Open Access Article
Open Access ArticleAn aluminium fluorosensor for the early detection of micro-level alcoholate corrosion†
Snigdha Roya,
Sanju Dasab,
Rini Majumdera,
Ambarish Ray‡
*b and
Partha Pratim Parui *a
*a
aDepartment of Chemistry, Jadavpur University, Kolkata 700032, India. E-mail: parthaparui@yahoo.com; Fax: +91-33-24146223; Tel: +91-9433490492
bDepartment of Chemistry, Maulana Azad College, Kolakta 700013, India. E-mail: r_ambarish@yahoo.co.in; Fax: +91-33-22268111; Tel: +91-9836650180
First published on 17th June 2020
Abstract
The detection of the dry alcoholate corrosion of aluminium is vital to design a corrosion resistive aluminium alloy for the storage and transportation of biofuel (methanol or ethanol). By synthesizing an Al3+ fluorescent probe operable in an alcoholic medium, we quantified the alcoholate corrosion in terms of the fluorometrically estimated soluble alkoxide (Al(OR)3) generation under nitrogen atmosphere. With time, a linear increase in corrosion with specific aluminium dissolution rate constants ∼2.0 and 0.9 μg per day per cm2 were estimated for aluminium and Al-7075 alloy, respectively. During open atmosphere monitoring, the adsorbed moisture converted small extent of Al(OR)3 to the insoluble Al(OH)3 at the alloy surface which retarded the alcoholate corrosion appreciably.
Switching over from conventional fossil fuel to biofuel is of current interest owing to the maximum utilization of eco-friendly non-conventional energy.1 Commercially produced less polluted biofuels such as methanol and ethanol, mixed with fossil fuels have an acceptable performance capacity for the gasoline engine.2 Moreover, in comparison to the gasoline, methanol and ethanol have much higher octane rating or compression ratio to resist the knocking for better thermal efficiency.3 Since most of the fuel tanks/pipes are made of aluminium or its alloys owing to its high strength-to-density ratio, the aluminium corrosion due to the formation of alkoxide (alcoholate or dry corrosion) during storage or even transportation of such bio-alcohols may cause leakage in the fuel tanks and in worst cases enough threat is speculated for fire and explosion.4 Mechanical overloads, alloy impurities even at elevated temperatures are further contenders for accelerating the alcoholate corrosion.5 However, a prolong exposure to the moisture retards the alcoholate corrosion by forming a protective layer of hydrated aluminium oxide in the metallic surface but moisture impurity in the fuel may damage the gasoline engine.6 Hence, a maintenance optimization is crucial in critical engineering disasters by detecting alcoholate corrosion as in its nascent state with minimizing the chance of water contamination.6,7
Several electrochemical and mechanical methods have been exploited for decades to propose aluminium alcoholate and other corrosions;6 yet the early detection of the alcoholate corrosion is still a challenging task due to the lack of sensitive analytical methods.6,8 Here, the fluorescence technique may act as a better alternative owing to its simplicity and high sensitivity.9 Till date, a large number of fluorescent probes for Al3+ have been exploited in the biological or environmental domain,10 but has never focused on alcoholate corrosion studies. Based on this requirement, we synthesized a fluorescent probe, namely HMBDC ((6Z)-6-(2-hydroxy-3-(hydroxymethyl)-5-methylbenzylideneamine)-2H-chromen-2-one), to detect alcoholate corrosion with μg-level detection ability along with its retarding signature in the presence of moisture in a judicious way. Such novel method may lead to an early detection of alcoholate corrosion in a simpler way.
