Open Access Article
Open Access ArticleAbscisic acid-enhanced starch accumulation of bioenergy crop duckweed (Spirodela polyrrhiza)†
Xuezhi Wanga,
Weihua Cui *ab,
Weiwu Huac and
Chuanping Feng
*ab,
Weiwu Huac and
Chuanping Feng a
a
aSchool of Water Resources and Environment, China University of Geosciences (Beijing), Beijing 100083, China. E-mail: cuiwh@cugb.edu.cn; Fax: +86 10 82321081; Tel: +86 10 82322281
bState Key Laboratory of Biogeology and Geology, China University of Geosciences (Beijing), Beijing 100083, China
cThe Journal Center, China University of Geosciences (Beijing), Beijing 100083, China
First published on 11th March 2020
Abstract
To meet the increasing energy consumption around the world and fight global climate change, there is an urgent need to explore renewable energy crops to replace the traditional energy sources. Duckweed (Spirodela polyrrhiza) is widely distributed in the world and has high starch and low lignin contents, which is perhaps an ideal feedstock for bioenergy production. To investigate the effects of abscisic acid (ABA) on duckweed biomass and starch accumulation, Spirodela polyrrhiza was cultivated at different ABA concentrations. The results showed that the highest starch content in duckweed (21.8% dry weight) was achieved in 1.0 × 10−2 mg L−1 ABA medium, 70.3% higher than that of the control medium without ABA. The number of starch granules in 1.0 × 10−2 mg L−1 ABA medium was far more than that in the control medium. The highest adenosine diphosphate (ADP)-glucose pyrophosphorylase (AGPase) activity was observed in the 1.0 × 10−2 mg L−1 ABA medium, which was caused by the up-regulation expression of ADP-glucose pyrophosphorylase 2 (APL2). Further investigations on cell ultra-structures and stomatal property of the duckweed indicated that ABA increased the number and size of starch granules and stomatal size in duckweed cells. These enhancements lead to a greatly improved energy flow in the aquatic plant from photosynthesis to carbon storage, making duckweed a potential renewable bioenergy crop.
1. Introduction
With the increasing consumption of fossil fuels, the energy crisis and global climate change around the world are becoming serious concerns.1 Biomass energy can effectively address the concerns because it is renewable and sustainable in global development. Thus, biomass energy production has significantly increased in the last decade and is expected to grow in the near future. A recent report indicates that the main feedstock for commercial bioenergy production is crops such as cassava,2 sweet sorghum3 and rice straw.4 However, the use of the crops to produce bioenergy leads to a competition against food and feed production, which makes the conversion of the crops for bioenergy production a controversial practice. Recently, many research efforts have been focused on the use of lignocellulosic biomass as a feedstock for the production of biofuels such as ethanol.5 However, the technologies for lignocellulosic biofuel production are still facing economic challenges. Therefore, it is critical to develop new technologies for renewable biofuel production in a cost-effective and environmentally friendly manner. It is important to explore new feedstock for bioenergy production that does not compete for farmland against food and feed production.Duckweed is a small aquatic plant that grows on water surface in many regions of the world and has a good potential as a sustainable feedstock for biofuel production.6 A floating duckweed can be much more easily harvested than microalgae. Duckweed also has advantages over many other energy crops. It grows very fast and accumulates biomass faster than most other potential energy crops. Duckweed could be harvested two to three times a week and produce dry biomass at a yield of 55 tons per hectare per year.7 Starch content of duckweed ranges from 3% to 75%, depending on duckweed species,8 growing conditions (temperature, pH, and nutrient concentrations),9 and developmental states (turion, resting frond, or growing frond).10 Duckweed has low levels of cellulose and lignin and can have a relatively high level of starch, which makes duckweed a potential starch feedstock for bio-ethanol production.11 Previous research reports indicated that duckweed starch could be readily converted to ethanol12 or butanol.13
Starch accumulation in duckweed is an essential concentration for duckweed-based biofuel production. Plant growth regulators could improve the yield of duckweed starch for bioethanol production.14 Liu et al.15 sprayed Landoltia punctata fronds with 800 mg L−1 uniconazole and found high starch accumulation, a dry-weight starch content of up to 48%. Abscisic acid (ABA), a terpenoid, is an important plant growth regulator, which controls plant growth and stress response.16 A high concentration (0.1 mM) of exogenous ABA reduced transport of sucrose into the grains and the starch synthesis ability of grains in wheat.17 Many duckweed species could grow biomass rapidly,18 so they were selected for studies on starch synthesis under ABA induction. In our previous study, we found that low ABA concentration increased duckweed starch accumulation in fronds of Spirodela polyrhiza.19 However, the understanding of the mechanism for ABA-induced starch accumulation in duckweed is very limited.
