Open Access Article
Open Access ArticleSolutions to the water flooding problem for unitized regenerative fuel cells: status and perspectives
Immanuel Vincent
,
Eun-Chong Lee
and
Hyung-Man Kim
 *
*
Department of Mechanical Engineering, High Safety Vehicle Core Technology Research Center, INJE University, 607 Eobang-Dong, Gimhae-si, Gyongsangnam-do 621-749, Republic of Korea. E-mail: mechkhm@inje.ac.kr; Fax: +82 55 324 1723; Tel: +82 55 320 3666
First published on 29th April 2020
Abstract
Unitized regenerative fuel cells (URFC) are capable of generating, storing, and releasing energy on demand in a sustainable manner. Water management is of vital importance to achieve maximum performance, durability, and round-trip efficiency in URFCs. However, URFCs suffer from critical issues related to their mode-switching process, water flooding, and membrane dehydration. The essential problem of water management is maintaining a subtle equilibrium between membrane drying and liquid water flooding to prevent membrane dehydration and ensure high URFC performance. This paper provides an overview of the operating principle of URFCs and describes the underlying phenomena related to water management issues. It also summarizes state-of-the-art studies of water management with a focus on recent developments and discusses the technical challenges of water management strategies. In addition, we propose a novel system design to address these critical water management issues. Overall, this review identifies the gaps in the research and development of URFC water management and identifies several essential future developments and research directions for future investigation.
1. Introduction
Electricity produced by sustainable energy sources, such as solar or wind, is able to meet an increasing share of global energy demand.1–3 However, climatic conditions such as cloud cover and low wind speeds can limit photovoltaic and wind power output.4–6 Thus, an effective and economical energy storage technology is needed to supply sustainably produced electricity without interruption.7–9 To counteract the irregularities in power output, cost-effective energy storage devices such as electrochemical batteries have been coupled with renewable energy sources to balance loads for full-time operation; such systems exhibit high round-trip efficiency.10–12 However, despite their cost-effectiveness and round-trip efficiency, batteries are not suitable for long-term operation due to their low efficiency and self-discharge; they can supply electricity for only a few days without being recharged.13–15 In addition, due to so-called battery health issues, they require frequent maintenance, and exhibit highly operation-dependent lifetimes (1–10 years) and low gravimetric energy densities (108–144 kJ kg−1). Clearly, a more suitable technology for uninterrupted power supply is required.16,17Recently, tremendous research and development efforts have been undertaken to develop alternative energy storage technologies for uninterrupted power supply. Storage of the produced energy as hydrogen has been proposed, as hydrogen is an excellent energy carrier that can be used for both energy harvesting and supplying an energy load.18,19 Regenerative fuel cells (RFCs) are an interesting potential hydrogen storage technology. RFCs are electrochemical devices that can store and convert energy using H2 as a flexible energy carrier; this technology is based on recent developments in fuel cell technology.20–22 These devices have the potential to provide low-loss storage on a season-to-season basis with a much longer lifetime than batteries. An RFC consists of an electrolyzer stack and a fuel cell stack; in many RFC designs, these stacks are implemented as separate devices. This sophisticated system allows for efficient energy storage and conversion. The role of the RFC system is to store energy by converting electrical energy into chemical energy (i.e., by producing the fuels hydrogen and oxygen); the energy stored in the reservoir as fuels can later be re-converted into electrical energy.
RFCs combine electrochemical storage and conversion, and can thus produce clean energy efficiently.23 Similarly, to batteries, they can effectively store and convert chemical energy into electrical energy. However, conventional batteries can only store <200 W h kg−1, while RFCs can store up to 400 to 800 W h kg−1.24 RFCs can also be charged and discharged without affecting their durability. RFCs have already been applied in many fields, including aerospace, aviation, grid supplementation, transportation, and military fields, and their coupling with wind and solar energy production has been proposed. Hence, RFCs could be used widely in electric vehicles, uninterrupted power supplies, and onsite energy storage systems for solar rechargeable vehicles.25
1.1 Unitized regenerative fuel cell
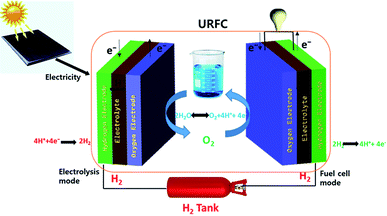
The unitized regenerative fuel cell (URFC) is a simplified version of a RFC in which both the electrolyzer and fuel cell stacks are combined into a single-unit electrochemical device, providing space and cost advantages.26 The specific energy is much higher in URFC which is 0.4–1.0 kW h kg−1 including the mass of the hydrogen and oxygen gas tanks. Further, URFCs can be totally charged and discharged without suffering the endurance compared with secondary batteries. The entire URFC is operated in one of two different modes: electrolysis cell (EC) mode or fuel cell (FC) mode.27 As its name implies, in electrolysis mode, water is split into H2 and O2 using electricity. The produced H2 can be stored in various forms, such as compressed gas cylinders, liquid organic hydrogen carriers (LOHC), or metal hydrides.28 The stored hydrogen is consumed to generate electricity on-site in “fuel cell mode” when the demand for electricity exceeds its production.Preliminary research into URFCs focused mainly on the development of the gas diffusion layer (GDL), flow field design, and operation under pressurized conditions.29–31 Various materials and modifications were tested in GDL development, and graphitized carbon,32 thin porous titanium metal with corrosion resistance,33 the metallic ceramics TiC and IrTiOx,34 foamy titanium,35 and carbon paper sprayed with IrO2 were found to be in particularly suitable for use as URFC GDLs. Other researchers investigated the design of the flow field, which is essential to both FC and EC mode operation.36–38 Serpentine,39 parallel,40 and mixed flow fields41 have been designed with the goal of providing suitable water management and enhancing URFC performance. Bhosale et al.40 also investigated the influence of the operating pressure on URFC performance. Thus far, significant progress has been made in research of these aspects of URFCs.
Despite the continuing research into URFC technology, the round-trip efficiency of URFCs is still lower than that of secondary batteries,26,42 mainly due to the water issues that occur when switching from fuel cell to electrolysis mode and vice versa.43 Other complications include the effects of water flooding, water starvation, and water transport between the components during fuel cell and electrolysis operation.44
1.2 Critical issues associated with conventional URFCs
As mentioned earlier, URFCs alternate between operation in fuel cell (FC) mode and electrolysis cell (EC) mode; several complications occur in the cell when the mode is changed. Mode switching is fundamental to URFCs, and is a major factor affecting their performance.44 The distribution of gases and water to the URFC is different in the different modes. Switching the operation mode also changes the mass and heat transport associated with the electrochemical reactions. In FC mode, mass transport is limited by the formation of the by-product water; while in EC mode, the feed side (the side at which the oxygen evolution reaction takes place) is filled with water, which also leads to mass transfer limitations.45 The production of water on the cathode side in FC mode can lead to water flooding, which negatively affects performance. On the other hand, after EC mode, water remains on the anode side.46 When the mode is switched from EC to FC, the water will block the distribution of the feed gas H2 to the anode.The excess water accumulated as a result of flooding in FC mode can affect the gas diffusion layer (GDL), and flow field plate (FFP) channel flooding reduces gas permeability, which in turn affects the limiting current density.47,48 Water transport also occurs due to the local pressure and concentration gradients, with localized fuel starvation occurring due to the mass transfer limitation.47,48 This phenomenon causes carbon corrosion on the BPP, which reduces performance. Consequently, the liquid water flooding that occurs if water is not removed periodically from the cell (from the cathode in particular) will lead to unpredictable, unreliable, and unrepeatable URFC performance under nominally identical operating conditions.47 On the other hand, removing the excess water from the cell can result in membrane drying, which reduces the performance of the URFC significantly.49 Mass transfer limitations will eventually lead to cell starvation and complete shutdown of the cell.50 Thus, while managing the membrane humidity to ensure good ionic conductivity is of foremost importance, the excess water that builds up must be removed from the cathode side.51 Accordingly, water management is a delicate task, as either an excess or deficiency of water can adversely impact the performance and lifetime of the URFC.43 Effective water management to maintain an appropriate balance between membrane humidity and the removal of the excess water produced in fuel cell mode is one of the most important issues in URFC design.
Maintaining the ideal water balance during dynamic operation has posed a significant challenge for URFC design and operation.52 Elaborate experimental diagnostics and schemes must be implemented to achieve consistent results, resulting in lower performance and limiting current densities.53 This paper provides an overview of the underlying phenomena linked to water management and some characterization methods and strategies to prevent their occurrence.