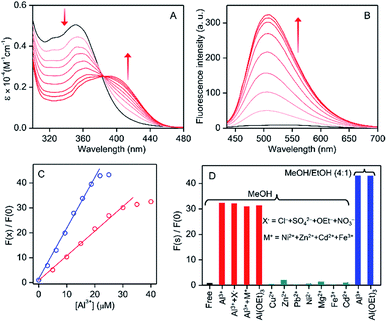
The non-fluorescent phenolic Schiff-base molecule containing a coumarin moiety (HMBDC) was prepared by condensing an equimolar mixture of 6-amino coumarin (6-ACO) and 2-hydroxy-3-(hydroxymethyl)-5-methylbenzaldehyde (HHMB) in dry ethanol (Scheme 1 and Fig. S1†) (c.f. ESI† for details). Among various organic solvents, the interaction of HMBDC with Al3+ was observed only in the alcoholic medium according to the UV-vis studies (Fig. S2†). In methanol, the absorption intensity at ∼353 nm for HMBDC (5 μM) decreased gradually with the continuous addition of Al(NO3)3 until saturated at ∼8-equiv., giving rise to a new peak at ∼406 nm, where an isosbestic point at ∼384 nm assures the formation of Al3+/HMBDC complex (1) (Fig. 1A). Upon optimization of the complex formation affinity in various ethanol/methanol mixed media, highest reactivity with the lowest saturated Al3+ concentration (∼5 equiv.) compared to that obtained in pure methanol was observed in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/ethanol-mixed medium (Fig. S3†). Most probably, more effective H-bonding interaction of the dimeric ethanol/methanol11 with 1 induces greater complex (1) stability, although the complex formation reactivity was much less in pure ethanol compared to the methanol medium (Fig. 1 and S2†).
1 methanol/ethanol-mixed medium (Fig. S3†). Most probably, more effective H-bonding interaction of the dimeric ethanol/methanol11 with 1 induces greater complex (1) stability, although the complex formation reactivity was much less in pure ethanol compared to the methanol medium (Fig. 1 and S2†).
In spite of the stronger H-bonding interactions of 1 with water compared to the methanol or ethanol, a complete dissociation of 1 in the presence of 20% (v/v) water in methanol (Fig. S4†) suggests that, in addition to the solvent assisted H-bonded structural stability of 1, alcohol molecule may also participate in the coordination with Al3+ to form 1. Indeed, the possible methanol coordination is reflected in the ESI-MS+ analysis (Fig. S5B†). In addition, the Job's plots in the absorption studies showed that the HMBDC formed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with Al3+ (Fig. S6†). To elucidate the probable structure of 1, we carried out the DFT-based theoretical calculation by considering the 1
1 stoichiometric complex with Al3+ (Fig. S6†). To elucidate the probable structure of 1, we carried out the DFT-based theoretical calculation by considering the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric Al3+/HMBDC complex with or without methanol coordination. A stable structure of 1 was obtained when the oxygen atom of the methanol molecule coordinates with Al3+ and other two coordination sites of Al3+ are occupied by the phenolic-oxygen and imine-nitrogen of HMBDC (Scheme 1, Fig. 2 and S7†). Facile coordination of those hard donor sites of HMBDC towards harder Al3+ is susceptible towards alcohol assisted stabilization of 1. The UV-vis absorbance at ∼402 nm for 1 computed from the time-dependent DFT (TD-DFT) calculations in methanol medium, where the HOMO (90) → LUMO (92) excitation nicely matched with the experimental absorbance at ∼406 nm (Fig. 1 and 2). However, monitoring of the 1H-NMR peak characterized for aldimine proton is a useful strategy to identify the bonding of the imine-N to Al3+.12 We observed that the aldimine proton peak intensity for HMBDC in CD3OD was quenched to a great extent with a considerable down-field shift from 8.80 to 8.88 ppm in the presence of Al3+ (Fig. S8†); the down-field shift is expected owing to the imine-N and Al3+ coordination, but intensity quenching does not follow the previous trend in the aprotic polar medium.12 The generation of a partial positive charge at the N-centre upon its binding with the Al3+ may enhance the acidity of the aldimine proton to become labile for participating in the H/D exchange in a protic medium (CD3OD), as reported previously for other allied systems.13 These results strongly suggest the imine-N and Al3+ bonding in 1. On the other hand, Al3+ induced large decrease in the IR intensity at ∼3300 cm−1 for phenolic-OH also supports the phenoxide coordination (Fig. S9†).