In general duckweed growth, energy is harvested during photosynthesis and transferred to biosynthesis of starch which is then accumulated in duckweed fronds. adenosine diphosphate (ADP) glucose pyrophosphorylase (AGPase) plays a key role in the regulation of starch content, which represents the main control of the starch synthesis rate.20 AGPase catalyzes the synthesis of adenosine diphosphate glucose (ADPGlc) and liberates inorganic pyrophosphate (PPi) from glucose 1-phosphate (Glc-1-P) and adenosine triphosphate (ATP).21 To better understand the mechanism of the ABA-induced starch accumulation in duckweed, AGPase activity was studied and starch granule, thylakoid, chloroplast, vacuole, and stomatal size and density of the duckweed were investigated in this study. To explore further insight into the relationship between ADP-glucose pyrophosphorylases (APLs) and starch content in duckweed fronds, 3 genes including APL1, APL2, and APL3 in large subunit (LS) of the AGPase in duckweed were investigated in this study.
2. Materials and methods
2.1 Duckweed cultivation and experimental procedure
Spirodela polyrrhiza was collected from Daoxiang Lake, Changping District, Beijing, China. Collected duckweed was washed using tap water for three times and precultured in enamel rectangle trays (450 × 350 × 25 mm) with whole a Hoagland nutrient solution (pH 7.0) for 7 days.22 The cultivation conditions and the duckweed species identification were documented in our previous publication.20 In our previous study we found that ABA at concentrations lower than 1.0 × 10−2 mg L−1 enhanced starch accumulation in duckweed Spirodela polyrrhiza.19 Therefore, two ABA concentrations (1.0 × 10−2 and 1.0 × 10−4 mg L−1) were selected in this study to investigate the mechanism for ABA-promoted starch accumulation in the duckweed. ABA was added into the Hoagland nutrient solution to achieve ABA concentrations of 1.0 × 10−4 and 1.0 × 10−2 mg L−1 in the experiment. A control group was used with no ABA addition in the same nutrient solution. Initially, fresh duckweed was transferred into 250 mL conical flasks. Each flask contained approximately 0.50 g fresh duckweed and 100 mL culture media. The flasks were then placed in a climate chamber (RDN-800B-5LED, Hangzhou Aipo Instrument Co., Ltd, Hangzhou, China) under the growth conditions of 16 h light (8000 lux)/8 h dark photoperiods at temperatures of 25 °C in light periods and 15 °C in dark periods. Distilled water was added into the flasks daily to compensate for evaporated water during the experiment. All the media for the duckweed cultivation were replaced with fresh new ones every other day to maintain constant ABA concentrations and sufficient nutrients. Duckweed was sampled every 2 days to analyze genes expressions, AGPase activity, and starch content in the experiment. Organelle structure of the duckweed including nucleus, mitochondrion, endoplasm reticulum, plastid, vacuole, and stomatal size and density were measured on Day 12.2.2 Measurement of starch content
Fresh duckweed samples were dried at 65 °C for 48 h. Dry duckweed was ground into powder (particle size of 1–2 mm) with a mortar. Determination of starch content in dry duckweed was adapted from Wrolstad et al.232.3 Transmission electron microscopy imaging
After cultivation for 12 days, randomly selected duckweed fronds were utilized to prepare the samples for transmission electron microscope (TEM) analysis. The fresh duckweed fronds were washed with deionized water and carefully blotted dry to remove the moisture on the surface of the duckweed fronds. Small portions (approximately 1 cm wide × 2 cm long) of the fronds were cut from their central parts using razor blades. Small lamina samples were fixed overnight in 2.5% glutaraldehyde (v/v) and washed three times with 0.1 M phosphate-buffered saline (PBS) (pH 7.4). The samples were post-fixed in 1% OsO4 (osmium(VIII) oxide) for 3 h, then washed three times using 0.1 M PBS (pH 7.4) in 10 min interval between two consecutive washes. Then, the samples were dehydrated with a graded acetone series (50, 60, 70, 80, 90, and 95%) in a 15–20 min interval and finally dehydrated with acetone (100%) for 20 min. The samples were filtrated and embedded overnight in Spurr's resin before polymerization. Ultrasections were obtained using a LEICA UC6 ultramicrotome and stained with uranyl acetate and lead phosphate. Images were observed and generated by a TEM (JEM-1230 JEOL, Tokyo, Japan).2.4 Scanning electron microscope imaging