1.3 Layout
This review article is structured as follows. Section 2 describes the theory behind URFCs and the basic principles involved. Section 3 discusses the significance of water issues, including their effect on the performance of URFCs. Section 4 summarizes conventional water management technologies and the various efforts that have been made to improve the performance of URFCs. Section 5 presents a proposed URFC model to address these critical water issues. Section 6 gives a summary and outlook of the state-of-the-art of water management technologies for URFC systems. Finally, recommendations to improve water management and suggestions for future research and development are given in Section 7.2. Principle of the URFC
The unitized regenerative fuel cell is an electrochemical device that can operate in either EC mode or FC mode.54 URFCs split water into hydrogen and oxygen in EC mode and produce electricity and water by combining the produced hydrogen and oxygen in FC mode. As in any electrochemical membrane process, the membrane electrode assembly is the key part of the device. Therefore, the membrane electrode assembly (MEA) should be described before further details of the URFC are discussed. In this review, we specifically consider the proton exchange membrane (PEM)-URFC.2.1 Membrane electrode assembly of a URFC
The MEA of a URFC is constructed from the components described below.55 In general, the PEM Nafion is used as a polymer electrolyte. The anode and cathode catalysts are typically IrRuOx and Pt, respectively. Porous Ti and graphite are used as the anode and cathode GDL. The entire MEA is housed between the two GDLs, and the complete set-up is placed between the Ti and bipolar plates, which are grooved to create a serpentine flow field. The oxygen side of the MEA is supplied with water to hydrate the membrane for better conductivity. The performance of the URFC is measured via charging and discharging cycles. During charging, the electrolysis process takes place; during discharge, the fuel cell process takes place. The charging (electrolysis) process is carried out using a constant voltage and constant current, and the discharge (fuel cell) process is carried out using a constant current. The polarization curves obtained are used to measure the performance of the URFC. The duration of the charging and discharging process can be changed according to the capacity of the storage medium. Fig. 1 illustrates the PEM-URFC components, and their reactants and products.2.2 Operating principle of URFC
| Anode: H2O → 2H+ + O2 + 2e− | (1) |
| Cathode: 2H+ + 2e− → H2 | (2) |
| Overall reaction: H2O (l) → H2 (g) + O2 (g) | (3) |
| Anode: H2 → 2H+ + 2e− | (4) |
| Cathode: O2 + 2H+ + 2e− → H2O | (5) |
| Overall reaction: H2 + O2 → H2O | (6) |
3. Water management issues associated with URFCs
As discussed above, four different electrochemical reactions are performed in a URFC: the OER and HER in EC mode, and the HOR and ORR in FC mode.58 Water is involved in all four of these electrochemical reactions. The performance of the system during both electrolysis and fuel cell operation is fundamentally linked to the transport of water in the electrochemical cell.59 The most common water transport problems during fuel cell and electrolysis operation are water flooding and water starvation.3.1 Water flooding and starvation
During FC mode, water is produced in the URFC at the cathode interface as a by-product of the oxygen reduction reaction (ORR). The produced water must be immediately removed through the gas diffusion layer to the cathode flow channels, or it will migrate from the cathode to the anode by back-diffusion.59 Such flooding causes a sharp increase in the ohmic loss due to wettability and pore size distribution. Residual water in the gas diffusion layer also hinders the flow of the reactant gas to the active sites and reduces the availability of the active catalytic sites. The loss of apparent catalytic surface area directly increases the cathodic ohmic potential at higher current densities. Thus, the overall performance of the URFC is negatively affected by water flooding.47Water starvation is another serious issue, and is a major cause of URFC failure.60 Water starvation leads to the absence of the reactant H2; due to the unavailability of H2 at the anode at the proper time, the carbon on the BPP begins to oxidize, and carbon corrosion may occur. The H2 cannot be oxidized by the water electrolysis to provide the required protons and electrons for the oxygen reduction reaction at the cathode. Water starvation mainly occurs under harsh operating conditions, such as sub-zero start-up and rapid load change with high fuel utilization. The reactions involved are the following:61
| 2H2O → O2 + 4H+ + 4e− | (7) |
| C + 2H2O → CO2 + 4H+ + 4e− | (8) |
Corrosion of the carbon GDL leads to the loss of platinum particles from the anode due to the weakness of the carbon support. The weak attachment between the platinum particles and carbon support also decreases the electronic conductivity of the catalyst layer. Finally, thPt clusters on the catalyst layer lead to severe reduction of the URFC performance. Fuel cell starvation is one of the main causes of early failure of PEM-URFCs.62 The main cause of water starvation is misaligned flow distribution due to poor design of the flow field, stack, and structure.63
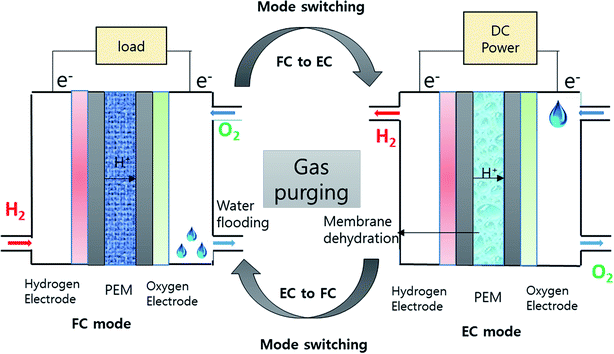
3.2 Mode switching
Switching between operation modes, i.e., from EC to FC mode or vice versa, is a unique and fundamental process in URFCs. In principle, switching the system between modes should not present any difficulties. The reactants and the products are different for each mode in the same electrochemical cell. Thus, when the operation mode is switched, the reactant and products are changed. In electrolysis mode, the reactant is water and the products are H2 and O2, while in fuel cell mode, H2 and O2 are the reactants and water is the product. When the system is switched from EC mode to FC mode, the H2 and O2 supplies are turned off, and both sides are purged with N2 gas to remove the residual liquid water remaining in the channels and the moisture on the cathode, where water flooding occurs during fuel cell operation.64 Likewise, when switching from EC to FC mode, the water supply to the anode is turned off and H2 and O2 are removed from the flow channel; gas purging is used to remove residual water on the anode side. The gas purging is carried out before switching from EC mode to FC mode to remove the residual liquid water remaining in the channels, GDLs, and membranes after operation. The removal of water from the anode and cathode helps to ensure smooth operation of both FC and EC mode.653.3 Effects of gas purging
During the gas purging step of mode switching, EC or FC operation is stopped to remove the retained water in the cell.59,66 The drying operation should completely remove water from the GDL and the membrane. The membrane, as well as the channel, GDL, and electrode, must be dried by the gas purge to prevent ice formation due to residual water in the membrane. This gas purging leads to dehydration of membrane and brittleness of the membrane.3.4 Membrane dehydration
The membrane must be hydrated for efficient proton conductivity. Protons move through the hydrated parts of the ionomer.67,68 The proton conductivity has been reported to be higher in wet conditions due to free movement of protons in the hydrated parts of the ionomer. The conductivity of a fully hydrated proton exchange membrane is 300% higher than when it is dry. Purging dries the membrane; in its dry state, protons cannot migrate across the membrane and its conductivity is decreased.68Membrane dehydration is a serious issue in URFC operation, and special attention is required to avoid this problem. During FC operation, dehydration generally occurs at the anode side.69 The membrane dehydration can originate from three different causes:70 (1) Insufficient hydration when the cell is fed with low humidity or dry reactant gases; (2) at higher current densities and temperature, formation of water at the cathode, will lack in water due to electro osmotic drag. (3) At high current density, back-diffusion is not sufficient to keep the membrane hydrated. Sharp increases in current density are another reason for membrane dehydration, as the electro-osmotic force pulls water molecules from the anode to the cathode.71
When the membrane is dehydrated, its pores shrink, which increases the rate of back-diffusion and leads to poor thermal management.72 In addition, the low ionic conductivity of the dry membrane inhibits the access of protons to the catalyst surface, so the number of possibility of reacting sites of interphase is reduced in the three-phase boundary layer, thus increasing the activation overpotential.73 Another major effect of membrane dehydration is decreased electrical conductivity, which increases ionic resistance and ohmic potential.74 Thus, a significant voltage drop and temporary shutdown of the URFC will occur. However, this temporary voltage drop can be recovered by rehumidification of the membrane. The recovery is strongly dependent on the thickness of membrane and the water diffusion coefficient. However, dehydration of the membrane for a prolonged period can lead to its permanent destruction.75 A dehydrated membrane can become brittle and form cracks, allowing the product and the reactant to come into contact with one another, which in turn leads to an undesirable reaction that forms hot spots in the membrane. These hot spots allow gas crossover and result in pinhole formation, which increases the crossover.70 Once the membrane has been dehydrated for a prolonged period, the destructive cycle will be initiated.76 Overall, membrane dehydration shortens the lifespan of a URFC. Fig. 2 shows the relationship between membrane flooding and membrane dehydration.
Notably, many researchers have investigated the development of water management techniques such as the use of polymer wicks in the storage system and modifications of the gas diffusion layer (GDL) and flow field plate (FFP) to improve water transport. Electroosmotic pumps have been used in PEM fuel cells, but have not been tested in URFCs. The next section describes in detail the efforts that have been made to improve overall URFC efficiency by improving water management in these devices.
4. Current water management strategies
As discussed earlier, water management in URFCs is addressed using gas purging or high heat vaporization to directly force water out of the system.77 Although these active management schemes are robust, they require additional energy and reduce the system reliability; parasitic losses associated with these techniques can reduce the net power output by as much as 35%.78 In order to avoid such losses, several alternative water management techniques have been demonstrated. Many water management systems have been designed. Various water management techniques, such the use of polymer wicks with a water storage system, modification of the GDL and FFP, the incorporation of a long channel length to induce large pressure gradients,79 the use of different channel geometries,78 and the removal of water by electro-osmotic micro-pumps,80,81 have been proposed.4.1 Polymer wicks
A passive internal water management strategy in which polymer wicks were used with a water storage system (WSS) was developed for PEM-based URFCs to improve round-trip efficiency.82 Polymer wicks are polymers that have the ability to absorb, desorb, and hold water.83 The polymer wick concept was adapted from PEMFCs.84 Polymer wicks were implemented in a URFC by Lele et al.85 The polymer wicks transport the water away from the MEA to a storage reservoir. The polymer wicks are mounted or directly engineered on the channel surface of the flow field to avoid the formation of liquid slugs.86–88 This new system uses the water removed from the device by the polymer wicks. Strickland et al.88 demonstrated that such a wick system could be successfully mounted in a PEMFC and could provide stable performance with a peak power density of 0.68 W cm−2 at low reactant delivery rates. Lele et al.89 developed a single-unit prototype regenerative PEM-URFC equipped with polymer wicks and a water storage reservoir. The key element of this technology is the polymer wicks with controlled pore size. The polymer wicks are linked to the water storage system (WSS), and passively transport water away from the cathode catalyst sites. The polymer wicks and the water storage system are employed in both EC (charge) and FC (discharge) mode, although with different roles.During EC mode (charging mode), the hydrogen generated by the forward PEM reaction (HER) at the cathode is absorbed by the capillaries of the polymer wicks and stored in the WSS, which acts as reservoir during EC operation. The passive transport scheme was designed and fabricated on the cathode flow field plate of the URFC. During discharging mode, the water produced at the catalyst sites is wicked away towards the water storage structure (WSS). During charging mode, water consumption at the catalyst sites drives the capillary flow away from the WSS. The role of the wicks during charging and discharging operation is depicted in Fig. 3.