1 stoichiometric Al3+/HMBDC complex with or without methanol coordination. A stable structure of 1 was obtained when the oxygen atom of the methanol molecule coordinates with Al3+ and other two coordination sites of Al3+ are occupied by the phenolic-oxygen and imine-nitrogen of HMBDC (Scheme 1, Fig. 2 and S7†). Facile coordination of those hard donor sites of HMBDC towards harder Al3+ is susceptible towards alcohol assisted stabilization of 1. The UV-vis absorbance at ∼402 nm for 1 computed from the time-dependent DFT (TD-DFT) calculations in methanol medium, where the HOMO (90) → LUMO (92) excitation nicely matched with the experimental absorbance at ∼406 nm (Fig. 1 and 2). However, monitoring of the 1H-NMR peak characterized for aldimine proton is a useful strategy to identify the bonding of the imine-N to Al3+.12 We observed that the aldimine proton peak intensity for HMBDC in CD3OD was quenched to a great extent with a considerable down-field shift from 8.80 to 8.88 ppm in the presence of Al3+ (Fig. S8†); the down-field shift is expected owing to the imine-N and Al3+ coordination, but intensity quenching does not follow the previous trend in the aprotic polar medium.12 The generation of a partial positive charge at the N-centre upon its binding with the Al3+ may enhance the acidity of the aldimine proton to become labile for participating in the H/D exchange in a protic medium (CD3OD), as reported previously for other allied systems.13 These results strongly suggest the imine-N and Al3+ bonding in 1. On the other hand, Al3+ induced large decrease in the IR intensity at ∼3300 cm−1 for phenolic-OH also supports the phenoxide coordination (Fig. S9†).
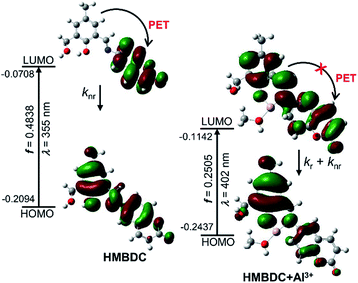 | ||
| Fig. 2 Frontier molecular orbital profiles including various UV-vis absorption parameters of HMBDC (left panel) and HMBDC/Al3+ complex (right panel) based on TD-DFT (B3LYP/6-31G(d)). | ||
The electronic distribution in the molecular orbital diagram (MO) of the HMBDC evaluated from the DFT calculation showed an intra-molecular photo-induced electron transfer (PET) from coumarin to the HHMB moiety, which makes the HMBDC non-fluorescent (Fig. 2). Al3+ induced an instantaneous increase in the fluorescence intensity for HMBDC (5 μM) in the alcoholic medium (methanol/ethanol or their mixture) due to the formation of 1 (Fig. 1B and S10†). A gradual fluorescence intensity increase at ∼506 nm (λex = 406 nm) of ∼30-fold for 8 equiv. of Al3+ and ∼40-fold for 5 equiv. of Al3+ was observed in the methanol and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol/ethanol medium, respectively (Fig. 1 and S10B†). According to the HOMO and LUMO electronic distributions for 1 in the DFT studies, the PET process in HMBDC was highly restricted upon its binding with Al3+ in 1, causing for the large increase in the fluorescence intensity (Fig. 2). However, the better fluorescence response (lower intensity-saturated Al3+ concentration and larger intensity increase) in the mixed medium than pure methanol may be associated with greater stability of 1, as described in the previous section (Fig. S2†). The fluorescence intensity increase remains invariant using other soluble Al(III)-salts (Fig. 1D and S11†), which eliminates the role of counter anions for the increasing intensity. To ascertain the Al3+ selectivity, we performed similar fluorescence studies with other potentially interfering cations but failed to produce any noticeable fluorescence (Fig. 1D and S12†). However, a linear intensity increase with the increase in the concentration of Al3+ up to 6 equiv. in methanol and 4 equiv. in the 4
1 (v/v) methanol/ethanol medium, respectively (Fig. 1 and S10B†). According to the HOMO and LUMO electronic distributions for 1 in the DFT studies, the PET process in HMBDC was highly restricted upon its binding with Al3+ in 1, causing for the large increase in the fluorescence intensity (Fig. 2). However, the better fluorescence response (lower intensity-saturated Al3+ concentration and larger intensity increase) in the mixed medium than pure methanol may be associated with greater stability of 1, as described in the previous section (Fig. S2†). The fluorescence intensity increase remains invariant using other soluble Al(III)-salts (Fig. 1D and S11†), which eliminates the role of counter anions for the increasing intensity. To ascertain the Al3+ selectivity, we performed similar fluorescence studies with other potentially interfering cations but failed to produce any noticeable fluorescence (Fig. 1D and S12†). However, a linear intensity increase with the increase in the concentration of Al3+ up to 6 equiv. in methanol and 4 equiv. in the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/ethanol mixed medium can be useful for a ratiometric detection of unknown concentration of Al3+ (Fig. 1C), where the limit of detection14 (LOD) of Al3+ with HMBDC in the methanol medium was found to be ∼0.5 μM (c.f. details in ESI†). Most importantly, HMBDC recognized Al3+ selectively from the mixture of various other cations, and also in presence of other soluble Al(III) salts, particularly, aluminium alkoxide (ethoxide) with similar accuracy (Fig. 1D and S12†). Therefore, the Al(III) sensing ability for an alcoholate corrosion with an aluminium alloy must not be perturbed due to the interference of other leached cations.