The randomly selected duckweed fronds after cultivation for 12 days were also used to prepare the samples for scanning electron microscope (SEM) analysis. As mentioned in a previous section, the fresh duckweed fronds were gently washed with deionized water, carefully blotted dry, and then cut into small sections from their central parts (approximately 1 cm wide × 2 cm long). The small sections were immediately fixed within 2 h in 2.5% glutaraldehyde (v/v) and washed three times with 0.1 M PBS. The samples were post-fixed in 1% OsO4 for 2 h, then washed three times with 0.1 M PBS (pH 7.4) in a 10 min interval between two consecutive washes. Then, the samples were dehydrated three times in a graded ethanol series (50, 60, 70, 80, 90, 95, and 100%) in a 15–20 min interval. The samples were dried with critical point dryer (Leica EM CPD 300, Leica Inc., Germany) and gilded with ion-plating apparatus (EIKO.IB-3., EIKO Inc., Tokyo, Japan). Images were generated using SEM (Hitachi S-3400N, Hitachi Inc., Tokyo, Japan).2.5 AGPase enzyme activity measurement
AGPase enzyme activity was measured with the enzyme assessment kit from Suzhou Keming Biological Technology Co., Ltd, Suzhou, China, following the specifications of the assessment kit (http://www.cominbio.com).2.6 Expression analyses of APL genes in Spirodela polyrrhiza
Quantitative real-time PCR (qPCR) was carried out as described by Wang.24 Total RNA was extracted from fresh frond samples with RNAiso for Polysaccharide-rich Plant Tissue (Takara, Dalian, China). The RNA quality and quantity were confirmed by analysis with Nanodrop 1000 (Thermo Fisher Scientific, USA). RNA first-strand cDNA synthesis was obtained with a kit of Prime Script™ 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). cDNA was diluted 80-fold. Real-time PCR was performed using the SYBR® Premix Ex Tap™ (Tli RNaseH Plus) (Takara, Dalian, China) following the manufacturer's standard instructions for 7500 RT-PCR (Thermo Fisher Scientific, USA). Gene expression values were calculated with calibrated cycle threshold (Ct) values normalized to a standard dilution series over all samples assayed.25 The duckweed primer sequences are presented in Table 1.26| Target gene | Primer sequence |
|---|---|
| APL1 | [F] CTCAGCGCGTCGTCCTCACTC |
| APL1 | [R] AAGAAGGTTGCAGTCAGAGAAG |
| APL2 | [F] ACTCGCCGGAGGTTCCTACTCG |
| APL2 | [R] GGGCTCTCCTACCCCTCACGAC |
| APL3 | [F] GATCGGGGCCACCTCGGCTTCT |
| APL3 | [R] CAGCGCGTCGTCGGACTGAAC |
2.7 Statistical analysis
All the data were subject to analysis of variance and expressed as means ± standard errors of three replicates. The correlation significance was verified using linear regression with p < 0.05 considered statistically significant. The results were analyzed for variance using SPSS statistical software (IBM, Chicago, IL, USA). For comparison, the differences in starch content and APL1, APL2, and APL3 expressions were checked for significance using Duncan's multiple range tests using various concentrations of ABA.3. Results
3.1 Effect of ABA on duckweed starch content