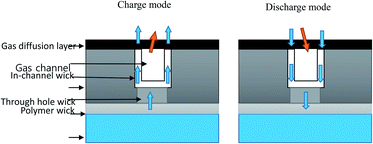 | ||
| Fig. 3 Schematic of the use of polymer wicks for improved passive water management (cross-sectional view). | ||
In principle, the driving force of water transport in the polymer wicks is the capillary pressure gradient across the two porous domains. In FC mode, the water is transported through the pores from the water formation sites at the cathode to the WSS through to prevent flooding of the channel. In EC mode, the water is pulled backward through the polymer wicks to the catalyst active sites for the production of hydrogen and water.
The integration of a wicking polymer in the URFC resulted an insignificant performance improvement, and flooding still persisted in even the medium current density range. The initial design of the polymer wicks and WSS by Lele et al.89 resulted in poor management of the water produced in the fuel cell; the major reason for the insufficient water removal was the long length of the channel. Due to the long channel length (12 mm) and transportation distance, water could not be completely removed. Lele et al.89 subsequently fabricated and implemented another short capillary with a channel length of 3 mm. Research into improved wick performance has also focused on high-resolution (∼1 μm) structures with well-defined geometries and minimal bubble formation. Lele et al.89 developed a fabrication process for porous polymer wicks in the originally proposed hydraulic via designed by utilizing a photoresist mold and bottom sealing plate. However, despite this advanced fabrication technique and the reduced channel length, the wicks still resulted in poor water management. The system was assumed to suffer from higher contact resistances based on the repeatability of the open circuit voltage. This suggested that the wicks alone could not transport all the produced water under certain operating regimes, possibly due to an insufficient gas pressure gradient across the capillaries.87,88
Further optimization of the porous polymer fabrication process and system design are still required for the successful implementation of polymer wicks and WSS. From an experimental point of view, comprehensive experiments could be performed to determine a mold release strategy applicable to ultra-thick photoresist structures (≥500 μm). Improved design of the polymer wicks and WSS and detailed system compression analysis could be helpful to achieve successful water management.
4.2 Gas diffusion layer
One of the main components of the URFC is GDL. The GDL is normally located between the catalyst layer and flow fields. The role of the GDL is to transport the reactant to the reaction site and extract the product while transferring electrons.The GDL plays three major roles in an URFC:30
(1) It provides a path for the reactant and products. During electrolysis operation, it helps to bring water to the catalyst layer from the flow field, and in fuel cell mode, it transports H2 and O2 from the flow fields to the catalyst layer. The GDL helps to distribute the reactant and the product evenly, especially under the ribs of flow field. The GDL also has an excellent ability to remove the product and transport it from the catalyst layer to the flow channel.
(2) It helps to transport to electrons between the flow field and the catalyst layer, and also provides a thermal pathway to transfer heat from the heater in both EC and FC mode.
(3) It provides additional structural strength to allow the MEA to withstand the pressure differentials in the EC and FC modes.
Switching from EC mode to FC mode is a challenging task because the residual water must be removed from the GDL and flow channel, but the membrane must remain hydrated. The gas purging applied when the mode is switched affects various properties of the GDL, such as its permeability, porosity, surface contact angle, electrical and thermal conductivity, mechanical strength and durability, porosity, and thickness.90 However, limited research is having been published so far regarding the role of the GDL in water management. In this section, we will discuss the effect of different properties of GDLs and possible treatments to improve the GDLs in terms of the purging characteristics when switching between modes.
One important drawback of the GDL in the URFC is the corrosion of the GDL in EC mode. Hence, it is worthwhile to investigate suitable materials and compositions for micro porous layers (MPLs) that can be applied to metal-based GDLs and to study their impact on the roundtrip efficiency (i.e., both FC and EC mode performance). MPLs can serve three vital roles in a URFC, namely, reducing contact resistance, providing effective water management, and hindering the loss of the catalyst layer.29
Selecting a GDL is challenging, because different GDL thicknesses are ideal for FC mode and EC mode. The thickness of the GDL can affect its mechanical properties and thus its support for the membrane, which is of great importance in URFCs. The thickness also strongly affects water management in the URFC. Thicker GDLs provide longer diffusion paths and lower permeability; however, they are more affected by flooding in FC mode and lead to higher thermal and electrical resistance. On the other hand, thinner GDLs offer high electrical conductivity and are suitable for higher humidity and current densities. Thinner GDLs are preferred for lower current densities and to maintain the hydration of the membrane.
Hwang et al.91 investigated the effects of the properties of GDLs incorporating Ti on URFC performance. Fig. 4 shows SEM images of GDLs with Ti fiber and sintered Ti powder. Their major objective was to understand the effect of the pores between the fibers. GDLs with larger pores can transport large amounts of the reactants with higher humidity and improve their distribution. Uneven pore diameter in the GDL improves water management in FC mode. The Ti particles used for were larger than the particles of the carbon support. The Ti coating on the GDL forms an oxide layer on the surface, which increases contact resistance and reduces performance. The use of corrosion-resistant materials such as TiN might avoid this problem; however, further investigation into the preparation and characterization of these materials is required.
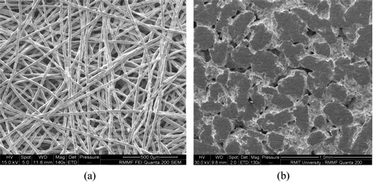 | ||
| Fig. 4 SEM images of sintered titanium fiber (a) and sintered titanium powder (b) GDLs.29 (This figure has been adapted from ref. 29 with permission from the Elsevier, copyright: 2019). | ||
Recently, a bimodal pore size distribution was proposed to improve the performance in FC mode.92 The effect of the bimodal pore size on the collection and removal of liquid water through the GDL was studied. The parts of the GDL consists of small hydrophobic pores (which should be ideally as small as possible) that are not filled with water and provide a pathway for the reactant gases.93,94 A moderate pore size gradient was found to beneficial for the removal of excess product from the catalyst layer. Sadeghifar et al.95 proposed an analytical model for the prediction of the thermal contact resistance between fibrous porous media and flat surfaces that is applicable to FC mode. However, this model did not take into account the effect on the URFC performance or water management. Generally, GDLs are subjected to pretreatment procedures or chemical modification of their structure to improve the URFC performance and water management. GDLs are commonly coated with Teflon and modified with an MPL on both sides. Most of these treatments enhance the water removal ability of the GDL and its ability to supply the reactants to the active area. Lettenmeier et al.96 found that loading Ti particles on the MPL of GDLs improved EC mode performance. Their research showed the MPL reduces mass transport limitation and achieved higher current densities. Water management at the higher densities of the EC mode improved; however, lower porosity was required close to the flow field and larger porosity was required for the effective removal of O2.
Overall, the round-trip efficiency of a URFC can be improved by improving the properties of the GDL. The porosity and fiber diameter of the GDL have a significant influence in both EC and FC mode. Treatments such as PTFE coating, Ti powder loading, and Pt coating of the GDL and the MPL significantly enhance the performance of the URFC in FC mode.97 However, the relationship between the properties of the GDLs, such as their permeability, diffusivity, and electrical and thermal conductivities, and their effects on URFC performance in EC and FC mode still need to be evaluated.
4.3 Design of flow channel
The bipolar plates in URFCs act as separators and current collectors, and promote even distribution of the reactants.98 They also provide structural support to the URFC stack and aid in heat and water management in the URFC. The bipolar plates should exhibit corrosion resistance in the URFC environment, low interfacial contact resistance (ICR), low surface energy, low gas permeability, light weight, good mechanical strength, and cost-effectiveness.99–101 As mentioned earlier, the flow channels are built on the bipolar plates. The most common and widely used layout for PEM-URFCs is the serpentine flow channel design,102 as it provides good performance and durability.103 The recent numerical modelling of serpentine flow fields has shown considerable cross-flow leakage between adjacent channels due to the pressure gradient along the channel direction. The design of the flow channel, which is responsible for the better overall cell performance achieved when using the serpentine flow channels.The flow field is a factor that directly affects the water management performance of the URFC.104 It is one of the factors that influences water flow and distribution during FC mode and EC mode. Modification of the flow fields on the bipolar plate (BPP) has been investigated to improve water management.38,105 However, at current, only limited reports of modification of the flow field on the BPPs of URFCs are available. Research into the design of the flow channel has taken into account the channel layout, configuration, cross-section, and length and their effects on the water removal performance and overall performance of the URFC. Experimental results have demonstrated that using the serpentine flow channel design, the water flooding phenomenon is effectively prevented, and the cell performance is reliable and repeatable under the same nominal operating conditions.106 In recent years, some research groups have attempted to image water transport within fuel cells.107 Weng et al. developed a transparent proton exchange membrane fuel cell in order to visualize the distribution of water and water flooding inside the cathode gas channels. The potential of water electrolysis is usually in the range of 1.6–2.0 V (vs. NHE), and it is conducted in an O2 atmosphere and at a pH of 2–4. The type of flow in the channel is another factor that determines the efficiency of the URFC. Turbulent flow is favorable for mass and heat transport; however, it leads to higher parasitic loss due to the high pressure drop. On the other hand, a typical laminar flow is more favorable for achieving sufficient mass transport of the reactant gases into the electrode for FC and EC mode electrochemical reactions under the most extreme fuel cell operating conditions (high current densities). Thus, it important to design the flow field to achieve laminar flow of the anode and cathode reactant gases.108 In flow field design, the relative pressure drop of the flow field must also be considered so that water can evaporate and be removed during gas purging. On the other hand, the membrane should remain fully saturated during purging in order to maintain its conductivity.