1 methanol/ethanol mixed medium can be useful for a ratiometric detection of unknown concentration of Al3+ (Fig. 1C), where the limit of detection14 (LOD) of Al3+ with HMBDC in the methanol medium was found to be ∼0.5 μM (c.f. details in ESI†). Most importantly, HMBDC recognized Al3+ selectively from the mixture of various other cations, and also in presence of other soluble Al(III) salts, particularly, aluminium alkoxide (ethoxide) with similar accuracy (Fig. 1D and S12†). Therefore, the Al(III) sensing ability for an alcoholate corrosion with an aluminium alloy must not be perturbed due to the interference of other leached cations.
The dry alcoholate corrosion of aluminium or its alloy while forming soluble alkoxide (Al(OR)3) can be detected upon incubation in an anhydrous alcoholic medium. However, under a condition of prolonged incubation, the contamination of trace amounts of moisture may also trigger the conversion of Al(OR)3 to Al(OH)3, followed by the hydrated alumina (Al2O3·xH2O) coating on the metallic surface.6 The formation of hydrated alumina can also be possible via the decomposition of Al(OR)3.6 To characterize the alcoholate corrosion as an exclusive process to the maximum limit, we minimized those wet-processes by allowing the corrosion under inert conditions. A previously grazed aluminium-sheet (dimension ∼3.5 × 1.5 × 0.2 cm3; surface area ∼12.5 cm2) was incubated for 18 days in 100 mL anhydrous methanol or methanol/ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent under nitrogen atmosphere by purging nitrogen every 24 h, where the small change in the solution volume if required was adjusted by injecting an appropriate amount of the nitrogen-saturated anhydrous solvent. The amount of Al(OR)3 (R = -Me, -Et) generated in the medium was estimated by monitoring the HMBDC (5 μM) fluorescence. After 10-fold dilution of the medium with the parent solvent, the amount of Al(OR)3 formed or the alcoholate corrosion was estimated in every 3 days interval according to the amount of Al3+ obtained from the time-dependent fluorescence responses (Fig. S13†) as per the linear calibration plots in Fig. 1C multiplied by the dilution factor. A linear increase in the normalized fluorescence intensity from ∼3.5 to 16.8 and ∼7.3 to 36.1 was observed with an increase in the incubation time period from 3 day to 18 day for methanol and methanol/ethanol (4
1) mixed solvent under nitrogen atmosphere by purging nitrogen every 24 h, where the small change in the solution volume if required was adjusted by injecting an appropriate amount of the nitrogen-saturated anhydrous solvent. The amount of Al(OR)3 (R = -Me, -Et) generated in the medium was estimated by monitoring the HMBDC (5 μM) fluorescence. After 10-fold dilution of the medium with the parent solvent, the amount of Al(OR)3 formed or the alcoholate corrosion was estimated in every 3 days interval according to the amount of Al3+ obtained from the time-dependent fluorescence responses (Fig. S13†) as per the linear calibration plots in Fig. 1C multiplied by the dilution factor. A linear increase in the normalized fluorescence intensity from ∼3.5 to 16.8 and ∼7.3 to 36.1 was observed with an increase in the incubation time period from 3 day to 18 day for methanol and methanol/ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) media (Fig. 3A and S13†), respectively, which correspond to the linear increase in the Al3+ amount in the medium from ∼3.2 to 16.6 μmol for either solvents (Fig. 3C). Indeed, the weight-loss of ∼0.47 mg i.e., ∼17.5 μmol was found to be closely similar with that of the increase in Al3+, revealing that not only the dry corrosion leads to the generation of Al3+ (Al(OR)3) as the only product, but also HMBDC is highly effective for an accurate estimation of the alcoholate corrosion. In addition, the nice correlation between the weight-loss and Al(OR)3 amount also reveals that the decomposition of alkoxide into insoluble alumina is negligibly small during the whole corrosion time-course.