Spirodela polyrhiza was cultured under controlled light and temperature conditions as described in the section of Materials and methods. Duckweed starch content in 0 (control), 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA media are shown in Fig. 1. As shown in Fig. 1, starch accumulation increased markedly at the first 8–10 days and then tended to be stable for the rest of the experiment. In the control group without ABA in the medium, starch content in the duckweed increased slightly and stabilized at 12.8% of dry duckweed on Day 10. In the 1.0 × 10−4 mg L−1 ABA medium, starch content in the duckweed also reached stability on Day 10, and its value was 16.9% of dry duckweed, 32.0% higher than that of the control group. In 1.0 × 10−2 mg L−1 ABA medium, starch content reached 21.5% on Day 8, which increased by 70.3% compared to the control group (Fig. 1). The starch contents in the absence and presence of ABA have significant differences (p < 0.05) and indicate clearly that ABA promotes the accumulation of starch in duckweed fronds. The data confirmed the finding in our previous study.19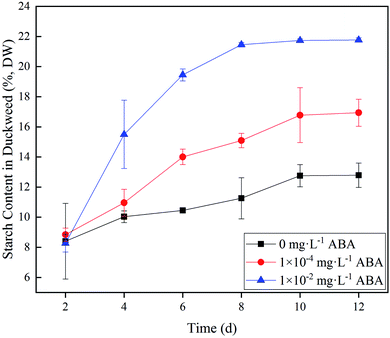 | ||
| Fig. 1 Duckweed starch content under three different abscisic acid (ABA) media: 0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1. DW – dry weight basis. | ||
3.2 Effect of ABA on AGPase enzyme activity
AGPase catalyzes the conversion of glucose 1-phosphate and ATP to ADP-glucose and pyrophosphate, which is the first rate-limiting step in starch biosynthesis process.27 Fig. 2 shows the difference of AGPase activities of the duckweed in three media (0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA). In the 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media, the AGPase activity increased along with the cultivation time and reached a plateau on Day 10. The AGPase activites were 3163 and 4076 nmol min−1 g−1 in 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media on Day 10, respectively, which increased by 41.9% and 82.8%, respectively, compared to the control group. Apparently, the presence of ABA improved the AGPase activity in Spirodela polyrrhiza, and thus enhanced starch synthesis metabolism in the duckweed.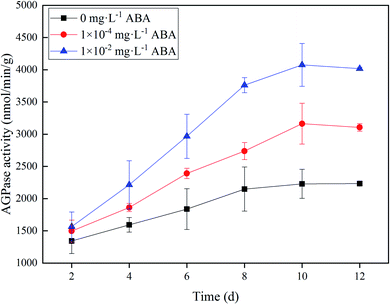 | ||
| Fig. 2 ADP-glucose pyrophosphorylase (AGPase) activity of duckweed in the media with 0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 abscisic acid (ABA). | ||
3.3 Impact of ABA on APL genes
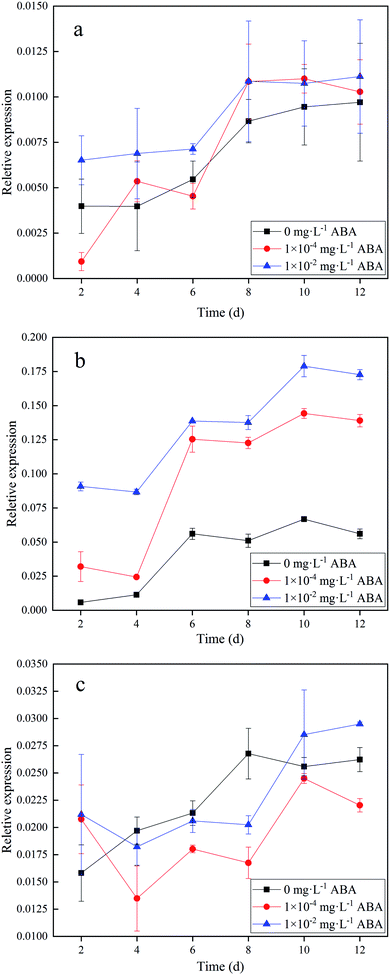
To understand the reason for the change of AGPase activity in duckweed, we measured the expressions of APL genes, which were mainly responsible for the AGPase activity. We applied qPCR to analyze the gene expressions. Specific primer pairs were used to distinguish transcripts from each gene. Fig. 3 shows the results of the expression of ADP-glucose pyrophosphorylases in the duckweed based on RNA in three different ABA media (0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA). As shown in Fig. 3a, there was no obvious increase in APL1 expression of the duckweed in the media with ABA addition, compared with the control group. APL1 expression in the duckweed increased with time in the first 8 days and then leveled off in all three media (Fig. 3a). The expression of APL1 in the duckweed did not have significant difference (p < 0.05) in three media. However, the expressions of APL2 in the duckweed growing in the different media were significantly different (Fig. 3b). As shown in Fig. 3b, the expressions of APL2 was not up-regulated in the initial stage, but increased from Day 4, and reached the maximum on the Day 10 (0.06, 0.14, and 0.18 in 0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA media, respectively). On Day 10, the APL2 gene expressions were strongly up-regulated by the addition of ABA in the media, which was 1.3 and 2.0 folds higher in 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media, respectively, than that in the control. Similar to the APL1 expressions, the APL1 expressions in the duckweed did not have significant difference in three media (Fig. 3c). Based on the data presented, we found that APL2 in Spirodela polyrhiza was the key to the synthesis of AGPase and the precursor of starch synthesis. Ventriglia et al. found the same results in Arabidopsis, i.e. APL2 contributed to the synthesis of AGPase.28 Our results have clearly shown that a low level of ABA in the media has promoted the expression of APL2 in duckweed and hence enhanced the AGPase activity, which has resulted in an improved starch accumulation.3.4 Effect of ABA on duckweed cell ultra-structures