Another research group79 designed and tested innovative flow channels in order to improve water management in a PEMFC. Their design involved channels slanted 20° downward to collect the liquid water that permeated from the GDL. This modification the flow field improved the hydration of the membrane and the pressure difference between the anode and cathode. In typical flow fields, the flow channels have rectangular or square-shaped cross-sections. In their investigation, the flow channel was modified into a slanted plate with trapezoidal grooves on the anode and cathode side. These slanted plates collect the permeated gas from the gas diffusion layers. The water is segregated into the bottom of the cell, and excess water is removed from the GDL by gravity. Better water management was achieved in certain orientations, i.e., an upward-slanting orientation for the cathode and a downward-slanting orientation for the anode. Downward-slanting channels at the anode side showed better performance than at the cathode side. The downward-slanting orientation at the anode increased the back-diffusion of water from the cathode to anode without increasing the total pressure gradient between the anode and cathode, which increased the hydration and thus the conductivity of the membrane. The performance of the URFC was improved by the downward-slanting orientation of the channels at the anode under extremely wet conditions and increased the power to a level comparable to that achieved without the modified flow channels under less wet conditions.
The BPP must be anticorrosive for efficient operation. For this reason, Ti is generally coated on the BPP to improve its corrosion resistance and mechanical strength.105 However, Ti is very passive and forms an oxide layer during electrolysis. This oxide layer increases the ICR and thus lowers the overall performance of the URFC. Recently, Jung et al.38 investigated the performance of carbon-based bipolar plates in URFCs. They also investigated the use of films of the transition metals Au and Pt on BPPs. They reported that the performance of the URFCs was satisfactory; however, the electrochemical properties of the coated materials were not reported.
In another study, BPPs were coated with a Ti–Ag film using pulsed bias arc ion plating technology109 and evaluated in a URFC. This technique was expected to improve the interfacial conductivity of the silver and of the modified BPP of the URFC. Pristine Ag-deposited Ti plates were used as reference materials. The URFC environment was simulated for the investigation. The treatment led to greatly improved performance, with ICR values as low as 4.3 mΩ cm−2 at 0.14 MPa. The simulated results demonstrated anticorrosion properties even at high potential. The contact angle between the Ti–Ag and Ti sample was 107°, which is favorable for water management in the URFC. Thus, Ti–Ag coatings are cost-effective and more environmentally friendly. Ti coating improves interfacial conductivity, corrosion resistance, and surface energy.105 However, its long-term corrosion properties must be investigated for commercialization. Further improvements to its structure, design, and process optimization are also required.
Mittelsteadt et al.110 developed composite plates for closed-loop fuel cell/electrolyzer power systems to address the water management issues. This novel BPP design avoids the need for external humidification and water/gas separation. The research group investigated a water permeable electrically conductive plate (WPECP) composite bipolar plate for URFCs. The WPECP exhibits higher electrical conductivity and water transport properties and lower gas permeability. When placed together with the separator plate, it forms an empty cavity. In EC mode, water fills the cavity, which helps to cool the stacks, and also acts as the reactant for the electrolysis operation. The water travels across the WPECP via the water gravity gradient to act as a reactant for electrolysis. In FC mode, the water produced as a product is continuously removed from the cathode chamber by a vacuum. The entire surface of the BPP helps to remove water when the system is switched between EC and FC mode. The FC mode allows the dead end H2 and O2 to feed. In this way, it avoids the peristatic pump losses that are often associated with the removal of the product water in URFCs.
The performance of the URFC with the WPECP was tested at 80 °C. Fig. 5 shows a schematic of the URFC build used in electrochemical testing (the top end plate is excluded on the right). The electrolysis operation was carried out at a current density of 400 mA cm−2, which is lower than that used during normal electrolysis operation. At this current density, the PEM dried out and was not able to carry oxygen to the electrode quickly enough. Evidence of flooding was observed under FC mode. The round-trip efficiency of the URFC was calculated at 300 mA cm−2 and found to be around 50%. Above this current density, the FC was heavily flooded at the cathode.
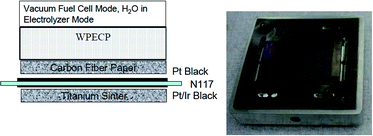 | ||
| Fig. 5 Schematic of the unitized regenerative fuel cell build used in electrochemical testing (top end plate excluded at right).110 (This figure has been adapted from ref. 110 with permission from the Electrochemical Society, copyright: 2005). | ||
This novel BPP design addresses water management, which is a major challenge in unitized systems, and the ratio between weight and volume allows an external stack to be avoided. It also has the advantages of being able to use water vapor as a reactant in EC mode and helping to protect from metal cation contamination.
4.4 Water electro-osmotic pump
In addition to these major water management techniques, several other methods have been used in PMFCs but not URFCs.80 Since research and development into URFCs is still at an early stage, water osmotic pumps have not yet been used in URFCs. However, in order to reduce parasitic losses, an electro-osmotic pump (EO) was implemented as a water management system.This technology avoids the high pressure gas purging process by integrating a low-power pump to directly remove water from the cathode channels and GDLs of PEMFCs.81 It is an active water management system that uses a hydrophilic porous cathode flow field with an electro-osmotic water pump for the removal of water, as shown in Fig. 6. The porous carbon cathode flow field is coupled with an electro-osmotic (EO) pump for water removal from a 25 cm2 PEMFC. This system involves pumping liquid water, which allows for low air flow rates, low air pressure, and parallel cathode architectures. The relatively low air flow rate leads to stable operation, which is an additional benefit of this method. The cell segmentation method was used to find the local current density of the PEMFC; this methodology can be used to reveal localized phenomena including oxygen depletion at the cathode and flooding events. The system was found to be consistently free of flooding even at an extended current density operation range and air stoichiometries as low as αair = 1.3. The study revealed the mechanisms and dynamics associated with EO pump-based recovery from catastrophic flooding. The EO pump required <1% of power consumption FC mode to recover from near-catastrophic flooding.
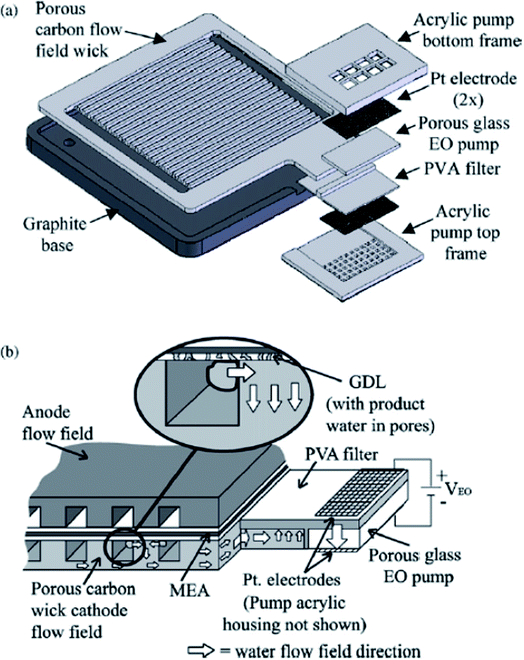 | ||
| Fig. 6 Exploded assembly view of the water management system on the (a) cathode-side featuring an EO pump, and (b) a schematic of water flow through the porous carbon wick to the EO pump.80 (This figure has been adapted from ref. 89 with permission from the Elsevier, copyright: 2007). | ||
Another method reported to improve water management is coupling the EO pump with the cathode flow fields.111 In this study, the machined cathode flow fields were thermally treated to increase their hydrophilicity. This porous carbon layer was placed on the non-porous plate, which exceeds out the MEA by 1 × 2 cm. This wick was coupled with a micro-osmotic pump. The micro-osmotic pump was placed above the cathodic region in the direction of the main peristaltic pump. The micro-osmotic pump consisted of an acrylic frame, mesh platinum pump anode, hydrophilic polyvinyl alcohol (PVA) filter component, porous borosilicate glass frit, mesh platinum pump cathode, and second acrylic frame.
In this system, the PVA links the porous carbon wick and the filter component, which helps to isolate the pump, and serves as a filter to prevent particles from entering. The electrodes are attached to the lead by wire clamps. The acrylic frame provides additional structural support to the MEA. During drying operation, water is removed from the cathode channel and GDL. A vacuum is created by the pressure differential generated by the EO pump. This vacuum removes the water from the fuel cell wicks; this water is pumped out by the external electro-osmotic pump. Segmentation techniques have been developed to evaluate the effects of gas evolution and mass transport losses by current mapping. The examination of partial MEA, sub-cells, and current mapping using segmentation was first developed by Stumper et al.112 This segmentation technology was adopted for the examination of the water management system. A printed circuit board was segmented between the anode circuit flow field and the current collector. An integrated system was used to link the electro-osmotic pump and the hydrophilic porous carbon flow field. The authors found that the flooding-free operation enabled the use of parallel cathode flow channels at lower concentration. In addition, water transport in the wick and pump system were examined by studying the steady-state and transient current densities in nine segments, and no evidence of flooding was found using the EO pump.