1) media (Fig. 3A and S13†), respectively, which correspond to the linear increase in the Al3+ amount in the medium from ∼3.2 to 16.6 μmol for either solvents (Fig. 3C). Indeed, the weight-loss of ∼0.47 mg i.e., ∼17.5 μmol was found to be closely similar with that of the increase in Al3+, revealing that not only the dry corrosion leads to the generation of Al3+ (Al(OR)3) as the only product, but also HMBDC is highly effective for an accurate estimation of the alcoholate corrosion. In addition, the nice correlation between the weight-loss and Al(OR)3 amount also reveals that the decomposition of alkoxide into insoluble alumina is negligibly small during the whole corrosion time-course.
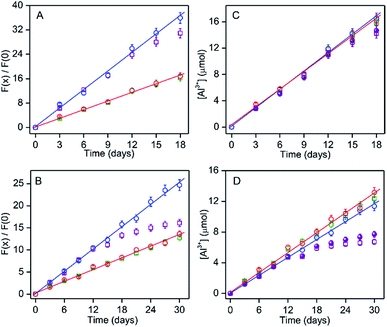 | ||
Fig. 3 (A and B) Extent of the fluorescence intensity increase due to corrosion-induced leached Al3+ (F(x)/F(0)) of HMBDC (5 μM) and (C and D) amount of Al3+ in the corrosion medium according to fluorescence response are plotted with various incubation times of pure aluminium sheet or its alloy (Al-7075) in different mediums/atmosphere conditions: nitrogen atmosphere in methanol (red) and methanol/ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (blue); open atmosphere in methanol (green) and methanol/ethanol (4 1) (blue); open atmosphere in methanol (green) and methanol/ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (purple). The data at nitrogen conditions are only fitted linearly. (A and B) The fluorescence intensity of HMBDC (5 μM) were monitored after the 10-fold dilution of the corrosion medium with the same solvent. (C and D) The amount of Al3+ estimated as the amount obtained from the normalized intensity with comparing the linear plots in Fig. 1C multiplied by the dilution factor 10. The actual amount of alcoholate corrosions for the mixed medium under open atmosphere are depicted by solid circle (purple). 1) (purple). The data at nitrogen conditions are only fitted linearly. (A and B) The fluorescence intensity of HMBDC (5 μM) were monitored after the 10-fold dilution of the corrosion medium with the same solvent. (C and D) The amount of Al3+ estimated as the amount obtained from the normalized intensity with comparing the linear plots in Fig. 1C multiplied by the dilution factor 10. The actual amount of alcoholate corrosions for the mixed medium under open atmosphere are depicted by solid circle (purple). | ||
However, under open atmospheric conditions maintained by air purging (average relative humidity ∼70%; average temperature 28 °C) in every 24 h interval while maintaining other similar experimental conditions and analysis protocol, the specific corrosion rate (∼2.0 μg per day per cm2) up to 12 days, was found to be closely similar to that detected under the nitrogen atmosphere (Fig. 3C and S13†). The results also indicate that the early stage of the alcoholate corrosion process (at least up to 12 days) for pure aluminium is not affected significantly by the atmospheric moisture content, although the final corrosion amount after 18 days incubation in normal atmosphere was slightly lower (∼84%) for the mixed medium compared to that obtained for pure methanol (Fig. 3C). The decrease in the Al(OR)3 amount can be affected by two processes: (a) Al(OR)3 to insoluble Al(OH)3 conversion due to the adsorbed moisture; (b) actual retardation of the corrosion rate due to the surface deposition of Al(OH)3. The extent of the conversion of Al(OR)3 to Al(OH)3 in the corrosion medium under the open air condition can be assessed by estimating the fluorescence intensity at every 3 day time interval in the absence of aluminium sheet (from day-3 to day-18) with the addition of same amount of Al(OEt)3 (3.2, 5.7, 8.0, 11.7, 14.2 and 16.4 μmol (final added amount) at day 0 (beginning of day 1), 3, 9, 12 and 15, respectively, in 100 mL mixed medium) as that of the alkoxide amount detected due to the corrosion under nitrogen condition (Fig. S14†). In comparison to the actual added Al(OEt)3, any decrease in the Al(OEt)3 amount upon such incubation should be added with the corrosion induced formation of Al(OR)3 amount under nitrogen condition for respective time interval to obtain the actual alcoholate corrosion. The actual corrosion was found to be slightly higher than that estimated from the corrosion-induced Al(OR)3 formation (Fig. 3C, solid symbol). According to the LOD of Al3+, the detection of the alcoholate corrosion amount as minimum as ∼0.1 μg mL−1 can be possible by monitoring the fluorescence response of HMBDC.