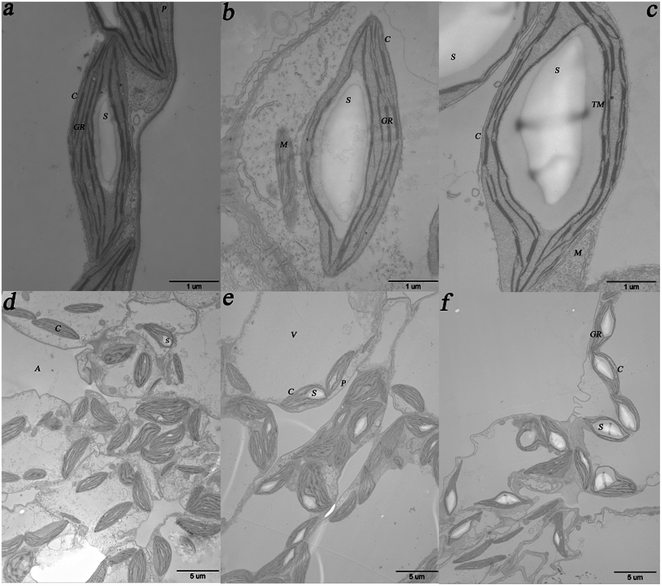
The results obtained from the TEM imaging analysis of the randomly selected duckweed fronds after cultivation for 12 days for imaging analysis are shown in Fig. 4. As shown in the figure, the kidney-shaped starch granules were wrapped in stacks of thylakoids membranes in the chloroplasts (Fig. 4a–c). Starch granules in the duckweed fronds cultivated in 0, 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media occupied approximately 18%, 36%, and 50% of the chloroplast area, respectively. Compared with the control, the area percentages of starch granules in the chloroplasts in the samples from 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media increased by 100% and 178%, respectively. Fig. 4a–c has also shown that the diameter of starch granules in the samples from 0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA media, are around 2.0, 3.5, and 4.3 μm, respectively. Compared with the control, the diameter of starch granules in the samples from 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media increased by 75% and 115%, respectively. The starch granule density (the number of starch granules per unit area) observed in the duckweed cultivated with the presence of low level of ABA was also higher than that in the control. In our experiment the control group had normal discal chloroplasts with a few small starch granules (0.006 μm−2) (Fig. 4d). On the other hand, the starch granule densities in duckweed from the 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media were around 0.012 and 0.013 μm−2, respectively (Fig. 4e and f) and increased by 100% and 117%, respectively in comparison with the control. These results revealed that ABA promoted the starch granule formation in duckweed cells. The increase of starch granule size contributed to the distortion of chloroplasts, the chloroplasts apparently became swollen, and a concomitant deformation of thylakoids were observed in this study (Fig. 4b and c).3.5 Effect of ABA on duckweed stomatal property
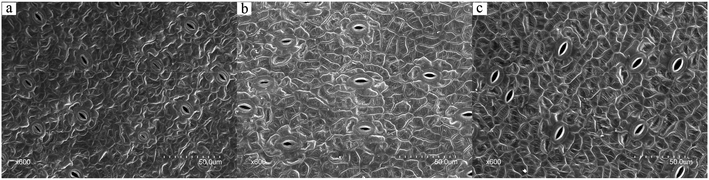
For photosynthesis and respiration of higher plants, carbon dioxide, oxygen, and steam need to go through epidermal stomatal pores to exchange. Gas exchange affects carbon dioxide absorption, photosynthesis, and biomass production.29 Carbon dioxide enters into the chloroplast of a plant and is fixed by Rubisco during daytime.30 Therefor, it is necessary to investigate the stomatal property of duckweed fronds. SEM observations of the upper epidermis ultra-structures of the duckweed fronds were used to analyze the stomatal morphological difference in different ABA media. Similar method was used by Sun et al. to investigate the stomatal size and density of potato leaves under different irrigation and phosphorus regimes.31Fig. 5 shows stomatal morphological difference of duckweed fronds in different ABA media (0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA). As shown in the figure, duckweed stomatal size increased with the increase of ABA concentration. In the control group, the average stomatal length was around 6.40 μm (Fig. 5a). In the 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media, the average stomatal length was around 6.72 and 9.40 μm, respectively (Fig. 5b and c), and increased by 5% and 46.8%, respectively, compared with the control group. Stomatal width of the duckweed from the media with low levels of ABA also increased in comparison with the control. The stomatal width of the duckweed was around 1.64 μm in the control group (Fig. 5a), and it was 1.95 and 2.76 μm in the media with 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA, respectively and 18.9% and 68.3% higher than that of the control group, respectively (Fig. 5b and c).