As flooding results in non-uniform flow, it produces an averaged current density. It was identified by determining which cell had the best performance in a partially flooded condition due to the fact that flooding losses intensify moving downstream. The inner channel showed better performance than the outer channels due to the thermal gradient of the plane, which increases the vapor pressure of the interior channels.
4.5 Hydrogen absorbing storage system
Coupling of a hydrogen storage system to the URFC is another potential method of water management. In URFCs, the hydrogen produced in EC mode is typically stored in an external medium, such as a storage tank, metal hydride, or LOHC. The need for gases to exit and enter the system complicates water management. Thus, Andrews et al.113 attempted to design a URFC with an integrated hydrogen storage system to avoid the need to introduce or remove hydrogen from the URFC. The hydrogen produced by the electrolyzer was stored in ionic form for use as a reactant for the fuel cell. However, the selection of a suitable storage material proved to be a bottleneck in this investigation; the development of a carbon storage material that could electrochemically store hydrogen would be useful. Activated carbon and a Nafion polymer composite storage electrode was selected. A special URFC with integrated solid-state hydrogen storage was designed. The initial goal was to determine the feasibility of hydrogen storage electrode as a proof-of-concept.114 The fabrication of URFC and the flow fields for the water transport showed in the Fig. 7 and 8. | ||
| Fig. 7 (a) Prepared multi-walled carbon nanotube electrode; (b) 3D mold used for fabrication of the electrode.114 (This figure has been adapted from ref. 114). | ||
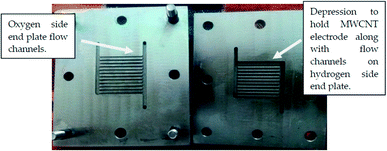 | ||
| Fig. 8 Photograph of the hydrogen- and oxygen-side end plates of the modified URFC.114 (This figure has been adapted from ref. 114). | ||
Before testing the device in a URFC, the composite material was scaled up and evaluated at different humidity levels to test the proton flow concept. The conductivity of the fully hydrated activated carbon and Nafion membrane was found to be in the range 0.04–0.11 S cm−1, which is considered to be a high value for proton conductivity. The high conductivity was attributed to the high water uptake of the composite material. The proton conductivity was found to increase with increasing relative humidity. The electron conductivity was found to be 0.5–2.3 S cm−1, demonstrating that this composite material can conduct both protons and electrons at the same time.
The electrode discharge value of the URFC with the storage electrode was found to be 0.67 mA h. It is relevant that at only 0.0002 wt% of hydrogen storage, this value is significantly to lower than that of other methods. The experiment was conducted using an hour of charge and discharge. During charging (electrolysis mode), the URFC was connected to a DC power source. Likewise, while discharging (fuel cell mode) the URFC was connected to an electrical load. Due to the chemical potential, the hydrogen bonds between the hydrogen atoms and the storage medium were broken, and the hydrogen was removed from the storage medium. During the HRR, the platinum black catalyst breaks the hydrogen atom down into H+ ions and electrons. The hydrogen ions travel towards the oxygen side through the PEM, and the electrons are transferred through electrical circuit, respectively, where they react with the oxygen produced during charging to reform water. The weight of the produced oxygen and hydrogen and the charging and discharging capacity in mA h g−1 were recorded and used to calculate the electrochemical hydrogen storage capacity of the fabricated MWCNT electrode.
Andrews et al.113 confirmed that the hydrogen can be stored in MWCNTs using the concept of a “proton battery”. The MWCNTs had a charging capacity of 670 mA h g−1 and the discharge capacity was found to be 661 mA h g−1. The equivalent storage capacities are 2.47 wt% and 2.45 wt%. However, the carbon cloth used as the GDL for the cathode and anode was found to be damaged by oxidation due to the fact that the carbon begins to oxidize at around 1.2 V. Thus, it is recommended to use porous titanium felt/frit as the anode GDL on the oxygen side.
5. Proposed novel hybrid AEM-URFC
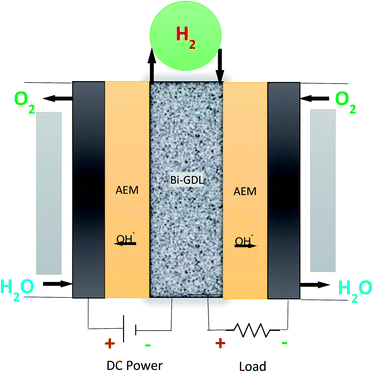
Recently, water splitting via a novel two-step electrochemical cycle was developed by several researchers.115,116 The half-cell reactions of water electrolysis, i.e., the OER and HER, were divided into two different reactions using a 3D intermediate electrode. A single electrochemical cell was divided by the 3D intermediate electrode, which acts as a cathode for the OER reaction and anode for HER reaction. The two-step reaction system drastically reduces the ohmic potential. In 3D particle electrodes, the reactions take place at the solid/liquid and solid/gas phase boundaries.117 This novel system has an intermediate electrode located between the positive and negative electrodes. The oxygen and hydrogen gas are generated separately through two electrochemical cycles in different chambers.In proposing a novel URFC design, we adopted this technology to avoid water management issues in the URFC. In addition to this, we propose the use an anion exchange membrane rather than a conventional (PEM) in the URFC. Tremendous efforts have been made to develop anion exchange membranes for EC and FC operation in order to replace the expensive platinum catalyst with a non-precious metal catalyst.118 For this reason, our design uses a novel 3D bi-GDL as an intermediate electrode and a pair of anion exchange membranes (AEM) instead of the conventional PEM.
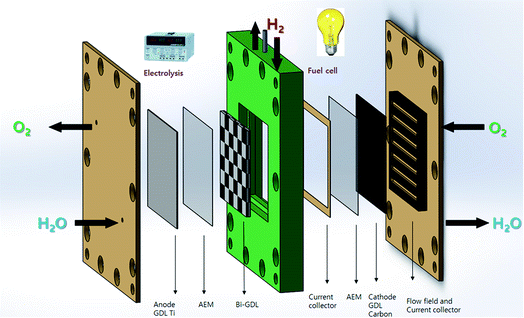
Our advanced novel bipolar GDL-assisted hybrid two step hybrid AEM-URFC design for research and development is shown schematically in Fig. 9. In this advanced URFC, the EC and FC compartments are separated as in a RFC. The electrolyzer and fuel cell chambers are separated by the bipolar GDL. However, the two units are connected by a single bipolar GDL. The bipolar GDL can act as a cathode for electrolysis and an anode for fuel cell operation. An intermittent current collector can be used to supply electricity to the electrolyzer and load to the fuel cell.
In EC mode, the OER and HER take place in the electrolyzer; likewise, in FC mode the ORR and HRR reaction take place in the fuel cell. This electrochemical device is divided into two separate chambers: the anode GDL membrane and bipolar GDL make up one unit, and the bipolar GDL, membrane, and cathode GDL constitute the other.
Because the EC and the FC operations are carried out in separate chambers, this hybrid URFC design eliminates the need for complicated switching between the EC and FC modes. Unlike in the conventional URFC, no reactant or product changes occur. Since the reactants and products are in separate chambers, there is no possibility of their cross-contamination. Purging is not required, which consequently avoids side effects such as dehydration of the membrane. The operating conditions of the EC and FC are different and can thus be easily achieved. Additionally, the simplified design of the hybrid URFC is more compact than that of the conventional DRFC.
An even greater advantage of this technology is that a Ti GDL can be used for electrolysis on the anode side, avoiding carbon corrosion on the bipolar flow field plates, while a carbon bipolar plate can be used for the HRR and ORR. Thus, in this URFC design, in the electrolysis section, Ti can be used as the anode GDL, and the flow field can be located on the fuel cell/ORR side. Hence, a graphite bipolar material can be used on the mounted flow field, providing efficient operation.
The URFC reactions could be carried out in two different ways, i.e., separated and simultaneous operation. We suggest that when the sunlight is available, simultaneous operation could be used. The hydrogen produced from the electrolysis reaction could then be partially stored in a hydrogen tank and partially supplied as a reactant to the fuel cell reaction. When sunlight is not available due to cloudy weather or at nighttime, the device could be operated as a fuel cell using the stored hydrogen as a reactant.
The removal of hydrogen as a product and its supply to the FC side as a reactant would be highly challenging. The advanced design of the URFC provides a solution to this issue; the hydrogen could be extracted from the middle of the device, as shown in Fig. 10.
Another complication would be using the intermittent current collector to supply electricity and electrical load to the bipolar GDL and membrane. A rectangular surface could be placed between the bi-GDL and fuel cell side membrane. This would ensure that the current collector was in contact with the bipolar GDL and not the membrane. This current collector would be connected to a Ni wire through a hole.
6. Summary and challenges
Research and development of URFCs is still in its preliminary stages. In this article, we have critically reviewed recent developments in water management techniques for PEM-based URFCs (since 2011). We have presented an overview of the state-of-art water management strategies developed by various researchers, and the pros and cons associated with these designs. Additionally, this review discussed conventional water management techniques and their drawbacks. New technologies, such as the implementation of polymer wicks, WSSs, modified GDLs, advanced sintered and mounted slanted flow fields, and porous cathode flow fields for the storage of H2 inside the URFC. Some technologies that have been used in PEM fuel cells but not URFCs, such as the use of an electro-osmotic pump to remove water directly from the GDL, have also been discussed. To date, the best round-trip efficiency was found for a URFC in which a water-permeable electrically conductive plate (WPECP) was used as the bipolar flow field, with a round-trip voltage efficiency of 50% at 300 mA cm−2. The overall performance of this water management technology is significantly higher than that of other water management technologies that have been reported.Gas purging is essential for switching the URFC from EC mode to FC mode and vice versa. The use of higher current densities in EC mode increases the amount of residual water on the hydrogen side, leading to a long purging time requirement for high start-up current density in FC mode. However, this reduces the performance of fuel cell operation. In conclusion, mode switching significantly affects the performance of both FC and EC modes. The water distribution will always be uneven at the beginning of electrolysis, so water starvation cannot be completely eliminated.
