueller, M. Marschall and S. B. Tsogoeva, Nat. Commun., 2017, 8, 1–9 CrossRef PubMed.
- (a) C. C. Cheng, D. F. Liu and T. C. Chou, Heterocycles, 1993, 35, 775–789 CrossRef CAS; (b) G. Shukla, A. K. Tiwari, V. K. Singh, A. Bajpai, H. Chandra and A. K. Mishra, Chem. Biol. Drug Des., 2008, 72, 533–539 CrossRef CAS PubMed; (c) M. A. El-Sherbeny, Drug Res., 2000, 50, 848–853 CAS.
- (a) M. A. Khalilzadeh, H. Kamiri-Maleh and V. K. Gupta, Electroanalysis, 2015, 27, 1766–1773 CrossRef CAS; (b) H. Karimi-Maleh, F. Tahernejad-Javazmi, V. K. Gupta, H. Ahmar and M. H. Asadi, J. Mol. Liq., 2014, 196, 258–263 CrossRef CAS.
- K. Arya, R. Tomar and D. S. Rawat, Med. Chem. Res., 2014, 23, 896–904 CrossRef CAS.
- L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed.
- (a) L. Weber, Drug Discovery Today, 2002, 7(2), 143–147 CrossRef CAS PubMed; (b) A. Domling, Curr. Opin. Chem. Biol., 2002, 6, 306–313 CrossRef CAS PubMed; (c) A. Domling, Curr. Opin. Chem. Biol., 2002, 4, 318–323 CrossRef.
- (a) R. V. A. Orru and d. M. Greef, Synthesis, 2003, 1471–1499 CrossRef CAS; (b) G. Balme, E. Bossharth and N. Monteiro, Eur. J. Org. Chem., 2003, 4101–4111 CrossRef CAS; (c) S. Brase, C. Gil and K. Knepper, Bioorg. Med. Chem., 2002, 10, 2415–2437 CrossRef CAS PubMed; (d) A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef CAS.
- C. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed.
- (a) W. Xu, Y. Jin, H. Liu, Y. Jiang and H. Fu, Org. Lett., 2011, 13, 1274–1277 CrossRef CAS PubMed; (b) M. Zhu, M. H. Kim, S. Lee, S. J. Bae, S. H. Kim and S. B. Park, J. Med. Chem., 2010, 53, 8760–8764 CrossRef CAS PubMed; (c) J. P. Wan and Y. J. Pan, Chem. Commun., 2009, 2768–2770 RSC; (d) R. D. Carpenter, K. S. Lam and M. J. Kurth, J. Org. Chem., 2007, 72, 284–287 CrossRef CAS PubMed.
- (a) K. Pericherla, P. Khedar, B. Khungar and A. Kumar, Chem. Commun., 2013, 49, 2924–2926 RSC; (b) Y. Yan, Y. Zhang, Z. Zha and Z. Wang, Org. Lett., 2013, 15, 2274–2277 CrossRef CAS PubMed; (c) M. Nagaraj, M. Boominathan, D. Perumal, M. S. Muthusubramanian and N. Bhuvanesh, J. Org. Chem., 2012, 77, 6319–6326 CrossRef CAS PubMed; (d) M. Krasavin, S. Shkavrov, V. Parchinsky and K. Bukhryakov, J. Org. Chem., 2009, 74, 2627–2629 CrossRef CAS PubMed.
- M. Kidwai, R. Chauhan and D. Bhatnagar, Sci. China: Chem., 2012, 55(10), 2154–2160 CrossRef.
- J. N. Sangshetti, D. K. Lokwani, R. S. Chouthe, A. B. Raval, F. A. K. Khan and D. B. Shinde, Med. Chem. Res., 2014, 23, 4893–4900 CrossRef CAS.
- S. Tu, S. Wu, S. Yan, W. Hao, X. Zhang, X. Cao, Z. Han, B. Jiang, F. Shi, M. Xia and J. Zhou, J. Comb. Chem., 2009, 11, 239–242 CrossRef CAS PubMed.
- (a) E. Georgescu, A. Nicolescu, F. Georgescu, F. Teodorescu, S. Shova, A. M. Macsim and C. Deleanu, Synthesis, 2015, 47, 1643–1655 CrossRef CAS; (b) E. Georgescu, A. Nicolescu, F. Georgescu, F. Teodorescu, D. Marinescu, A. M. Iurascu-Macsim and C. Deleanu, Beilstein J. Org. Chem., 2014, 10, 2377–2387 CrossRef PubMed; (c) A. Nicolescu, C. Deleanu, E. Georgescu, F. Georgescu, A. M. Iurascu, S. Shova and P. Filip, Tetrahedron Lett., 2013, 54, 1486–1488 CrossRef CAS.
- V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, I. V. Knyazeva, U. Groth, T. N. Glasnov and C. O. Kappe, J. Org. Chem., 2008, 73, 5110–5118 CrossRef CAS PubMed.
- (a) H. Tripathy and N. Mahapatra, Indian J. Chem., 1970, 8, 586–587 CAS; (b) S. N. Pandeya, D. Sriram, G. Nath and E. DeClercq, Eur. J. Pharm. Sci., 1999, 9, 25–31 CrossRef CAS PubMed.
- L. Chen, T. Chung, B. D. Narhe and C. Sun, ACS Comb. Sci., 2016, 18, 162–169 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and copies of NMR, mass spectra. See DOI: 10.1039/d0ra01066a |
| This journal is © The Royal Society of Chemistry 2020 |