4. Discussion
In starch biosynthesis, AGPase is a key regulatory enzyme.32 AGPase is composed of two closely related (small subunit (SS) and large subunit (LS)) but different types of subunits in higher plants.17 The SS is responsible for the catalytic activity, while the LS plays a regulatory function.33 SS and LS originate from a common ancestral gene, so they share many amino acids in plants.34 In this study, we selected the LS for investigation because the LS regulates the activity of the SS.35 Furthermore, SS has been subjected to a stricter evolutionary constraint than LS in angiosperms, as the former is less tissue-specific and must interact with different LS.36 The expression of APL2 has obviously positive correlation with ABA concentration. In order to further explore the mechanism of low ABA induced starch accumulation in duckweed, the AGPase activity was investigated under different culture media. Correlation coefficient of AGPase activity and APL2 expression in 0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA media was 0.91 (p = 0.01), 0.93 (p = 0.007), and 0.93 (p = 0.006), respectively (Table S1†). Ventriglia et al. also indicated that APL subunits control the synthesis of starch by catalyzing AGPase activity in Arabidopsis Columbia ecotype.28 Up-regulation of APL2 gene expression could increase AGPase activity in 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media, which catalyzed the starch synthesis. APL2 plays a major role in the synthesis of starch with a low-level ABA addition in the media. The results indicated that AGPase activity was mainly regulated by APL2 gene. AGPase activity gradually increased and accelerated starch synthesis until Day 10. The correlation coefficients of starch content and AGPase activity in 0, 1.0 × 10−4, and 1.0 × 10−2 mg L−1 ABA media was 0.96 (p = 0.002), 0.99 (p < 0.001), and 0.95 (p = 0.004), respectively (Table S2†). This suggests that a low-level ABA increases the synthesis of starch by enhancing the activity of AGPase, which is a result of the upregulation expression of APL2.The plant hormone ABA was involved in the regulation of various plant developmental processes, including the stimulation of maize yield and induction of seed dormancy.37 Previous research has demonstrated that there are strong correlations between ABA concentration and the capacity of starch synthesis in barley seed development.38 In this study, we added exogenous ABA to induce starch accumulation in duckweed fronds. Starch content reached 16.9% and 21.8% (dry weight) in 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media on Day 12, respectively. Addition of low-level ABA can improve the starch accumulation in duckweed fronds and make duckweed a potential energy crop. In 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media, starch granule diameter was around 3.5 and 4.3 μm (estimated with TEM), respectively. However, starch granules from corn, rice, and wheat can reach a size of 25, 20, and 30 μm, respectively.39 Franco et al.40 reported that larger starch granules were more difficultly hydrolyzed into sugars than smaller ones, regardless of botanical source. The starch granules in duckweed is much smaller than those in corn, rice, and wheat, which means that the starch in duckweed can be more easily hydrolyzed into sugars than the starch in corn, rice, and wheat. Thus, duckweed can be potentially a better feedstock for biofuel production than corn, rice, and wheat. Moreover, thylakoid degradation was observed in 1.0 × 10−4 and 1.0 × 10−2 mg L−1 ABA media. Wang and Messing reported that intercellular airspace deficiency and smaller vacuoles were observed in turion of Spirodela polyrrhiza, leading to the turion sinking in deep water.26 In the present study, there was no difference in intercellular airspace between the ABA added groups and the control group (Fig. 4). These results explained why no turion formation was observed in deep water.
ABA is one of the most important hormones which is used to control opening and closing the stomata.41 In this study, we found that stomatal aperture increased with the increase of ABA concentration. In the model glyophyte Arabidopsis thaliana, Stefan et al. found that ABA triggered the initial steps of carbon mobilization.42 Larger stomata would contribute to the enhanced photosynthesis rate, which increased carbon storage under higher ABA concentration.43 The stomata had the greatest effect on photosynthetic function, which reflects the ability of mesophyll photosynthesis by the net rate of carbon dioxide assimilation.44 Photosynthetic activity of plant could affect its growth and ability for carbon-energy fixation,45 which contribute to accumulate more starch content of Spirodela polyrrhiza under higher ABA concentration.