In conventional URFC systems, the development of methods to maintain the subtle equilibrium between the water flooding, gas purging, and membrane dehydration will be crucial for water management. However, this optimum balance depends on the reactant stream humidification, flow field layout, and structural wetting properties of the GDL and MPL. Extensive research has been carried out to eliminate the water management issues, including visualization of the liquid water distribution, prediction using numerical modelling, experimental measurements, and optimization of strategies for water management, including optimization of the operating conditions, cell system design, and the structure and material of the MEA. Polymer-wick-assisted URFC prototypes exhibited poor water management due to insufficient removal of water from the cathode due to the long transport length. The fabrication of polymer wicks and WSS also require further improvement to enhance water removal.
Several GDL designs for improved water management were also studied. The major problem in this area is the corrosion of the GDL due to its carbon-based layer. However, this can be alleviated by selection of an alternative material for the GDL. The use of a platinum gas diffusion layer would not only mitigate the corrosion of the GDL, but also help to improve the hydrogen and oxygen dissociation rates during FC mode.
Switching from EC to FC mode in a URFC is a challenging task, as the excess water must be removed by gas purging, but the membrane must also remain hydrated. The properties of the GDL, such as its porosity, thickness, and water vapor diffusivity, affect the purging requirements in terms of the flow field and diffusivity. Little investigation of the role of the GDL properties in gas purging has been reported; further investigation of the effect of the GDL properties on the purging characteristics during mode switching is recommended.
Most investigation into water management techniques has been carried out via experimental studies. Modelling and simulation of the component, stack, and transport phenomena of URFCs should be developed. This would improve the understanding of the different components, operating conditions, mechanisms of mass transport, and stack assembly, as well as their effects on the overall performance of URFCs.
Dedicated studies are still needed in order to develop effective water management strategies in URFC in both EC and FC mode. Systematic mathematical modelling and validation with the experimental results should result in improved designs with higher performance and lower cost.
7. Recommendations
Water management in URFCs is crucial, and it can be addressed in various ways to improve the overall round trip efficiency. Reducing parasitic power loss would reduce the cost and improve the stability of the overall system. Strategies to prevent water flooding should be developed, possibly involving the use of advanced materials to design novel porous GDLs, bipolar materials, and flow fields with individual channels for water and gas transport. The design and development of a novel URFC in which the reactants and products of the process do not need to be changed would also represent a promising method to completely eliminate the water flooding that plagues URFCs.We recommend that further research into water management should be focused within four primary areas:
Control of flooding and starvation
(1) A physical model for the role of the MPL with good water pore and good agreement should be developed. Additionally, the rheology of the flooding can be observed using environmental scanning electron microscopy (ESEM).
(2) These techniques would allow pore-network analysis of water transport in the GDL, which should help to demonstrate the water transport behavior and the hydrophobic behavior.
(3) Minimizing the pore size of the MPL on the cathode layer (CL) side reduces the saturation level of interfacial water droplets, but complicates subsequent collection of the liquid water from the CL. Thus, finding the optimum pore size is important.
(4) Application of a PTFE coating on the channel surface could be applied to reduce the interface and as a way to increase the liquid connectivity in the under-the-channel location.
Mode switching of between fuel cell and electrolysis operation
(1) In the beginning of electrolysis mode, a large inlet velocity should be applied to enhance mass transfer and improve cell performance. This large inlet velocity increases the amount of water available as a reactant and prevents water starvation at the oxygen catalyst layer. Thus, the performance of the electrolysis cell will be improved.
(2) The amount of water that should be pumped into the oxygen flow channel prior to EC operation decreases with increasing current density. Thus, in EC mode, a stable flow of water should be pumped into the oxygen channels.
(3) We recommend the application of pre-reactant switching to consume the water retained at the end of electrolysis mode by providing sufficient time between the reactant and current transitions, which may lead to smooth mode switching at low current densities. Increasing the oxygen flow rate also favors smooth startup of FC mode.
(4) The interval between FC and EC operation should be long, with a voltage increase before the electricity supply to consume the residual water. This method is more suitable for low-current-density FC mode startup, rather than high current density.
Improvement of existing polymer wick, GDL, and flow field designs
(1) Polymer wicks could be enhanced by using porous polymer fabrication and optimization of their design. Soft bake parameters, as well as mold-release strategies applicable to ultra-photoresist structures, should be developed and utilized. Prior to fabrication, the use of a semi-analytical model for numerical simulation is preferred, as is a short transport length.
(2) The water management of the URFC may be improved by improving physical properties of GDL. It is worth to find out advanced material and compositions for the micro porous layer. The metal based GDL has an impact on the round trip efficiency. Development of mathematical modelling with good agreement with the experimental round trip efficiency.
(3) Advanced materials and composition for MPLs that could be applied to metal-based GDLs should be developed, and their impact on the roundtrip efficiency should be studied. When Ti is used on the GDL, it formed an oxide layer at the surface, which increased the contact resistance and thus reduced the overall performance. Materials that are suitable for EC and FC operation, such as TiN, should be developed.80
Additionally, our novel URFC design eliminates the conventional product and reactant change of the URFC. Since the EC and FC half-reactions take place in different chambers, mode switching is not required. Different operating conditions favorable to the EC and FC could be applied in the respective chambers.
The performance of the URFC depends on water management in EC and FC mode. Electrochemical compression is another factor that affects the water management and water and gas balance; this could be improved by optimizing the component, cell, and stack design for high pressure operation; improvement of the membrane properties would be especially useful in this regard. In parallel to controlling flooding and membrane dehydration, the development of GDL components, advanced flow field design, improved switching operation, and novel URFCs with separate reaction chambers for EC and FC operation should be investigated.
Abbreviations
| AEM | Anion exchange membrane |
| BPP | Bipolar plate |
| EC | Electrolysis |
| EO | Electroosmotic pump |
| ESEM | Environmental scanning electron microscope |
| FC | Fuel cell |
| FFP | Flow field plate |
| GDL | Gas diffusion electrode |
| HER | Hydrogen evolution reaction |
| ICR | Interfacial contact resistance |
| MEA | Membrane electrode assembly |
| MPL | Micro porous layer |
| OER | Oxygen evolution reaction |
| MWCNT | Multi wall carbon nanotube |
| ORR | Oxygen reduction reaction |
| PEM | Proton exchange membrane |
| PEMFC | Proton exchange membrane fuel cell |
| PVA | Polyvinyl alcohol |
| RFC | Regenerative fuel cell |
| URFC | Unitized regenerative fuel cell |
| WSS | Water storage system |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Brain Pool Fellowship (2019H1D3A2A02102994) from the Human Resource Development and partially by the Basic Science Research Program through the National Research Foundation of Korea (NRF) from the Ministry of Education (No. 2019R1I1A3A03050441).References
- N. L. Panwar, S. C. Kaushik and S. Kothari, Renewable Sustainable Energy Rev., 2011, 1513–1524, DOI:10.1016/j.rser.2010.11.037.
- V. Khare, S. Nema and P. Baredar, Renewable Sustainable Energy Rev., 2016, 23–33, DOI:10.1016/j.rser.2015.12.223.
- P. Nema, R. K. Nema and S. Rangnekar, Renewable Sustainable Energy Rev., 2009, 2096–2103, DOI:10.1016/j.rser.2008.10.006.
- P. E. Bett and H. E. Thornton, Renewable Energy, 2016, 96–110, DOI:10.1016/j.renene.2015.10.006.
- N. Armaroli and V. Balzani, Energy Environ. Sci., 2011, 3193–3222, 10.1039/C1EE01249E.
- K. Juodkazis, J. Juodkazytė, E. Jelmakas, P. Kalinauskas, I. Valsi Unas, P. Miĕ Cinskas and S. Juodkazis, J. Opt. Soc. Am., 2010, 147–160, DOI:10.1364/OE.20.00A678.
- S. Weitemeyer, D. Kleinhans, T. Vogt and C. Agert, Renewable Energy, 2015, 14–20, DOI:10.1016/j.renene.2014.09.028.
- N. Kittner, F. Lill and D. M. Kammen, Nat. Energy, 2017, 17125–17130, DOI:10.1038/nenergy.2017.125.
- S. Becker, B. A. Frew, G. B. Andresen, T. Zeyer, S. Schramm, M. Greiner and M. Z. Jacobson, Energy, 2014, 443–458, DOI:10.1016/j.energy.2014.05.067.
- J. B. Goodenough, Energy Environ. Sci., 2014, 14–18, 10.1039/C3EE42613K.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 928–935, DOI:10.1126/science.1212741.
- T. M. I. Mahlia, T. J. Saktisahdan, A. Jannifar, M. H. Hasan and H. S. C. Matseelar, Renewable Sustainable Energy Rev., 2014, 532–545, DOI:10.1016/j.rser.2014.01.068.
- A. R. Dehghani-Sanij, E. Tharumalingam, M. B. Dusseault and R. Fraser, Renewable Sustainable Energy Rev., 2019, 192–208, DOI:10.1016/j.rser.2019.01.023.
- J. B. Goodenough and Y. Kim, J. Power Sources, 2011, 6688–6694, DOI:10.1016/j.jpowsour.2010.11.074.