Alcoholate corrosion in a widely used aluminium alloy, Al-7075 (composition: Al, 90%; Zn, 5.5%; Mg, 2.5%; Cu, 1.5 and Si, 0.5%) was also studied. The previously grazed alloy sheet with same dimension and surface area as that of the pure aluminium sheet was incubated in 100 mL anhydrous methanol or 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/ethanol under nitrogen as well as normal atmospheric conditions. The amount of the alcoholate corrosion in every 3 days interval up to 30 days was estimated by evaluating the fluorescence response of HMBDC (Fig. 3B and S15†). In comparison to the pure aluminium sheet, the increase in corrosion from ∼1.5 to 4.0 μmol evaluated from the increase in the normalized fluorescence intensity (1.65 to 5.90 in methanol; 2.64 to 10.40 in methanol/ethanol (4
1 methanol/ethanol under nitrogen as well as normal atmospheric conditions. The amount of the alcoholate corrosion in every 3 days interval up to 30 days was estimated by evaluating the fluorescence response of HMBDC (Fig. 3B and S15†). In comparison to the pure aluminium sheet, the increase in corrosion from ∼1.5 to 4.0 μmol evaluated from the increase in the normalized fluorescence intensity (1.65 to 5.90 in methanol; 2.64 to 10.40 in methanol/ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture) with the increase in the incubation time from day-3 to day-12 follows a similar linear relation regardless of the solvent compositions and atmospheric conditions (Fig. 3B and D), while the intrinsic rate of corrosion ∼0.95 μg per day per cm2 was more than 2-fold slower (Fig. 3C and D). The lower rate constant value for the alloy compared to pure aluminium indicates that the contamination of other metals in the alloy resists the early stage alcoholate corrosion process. However, under normal atmospheric condition, the corrosion amount vs. time relation deviates from the linearity after 12 days. Importantly, after 30 days of incubation, a large reduction in the Al(OR)3 amount from ∼11.38 to 6.64 μmol was estimated for the mixed medium, but the change was only from ∼13.20 to ∼12.33 μmol for pure methanol (Fig. 3D). By determining the hydration-induced conversion amount of Al(OR)3 to Al(OH)3 according to the procedure, as described before (Fig. S16†), the actual alcoholate corrosion was found to decrease from ∼11.38 to 7.70 μmol by changing the condition from nitrogen to open atmosphere after 30 days (Fig. 3D, solid symbol). Our study reveals that in comparison to pure methanol, the formation of Al(OH)3 under open atmospheric condition retards the alcoholate corrosion largely due to the presence of more hygroscopic ethanol.15 The deposition of Al2O3·xH2O onto the alloy-surface is responsible for resisting the further alcoholate corrosion6 (Fig. 3D). In fact, the generation of more surface pits owing to the higher extent of the alcoholate corrosion in methanol over the mixed medium was also detected by naked eye (Fig. S17†). The surface morphology in the SEM studies showed that the alloy surface was little bit smoother after the corrosion in the mixed medium (Fig. S18†), justifying our proposition for the surface deposition of Al2O3·xH2O. On the other hand, cyclic voltammetric studies in the corrosion medium exposed to normal atmospheric conditions identified an irreversible cathodic peak at ∼−0.7 V due to the formation of insoluble Al(OH)3 in addition to the conversion from Al to Al3+, but such irreversible peak was not observed for the medium exposed to nitrogen (Fig. S19†). Moreover, the formation of white gelatinous precipitate of Al(OH)3 in the mixed medium was clearly visible by naked eye under normal atmospheric conditions (Fig. S17B†). All those results strongly support that the initiation of the wet-process by forming Al(OH)3 inhibits the alcoholate corrosion rate.