5. Conclusions
This study demonstrated that ABA induced enhanced starch accumulation in duckweed Spirodela polyrrhiza. ABA up-regulates the expression of APL2 gene which enhance the AGPase activity and result in larger and more starch granules in the duckweed fronds. The cell ultra-structures and stomatal morphology of the duckweed also indicate that ABA increases the number and size of starch granules and stomatal size and density. Spirodela polyrrhiza induced by ABA had higher content of starch (more and larger size starch granules), which provides a solid foundation for developing Spirodela polyrrhiza as an alternative crop for biofuel production. These results provide valuable information for further investigation of ABA application on duckweed starch accumulation in laboratory and outdoor ponds, and eventually make Spirodela polyrrhiza a promising alternative feedstock for biofuel production.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 51309206). The authors would like to thank Prof. Jay J. Cheng at North Carolina State University for his kind help in polishing the English language in this article.References
- W. Cui and J. J. Cheng, Plant Biol., 2015, 17, 16–23 CrossRef PubMed.
- B. Escaramboni, E. G. F. Núñez, A. F. A. Carvalho and P. D. O. Neto, Ind. Crops Prod., 2018, 112, 368–377 CrossRef CAS.
- N. B. Appiah-Nkansah, K. Zhang, W. Rooney and D. Wang, Ind. Crops Prod., 2018, 111, 247–253 CrossRef CAS.
- Y. Deng, L. Qiu, Y. Yao and M. Qin, RSC Adv., 2018, 8, 22643–22651 RSC.
- N. Sarkar, S. K. Ghosh, S. Bannerjee and K. Aikat, Renewable Energy, 2012, 37, 19–27 CrossRef CAS.
- G. Chen, Y. Fang, J. Huang, Y. Zhao, Q. Li, F. Lai, Y. Xu, X. Tian, K. He, Y. Jin, L. Tan and H. Zhao, RSC Adv., 2018, 8, 17927–17937 RSC.
- G. Oron, Agric. Water Manag., 1994, 26, 27–40 CrossRef.
- M. S. Reid and R. L. Bieleski, Plant Physiol., 1970, 46, 609–613 CrossRef CAS PubMed.
- J. Xu, H. Cui, J. J. Cheng and A.-M. Stomp, Biosyst. Eng., 2011, 110, 67–72 CrossRef.
- R. D. Pankey, H. N. Draudt and N. W. Desrosier, J. Food Sci., 1965, 30, 627–631 CrossRef CAS.
- X. Zhao, G. K. Moatesa, N. Wellnera, S. R. A. Collinsa, M. J. Colemanb and K. W. Waldrona, Carbohydr. Polym., 2014, 111, 410–418 CrossRef CAS PubMed.
- Q. Chen, Y. Jin, G. Zhang, Y. Fang, Y. Xiao and H. Zhao, Energies, 2012, 5, 3019–3032 CrossRef CAS.
- H. Su, G. Xu, H. Chen and Y. Xu, ACS Sustainable Chem. Eng., 2015, 3, 2002–2011 CrossRef CAS.
- P. Ziegler, K. S. Sree and K.-J. Appenroth, Environ. Sci. Pollut. Res., 2019, 26, 14797–14822 CrossRef PubMed.
- Y. Liu, Y. Fang, M. Huang, Y. Jin, J. Sun, X. Tao, G. Zhang, K. He, Y. Zhao and H. Zhao, Biotechnol. Biofuels, 2015, 8, 1–12 CrossRef PubMed.
- K. N. Baron, GRAM Genes and Abscisic Acid (ABA) Metabolism in the Reproductive of Arabidopsis Thaliana, Dissertation, University of Manitoba, 2013.
- S. Bhatia and R. Singh, Plant Growth Regul., 2002, 36, 97–104 CrossRef CAS.
- E. I. Iatrou, E. Kora and A. S. Stasinakis, Environ. Technol., 2019, 40, 2649–2656 CrossRef CAS PubMed.
- X. Wang, W. Cui, W. Hu and C. Feng, BioEnergy Res., 2017, 10, 417–426 CrossRef CAS.
- G. Yu, Y. Lv, L. Y. Shen, Y. Wang, Y. Qing, N. Wu, Y. Li, H. Huang, N. Zhang, Y. Liu, Y. Hu, H. Liu, J. Zhang and Y. Huang, Int. J. Mol. Sci., 2019, 20, 986 CrossRef CAS PubMed.