- Z. Yang, J. Zhang, M. C. W. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 3577–3613, DOI:10.1021/cr100290v.
- F. D. Kong, S. Zhang, G. P. Yin, N. Zhang, Z. B. Wang and C. Y. Du, Electrochem. Commun., 2012, 63–66, DOI:10.1016/j.elecom.2011.11.002.
- W. Yao, J. Yang, J. Wang and Y. Nuli, Electrochem. Commun., 2007, 1029–1034, DOI:10.1016/j.elecom.2006.12.017.
- S. Ould Amrouche, D. Rekioua, T. Rekioua and S. Bacha, Int. J. Hydrogen Energy, 2016, 20914–20927, DOI:10.1016/j.ijhydene.2016.06.243.
- P. Hayes and J. Arevalo, ABB Rev., 2015, 43–49, DOI:10.1016/b978-0-12-416714-8.00006-8.
- K. Park, J. Lee, H. M. Kim, K. S. Choi and G. Hwang, Sci. Rep., 2014, 4592–4597, DOI:10.1038/srep04592.
- J. Andrews and A. K. Doddathimmaiah, in Materials for Fuel Cells, 2008, pp. 344–385, DOI:10.1533/9781845694838.344.
- S. H. Kim, O. J. Kwon, D. Hyon, S. H. Cheon, J. S. Kim, B. H. Kim, S. T. Hwang, J. S. Song, M. T. Hwang and B. S. Oh, Int. J. Hydrogen Energy, 2013, 8415–8421, DOI:10.1016/j.ijhydene.2013.04.020.
- X. Li, Y. Xiao, Z. Shao and B. Yi, J. Power Sources, 2010, 4811–4815, DOI:10.1016/j.jpowsour.2010.02.045.
- F. Mitlitsky, B. Myers and A. H. Weisberg, Energy Fuels, 1988, 56–71, DOI:10.1021/ef970151w.
- W. Smith, J. Power Sources, 2000, 86, 74–83 CrossRef CAS.
- Y. Wang, D. Y. C. Leung, J. Xuan and H. Wang, Renewable Sustainable Energy Rev., 2016, 961–977 CrossRef.
- M. Gabbasa, K. Sopian, A. Fudholi and N. Asim, Int. J. Hydrogen Energy, 2014, 17765–17778 CrossRef CAS.
- B. Paul and J. Andrews, Renewable Sustainable Energy Rev., 2017, 585–599 CrossRef.
- R. Omrani and B. Shabani, Int. J. Hydrogen Energy, 2019, 3834–3860 CrossRef CAS.
- T. Sadhasivam, K. Dhanabalan, S. H. Roh, T. H. Kim, K. W. Park, S. Jung, M. D. Kurkuri and H. Y. Jung, Int. J. Hydrogen Energy, 2017, 4415–4433 CrossRef CAS.
- Y. Zhang, Y. Ding and X. Zhao, J. Renewable Sustainable Energy, 2018, 44302–44307, DOI:10.1063/1.5024702.
- S. H. Roh, T. Sadhasivam, H. Kim, J. H. Park and H. Y. Jung, Int. J. Hydrogen Energy, 2016, 20650–20659, DOI:10.1016/j.ijhydene.2016.09.062.
- S. Y. Zhang, W. Zhang, T. Wang, X.-r. Wang and X. Liu, Chin. J. Power Sources, 2011, 7, 795–798 Search PubMed.
- T. Sadhasivam, S. H. Roh, T. H. Kim, K. W. Park and H. Y. Jung, Int. J. Hydrogen Energy, 2016, 18226–18230, DOI:10.1016/j.ijhydene.2016.08.092.
- G. Chen, H. Zhang, H. Zhong and H. Ma, Electrochim. Acta, 2010, 8801–8807, DOI:10.1016/j.electacta.2010.07.103.
- C. M. Hwang, H. Ito, T. Maeda, A. Nakano, A. Kato and T. Yoshida, ECS Trans., 2012, 805–815 Search PubMed.
- U. Wittstadt, E. Wagner and T. Jungmann, J. Power Sources, 2005, 555–562, DOI:10.1016/j.jpowsour.2005.02.068.
- H. Y. Jung, S. Y. Huang, P. Ganesan and B. N. Popov, J. Power Sources, 2009, 972–975, DOI:10.1016/j.jpowsour.2009.06.030.
- X. R. Zhang, R. J. Zhu and W. Zhang, et al., C. Nat Conf Chem Physic Power., 2013, pp. 469–470 Search PubMed.
- A. C. Bhosale, S. R. Mane, D. Singdeo and P. C. Ghosh, Energy, 2017, 256–263, DOI:10.1016/j.energy.2017.01.031.
- S. W. Li and S. Sui, Chin. J. Power Sources, 2011, 35, 1235–1239 Search PubMed.
- Y. Wang, D. Y. C. Leung, J. Xuan and H. Wang, Renewable Sustainable Energy Rev., 2017, 961–977 Search PubMed.
- U. A. Hasran, A. M. Pauzi, S. Basri and N. A. Karim, J. Kejuruter., 2018, 37–46, DOI:10.17576/jkukm-2018-si1(1)-06.
- X. M. Yuan, H. Guo, J. X. Liu, F. Ye and C. F. Ma, Energy, 2018, 162, 1041–1051 CrossRef CAS.
- J. X. Liu, H. Guo, X. M. Yuan, F. Ye and C. F. Ma, Int. J. Hydrogen Energy, 2019, 15926–15932, DOI:10.1016/j.ijhydene.2018.05.113.
- Q. Guo, H. Guo, F. Ye, C. F. Ma, Q. Liao and X. Zhu, Int. J. Energy Res., 2019, 2678–2693, DOI:10.1002/er.4319.
- N. Yousfi-Steiner, P. Moçotéguy, D. Candusso, D. Hissel, A. Hernandez and A. Aslanides, J. Power Sources, 2008, 260–274 CrossRef CAS.
- H. Li, Y. Tang, Z. Wang, Z. Shi, S. Wu, D. Song, J. Zhang, K. Fatih, J. Zhang, H. Wang, Z. Liu, R. Abouatallah and A. Mazza, J. Power Sources, 2008, 103–117 CrossRef.
- J. Wu, X. Z. Yuan, J. J. Martin, H. Wang, J. Zhang, J. Shen, S. Wu and W. Merida, J. Power Sources, 2008, 104–119 CrossRef.
- M. V. Williams, H. R. Kunz and J. M. Fenton, J. Electrochem. Soc., 2005, 635–644, DOI:10.1149/1.1860034.
- O. S. Ijaodola, Z. El-Hassan, E. Ogungbemi, F. N. Khatib, T. Wilberforce, J. Thompson and A. G. Olabi, Energy, 2019, 246–267, DOI:10.1016/j.energy.2019.04.074.
- P. Millet, R. Ngameni, S. A. Grigoriev and V. N. Fateev, Int. J. Hydrogen Energy, 2011, 4156–4163, DOI:10.1016/j.ijhydene.2010.06.106.
- B. Andreaus, A. J. McEvoy and G. G. Scherer, Electrochim. Acta, 2002, 2223–2229 CrossRef CAS.
- S. Park, Y. Shao, J. Liu and Y. Wang, Energy Environ. Sci., 2012, 9331–9344 RSC.
- G. Chen, H. Zhang, J. Cheng, Y. Ma and H. Zhong, Electrochem. Commun., 2008, 1373–1376, DOI:10.1016/j.elecom.2008.07.002.
- F. Barbir, T. Molter and L. Dalton, Int. J. Hydrogen Energy, 2005, 351–357, DOI:10.1016/j.ijhydene.2004.08.004.
- J. J. Chen and D. G. Xu, Int. Lett. Chem., Phys. Astron., 2015, 47, 165–177 Search PubMed.
- J. W. Desmond Ng, Y. Gorlin, T. Hatsukade and T. F. Jaramillo, Adv. Energy Mater., 2013, 1–6, DOI:10.1002/aenm.201300492.
- H. Ito, T. Maeda, A. Nakano, C. M. Hwang, M. Ishida, N. Yokoi, Y. Hasegawa, A. Kato and T. Yoshida, ECS Trans., 2010, 33, 945–954 CAS.
- D. Liang, Q. Shen, M. Hou, Z. Shao and B. Yi, J. Power Sources, 2009, 847–853, DOI:10.1016/j.jpowsour.2009.06.059.
- Z. Y. Liu, B. K. Brady, R. N. Carter, B. Litteer, M. Budinski, J. K. Hyun and D. A. Muller, J. Electrochem. Soc., 2008, 979–984, DOI:10.1149/1.2956198.
- X. M. Yuan, H. Guo, F. Ye and C. F. Ma, 7th Int. Conf. Renew. Energy Res. Appl., 2012, vol. 5, pp. 814–818 Search PubMed.
- A. Taniguchi, T. Akita, K. Yasuda and Y. Miyazaki, J. Power Sources, 2004, 42–49, DOI:10.1016/j.jpowsour.2003.12.035.
- H. Y. Li, H. Guo, F. Ye and C. F. Ma, Int. J. Energy Res., 2018, 42, 3378–3389 CrossRef CAS.
- H. Guo, Q. Guo, F. Ye, C. F. Ma, Q. Liao and X. Zhu, Energy Convers. Manage., 2019, 188, 27–39 CrossRef.
- X. M. Yuan, H. Guo, F. Ye and C. F. Ma, Energy Convers. Manage., 2019, 186, 258–266 CrossRef CAS.
- K. Teranishi, S. Tsushima and S. Hirai, Electrochem. Solid-State Lett., 2005, 8, 281–284 CrossRef.