1) mixture) with the increase in the incubation time from day-3 to day-12 follows a similar linear relation regardless of the solvent compositions and atmospheric conditions (Fig. 3B and D), while the intrinsic rate of corrosion ∼0.95 μg per day per cm2 was more than 2-fold slower (Fig. 3C and D). The lower rate constant value for the alloy compared to pure aluminium indicates that the contamination of other metals in the alloy resists the early stage alcoholate corrosion process. However, under normal atmospheric condition, the corrosion amount vs. time relation deviates from the linearity after 12 days. Importantly, after 30 days of incubation, a large reduction in the Al(OR)3 amount from ∼11.38 to 6.64 μmol was estimated for the mixed medium, but the change was only from ∼13.20 to ∼12.33 μmol for pure methanol (Fig. 3D). By determining the hydration-induced conversion amount of Al(OR)3 to Al(OH)3 according to the procedure, as described before (Fig. S16†), the actual alcoholate corrosion was found to decrease from ∼11.38 to 7.70 μmol by changing the condition from nitrogen to open atmosphere after 30 days (Fig. 3D, solid symbol). Our study reveals that in comparison to pure methanol, the formation of Al(OH)3 under open atmospheric condition retards the alcoholate corrosion largely due to the presence of more hygroscopic ethanol.15 The deposition of Al2O3·xH2O onto the alloy-surface is responsible for resisting the further alcoholate corrosion6 (Fig. 3D). In fact, the generation of more surface pits owing to the higher extent of the alcoholate corrosion in methanol over the mixed medium was also detected by naked eye (Fig. S17†). The surface morphology in the SEM studies showed that the alloy surface was little bit smoother after the corrosion in the mixed medium (Fig. S18†), justifying our proposition for the surface deposition of Al2O3·xH2O. On the other hand, cyclic voltammetric studies in the corrosion medium exposed to normal atmospheric conditions identified an irreversible cathodic peak at ∼−0.7 V due to the formation of insoluble Al(OH)3 in addition to the conversion from Al to Al3+, but such irreversible peak was not observed for the medium exposed to nitrogen (Fig. S19†). Moreover, the formation of white gelatinous precipitate of Al(OH)3 in the mixed medium was clearly visible by naked eye under normal atmospheric conditions (Fig. S17B†). All those results strongly support that the initiation of the wet-process by forming Al(OH)3 inhibits the alcoholate corrosion rate.
In conclusion, a phenolic Schiff-base consisting of a coumarin unit as a fluorescent sensor for Al3+ operable only in the alcoholic medium is synthesized to monitor dry alcoholate corrosion. The photo-induced electron transfer process in the probe molecule exhibits Al3+ induced large increase of fluorescence intensity, lifted by its complexation with Al3+, which was further stabilized by the coordination and H-bonding interaction with the solvent molecule. The alcohol specific complex formation and subsequent fluorescence generation was suitably tuned to monitor the alcoholate corrosion by fluorometrically estimating aluminium alkoxide formation with a sensitivity of ∼10 μg L−1. However, the simultaneous participation of small extent of the wet-process (Al(OR)3 to Al(OH)3 conversion) and its deposition in metal surface, particularly for the alloy, inhibits the dry alcoholate corrosion. The alloy specific detection of the early stage alcoholate corrosion is in progress to obtain suitable material useful as a biofuel container.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study is partially supported by UGC and government of West Bengal for financial support under RUSA 2.0 scheme (PPP; No: 5400-F(Y)). SR and RM acknowledge UGC for the SRF fellowship. Authors also acknowledge JU and MA College, for departmental facilities. We are thankful to Dr N. R. Singha (GCELT) for TGA studies.Notes and references
- (a) J. Xuan, D. Y. C. Leung, M. Ni and M. K. H. Leung, Renew. Sustain. Energy Rev., 2009, 13, 1301 CrossRef CAS; (b) F. H. Shinya and Y. KenjiImou, Bioresour. Technol., 2010, 101, 109 CrossRef PubMed.