- M. Stitt and S. C. Zeeman, Starch turnover: pathways, regulation and role in growth, Curr. Opin. Plant Biol., 2012, 15, 282–292 CrossRef CAS PubMed.
- D. R. Hoagland and D. I. Arnon, The Water-Culture Method for Growing Plants Without Soil, California, USA, 1950 Search PubMed.
- R. E. Wrolstad, T. E. Acree, E. A. Decker, M. H. Penner, D. S. Red, S. J. Schwartz, C. F. Shoemaker, D. Smith and P. Sporns, Starch and Starch Derivatives, Handbook of food analytical chemistry, Hoboken, New Jersey, 2004 Search PubMed.
- X. Wang, RNA, 2009, 15, 716–723 CrossRef CAS PubMed.
- A. Zdepski, W. Wang, H. D. Priest, F. Ali, M. Alam, T. C. Mockler and T. P. Michael, Trop. Plant Biol., 2008, 1, 236–245 CrossRef PubMed.
- W. Wang and J. Messing, BMC Plant Biol., 2012, 12, 1–14 CrossRef PubMed.
- M. A. Ballicora, A. A. Iglesias and J. Preiss, Photosynth. Res., 2004, 79, 1–24 CrossRef CAS PubMed.
- T. Ventriglia, M. L. Kuhn, M. T. Ruiz, R. P. Marina, F. Valverde, M. A. Ballicora, J. Preiss and J. M. Romero, Plant Physiol., 2008, 148, 65–76 CrossRef CAS PubMed.
- J. R. Evans, R. Kaldenhoff, B. Genty and I. Terashima, J. Exp. Bot., 2009, 60, 2235–2248 CrossRef CAS PubMed.
- I. Andersson and A. Backlund, Plant Physiol. Biochem., 2008, 46, 275–291 CrossRef CAS.
- Y. Sun, F. Yan, X. Cui and F. Liu, J. Plant Physiol., 2014, 171, 1248–1255 CrossRef CAS.
- J. Preiss, C. Lammel and A. Sabraw, Plant Physiol., 1971, 47, 104–108 CrossRef CAS PubMed.
- R. J. Spreitzer, S. R. Peddi and S. Satagopan, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17225–17230 CrossRef CAS PubMed.
- M. R. Bhave, S. Lawrence, C. Barton and L. C. Hannah, Plant Cell, 1990, 2, 581–588 CAS.
- S. K. Hwang, S. Hamada and T. W. Okita, FEBS Lett., 2006, 580, 6741–6748 CrossRef CAS PubMed.
- N. Georgelis, E. L. Braun, J. R. Shaw and L. C. Hannaha, Plant Cell, 2007, 19, 1458–1472 CrossRef CAS.
- I. Ahmad, M. Kamran, S. Ali, T. Cai, B. Bilegjargal, T. N. Liu and Q. F. Han, Environ. Sci. Pollut. Res., 2018, 25, 33225–33239 CrossRef CAS.
- C. Seiler, V. T. Harshavardhan, K. Rajesh, P. S. Reddy, M. Strickert, H. Rolletschek, U. Scholz, U. Wobus and N. Sreenivasulu, J. Exp. Bot., 2011, 62, 2615–2632 CrossRef CAS PubMed.
- M. Gupta, A. S. Bawa and A. D. Semwal, Int. J. Food Prop., 2009, 12, 587–604 CrossRef.
- C. M. L. Franco, C. F. Ciacco and D. Q. Tavares, Starch Staerke, 1998, 50, 193–198 CrossRef CAS.
- G. A. Daszkowska and I. Szarejko, Front. Plant Sci., 2013, 4, 1–16 Search PubMed.
- K. Stefan, K. Julia, D. S. Silvia, K. Joachim and J. Claudia, PLoS One, 2008, 3, e3935 CrossRef PubMed.
- K. Shah, N. U. Amin, I. Ahmad, G. Ara, M. U. Rahman, X. Zuo, L. Xing and X. Ren, Environ. Sci. Pollut. Res., 2019, 26, 19490–19501 CrossRef CAS PubMed.
- Y. Chen, N. Wu, H. Mao, J. Zhou, Y. Su, Z. Zhang, H. Zhang and S. Yuan, RSC Adv., 2019, 9, 19243–19252 RSC.
- X. Zhang, J. Cheng, H. Lu, F. Chu, J. Xu, X. Wang and K. Cen, RSC Adv., 2019, 9, 26495–26502 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00269k |
| This journal is © The Royal Society of Chemistry 2020 |