- H. Meng and C. Y. Wang, J. Electrochem. Soc., 2005, 152, 1733–1741 CrossRef.
- W. Schmittinger and A. Vahidi, J. Power Sources, 2008, 1–14 CrossRef CAS.
- X. Huang, R. Solasi, Y. Zou, M. Feshler, K. Reifsnider, D. Condit, S. Burlatsky and T. Madden, J. Polym. Sci., Part B: Polym. Phys., 2006, 2346–2357, DOI:10.1002/polb.20863.
- G. Li and P. G. Pickup, Electrochem. Solid-State Lett., 2006, 249–251, DOI:10.1149/1.2186023.
- J. Yu, T. Matsuura, Y. Yoshikawa, M. N. Islam and M. Hori, Electrochem. Solid-State Lett., 2005, 156–158, DOI:10.1149/1.1854781.
- D. Spernjak, A. K. Prasad and S. G. Advani, J. Power Sources, 2007, 334–344, DOI:10.1016/j.jpowsour.2007.04.020.
- R. E. White and T. V. Nguyen, J. Electrochem. Soc., 1993, 2178–2186, DOI:10.1149/1.2220792.
- F. N. Büchi and S. Srinivasan, J. Electrochem. Soc., 1997, 2767–2771, DOI:10.1149/1.1837893.
- Y. Sone, P. Ekdunge and D. Simonsson, J. Electrochem. Soc., 1996, 1254–1257, DOI:10.1149/1.1836625.
- C. Song, Y. Tang, J. L. Zhang, J. Zhang, H. Wang, J. Shen, S. McDermid, J. Li and P. Kozak, Electrochim. Acta, 2007, 2552–2561, DOI:10.1016/j.electacta.2006.09.008.
- X. Li, I. Sabir and J. Park, J. Power Sources, 2007, 933–942, DOI:10.1016/j.jpowsour.2006.10.015.
- N. Bunmark, S. Limtrakul, M. W. Fowler, T. Vatanatham and J. Gostick, Int. J. Hydrogen Energy, 2010, 6887–6896, DOI:10.1016/j.ijhydene.2010.04.027.
- D. G. Strickland, S. Litster and J. G. Santiago, J. Power Sources, 2007, 272–281, DOI:10.1016/j.jpowsour.2007.08.059.
- S. Litster, C. R. Buie, T. Fabian, J. K. Eaton and J. G. Santiago, J. Electrochem. Soc., 2007, 1049–1058, DOI:10.1149/1.2766650.
- S. S. Lele, M. A. Sizemore and D. Fabris, ASME 2014 12th Int. Conf. Fuel Cell Sci. Eng. Technol. Fuel cell 2014 Collocated with ASME 2014 8th Int. Conf. Energy Sustain., 2014, pp. 1–8 Search PubMed.
- S. H. Ge, X. G. Li and I. M. Hsing, J. Electrochem. Soc., 2004, 523–528, DOI:10.1149/1.1781591.
- V. Shkolnikov, D. G. Strickland, D. P. Fenning and J. G. Santiago, Sens. Actuators, B, 2010, 1667–1675, DOI:10.1016/j.snb.2010.08.040.
- S. S. Lele, M. A. Sizemore, S. S. Zalawadia, A. P. Zabalegui, A. H. Tabrizi and D. Fabris, ASME 2013 11th Int. Conf. Fuel Cell Sci. Eng. Technol. Collocated with ASME 2013 Heat Transf. Summer Conf. ASME 2013 7th Int. Conf. Energy Sustain. Fuel cell 2013, 2013, pp. 1–7 Search PubMed.
- S. Ge, X. Li and I. M. Hsing, Electrochim. Acta, 2005, 1909–1916, DOI:10.1016/j.electacta.2004.08.044.
- D. G. Strickland and J. G. Santiago, ECS Trans., 2009, 303–309 CAS.
- D. G. Strickland and J. G. Santiago, J. Power Sources, 2010, 1667–1675, DOI:10.1016/j.jpowsour.2009.09.034.
- S. Lele, Passive Unitized Regenerative Fuel Cell (PUReFC) for Energy Storage Applications, PhD Thesis, 2016, http://scholarcommons.scu.edu/mech_mstr.
- H. Ito, T. Maeda, A. Kato, T. Yoshida and O. Ulleberg, ECS Trans., 2009, 1979–1990 CAS.
- C.-M. Hwang, M. Ishida, H. Ito, T. Maeda, A. Nakano, A. Kato and T. Yoshida, Journal of International Council on Electrical Engineering, 2012, 2, 171–177 CrossRef.
- C. Simon, D. Kartouzian, D. Müller, F. Wilhelm and H. A. Gasteiger, J. Electrochem. Soc., 2017, 164, F1697–F1711 CrossRef CAS.
- J. Benziger, J. Nehlsen, D. Blackwell, T. Brennan and J. Itescu, J. Membr. Sci., 2012, 98–106, DOI:10.1016/j.memsci.2005.03.049.
- J. Cho, H. Oh, J. Park, K. Min, E. Lee and J. Y. Jyoung, Int. J. Hydrogen Energy, 2014, 495–504, DOI:10.1016/j.ijhydene.2013.10.057.
- H. Sadeghifar, N. Djilali and M. Bahrami, J. Power Sources, 2014, 51–59, DOI:10.1016/j.jpowsour.2014.04.149.
- P. Lettenmeier, S. Kolb, F. Burggraf, A. S. Gago and K. A. Friedrich, J. Power Sources, 2016, 153–158, DOI:10.1016/j.jpowsour.2016.01.100.
- R. Omrani and B. Shabani, Energy Procedia, 2019, 574–581 CrossRef CAS.
- S. S. Dihrab, K. Sopian, M. A. Alghoul and M. Y. Sulaiman, Renewable Sustainable Energy Rev., 2009, 13, 1663–1668 CrossRef CAS.
- H. Tawfik, Y. Hung and D. Mahajan, J. Power Sources, 2007, 755–767 CrossRef CAS.
- N. De Las Heras, E. P. L. Roberts, R. Langton and D. R. Hodgson, Energy Environ. Sci., 2009, 206–214 RSC.
- R. A. Antunes, M. C. L. Oliveira, G. Ett and V. Ett, Int. J. Hydrogen Energy, 2010, 3632–3647 CrossRef CAS.
- S. S. Dihrab, K. Sopian and A. Zaharim, WSEAS Trans. Environ. Dev., 2008, 4, 1151–1160 CAS.
- P. V. Suresh, S. Jayanti, A. P. Deshpande and P. Haridoss, Int. J. Hydrogen Energy, 2011, 6067–6072, DOI:10.1016/j.ijhydene.2011.01.147.
- J. Fall, D. Humphreys and S. M. Guo, J. Fuel Cell Sci. Technol., 2009, 6, 0310031–0310035 CrossRef.
- H. Zhang, M. Hou, G. Lin, Z. Han, Y. Fu, S. Sun, Z. Shao and B. Yi, Int. J. Hydrogen Energy, 2011, 5695–5701, DOI:10.1016/j.ijhydene.2011.01.154.
- A. Hermann, T. Chaudhuri and P. Spagnol, Int. J. Hydrogen Energy, 2005, 1297–1302 CrossRef CAS.
- B. Eriksson, H. Grimler, A. Carlson, H. Ekström, R. Wreland Lindström, G. Lindbergh and C. Lagergren, Int. J. Hydrogen Energy, 2019, 4930–4939, DOI:10.1016/j.ijhydene.2018.12.185.
- P. Forysinski, C. Oloman, S. Kazemi, T. Nickchi and A. Usgaocar, J. Power Sources, 2019, 414, 366–376 CrossRef CAS.
- Y. Fu, M. Hou, G. Lin, J. Hou, Z. Shao and B. Yi, J. Power Sources, 2008, 4930–4939, DOI:10.1016/j.jpowsour.2007.10.038.
- C. K. Mittelsteadt and W. Braff, ECS Trans., 2008, 16, 1891–1899 CAS.
- C. R. Buie, J. D. Posner, T. Fabian, S. W. Cha, D. Kim, F. B. Prinz, J. K. Eaton and J. G. Santiago, J. Power Sources, 2006, 191–202, DOI:10.1016/j.jpowsour.2006.03.021.
- J. Stumper, S. A. Campbell, D. P. Wilkinson, M. C. Johnson and M. Davis, Electrochim. Acta, 1998, 3629–3862, DOI:10.1016/s0013-4686(98)00137-6.
- J. Andrews, Appl. Mech. Mater, 2013, 693–697 Search PubMed.
- D. Kapoor, A. S. Oberoi and P. Nijhawan, Processes, 2019, 7, 1–19 CrossRef.
- H. Dotan, A. Landman, S. W. Sheehan, K. D. Malviya, G. E. Shter, D. A. Grave, Z. Arzi, N. Yehudai, M. Halabi, N. Gal, N. Hadari, C. Cohen, A. Rothschild and G. S. Grader, Nat. Energy, 2019, 49, 1–19, DOI:10.1038/s41560-019-0462-7.
- L. Chen, X. Dong, Y. Wang and Y. Xia, Nat. Commun., 2016, 11741–11749, DOI:10.1038/ncomms11741.
- I. Vincent, B. Choi, M. Nakoji, M. Ishizuka, K. Tsutsumi and A. Tsutsumi, Int. J. Hydrogen Energy, 2018, 10240–10248, DOI:10.1016/j.ijhydene.2018.04.087.
- I. Vincent and D. Bessarabov, Renewable Sustainable Energy Rev., 2018, 1690–1704, DOI:10.1016/j.rser.2017.05.258.
| This journal is © The Royal Society of Chemistry 2020 |