- (a) L. Matejovsky, J. Macak, M. Pospisil, P. Baros, M. Stas and A. Krausova, Energy Fuels, 2017, 31, 10880 CrossRef CAS; (b) R. H. Borgwardt, Ind. Eng. Chem. Res., 1998, 37, 3760 CrossRef CAS; (c) B. Shayan, S. M. Seyedpour, F. Ommi, S. H. Moosavy and M. Alizadeh, Int. J. Automot. Mech. Eng., 2011, 1, 3 Search PubMed; (d) J. H. Lunsford, Catal. Today, 2000, 63, 165 CrossRef CAS.
- M. B. Çelik, B. Ozdalyan and F. Alkan, Fuel, 2011, 90, 1591 CrossRef.
- K. Wagner, K. Eppel, T. Troßmann and C. Berger, Energy Mater. Mater. Sci. Eng. Energy Syst., 2008, 3, 227 CrossRef.
- K. Eppel, M. Scholz,T. Troßmann and C. Berger, Proceedings: EUROCORR, 2009, Nice, p. 9 Search PubMed.
- J. Linder, thesis on, Alcoholate corrosion of aluminium in ethanol blends, Master thesis, KTH Royal Institute of Technology, Div. Surface and Corrosion Science, Stockholm, 2012.
- (a) P. B. L. Fregolente, M. R. Wolf Maciel and L. S. Oliveira, Braz. J. Chem. Eng., 2015, 32, 895 CrossRef CAS; (b) S. K. Thangavelu, A. S. Ahmed and F. N. Ani, Int. J. Energy Res., 2016, 40, 1704 CrossRef CAS.
- (a) L. Kruger, F. Tuchscheerer, M. Mandel, S. Muller and S. Liebsch, J. Mater. Sci., 2012, 47, 2798 CrossRef; (b) O. Seri and Y. Kido, Mater. Trans., 2009, 50, 1433 CrossRef CAS; (c) Corrosion resistance of aluminium and protective measures where appropriate, First Edition: 2011 © AFSA Compiled and published by the Aluminium Federation of South Africa; (d) G. R. Kramer, C. M. Mendez and A. E. Ares, Mat. Res., 2018, 21, 1 Search PubMed.
- (a) S. Das, Y. Sarkar, S. Mukherjee, J. Bandyopadhyay, S. Samanta, P. P. Parui and A. Ray, Sens. Actuators, B, 2015, 209, 545 CrossRef CAS; (b) P. P. Parui, A. Ray, S. Das, Y. Sarkar, T. Paul, S. Roy, R. Majumder and J. Bandyopadhyay, New J. Chem., 2019, 43, 3750 RSC; (c) P. A. Anna, P. Paul and W. R. David, Anal. Chem., 1997, 69, 1635 CrossRef PubMed.
- (a) A. Gupta and N. Kumar, RSC Adv., 2016, 6, 106413 RSC; (b) Q. Jiang, M. Li, J. Song, Y. Yang, X. Xu, H. Xu and S. Wang, RSC Adv., 2019, 9, 10414 RSC; (c) H. L. Nguyen, N. Kumar, J.-F. Audibert, R. Ghesami, J.-P. Lefevre, M.-H. Ha-Thi, C. Mongin and I. Leray, New J. Chem., 2019, 43, 15302 RSC.
- I. A. Finneran, P. B. Carroll, G. J. Mead and G. A. Blake, Phys. Chem. Chem. Phys., 2016, 18, 22565 RSC.
- S. Sahana, S. Bose, S. K. Mukhopadhyay and P. K. Bharadwaj, J. Lumin., 2016, 169, 334 CrossRef CAS.
- (a) R. Casasnovas, M. Adrover, J. Ortega-Castro, J. Frau, J. Donoso and F. Muñoz, J. Phys. Chem. B, 2012, 116, 10665 CrossRef CAS PubMed; (b) B. Setner, M. Wierzbicka, L. Jerzykiewicz, M. Lisowski and Z. Szewczuk, Org. Biomol. Chem., 2018, 16, 825 RSC.
- V. Thomsen, D. Schatzlein and D. Mercuro, Spectroscopy, 2003, 18, 112 CAS.
- B. Tan, P. Melius and P. Ziegler, J. Chromatogr. Sci., 1982, 20, 213 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00619j |
| ‡ Department of Chemistry, Barasat Govt. College, Kolkata 700124, India |
| This journal is © The Royal Society of Chemistry 2020 |