Open Access Article
Open Access ArticleEfficient synthesis of 1-iodoalkynes via Al2O3 mediated reaction of terminal alkynes and N-iodosuccinimide†
Ming Yao *a,
Jingjing Zhangab,
Sen Yanga,
Hangxing Xiong*ab,
Li Liab,
E. Liua and
Hong Shia
*a,
Jingjing Zhangab,
Sen Yanga,
Hangxing Xiong*ab,
Li Liab,
E. Liua and
Hong Shia
aJingchu University of Technology, 33 Xiangshan Road, Jingmen, Hubei 448000, P. R. China. E-mail: yaomingcep@jcut.edu.cn
bWuhan Institute of Technology, 206 Guanggu First Road, Wuhan, Hubei 430205, P. R. China
First published on 23rd January 2020
Abstract
Iodination of terminal alkynes using N-iodosuccinimide (NIS) in the presence of γ-Al2O3 was developed to afford 1-iodoalkynes with good to excellent yields (up to 99%). This described approach featured excellent chemoselectivity, good functional group tolerance, and utilization of an inexpensive catalyst.
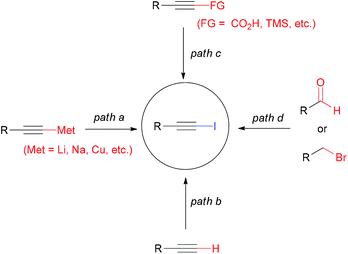
1-Iodoalkynes are both highly versatile synthons in organic synthesis and valuable building blocks in materials and polymer sciences.1,2 Traditional procedures for generating 1-iodoalkynes include iodination of metal acetylides (Scheme 1, path a),3 direct iodination of terminal alkynes (path b),4 iodination of propiolic acid/trialkylsilylacetylides (path c),5,6 and a two-step homologation/iodination or elimination sequence from aldehydes or benzylic bromides (path d).7,8 Among these achievements, path b, the preparation of 1-iodoalkynes from terminal alkynes, is highly desirable. In this regard, various iodinating conditions, including nBuLi/I2,4a EtMgBr/I2,4b I2/DMAP,4c NIS/AgNO3,4d NIS/BnN3,4e NIS/DBU,4f NIS/Ag/C3N4,4g KI/CuI/PhI(OAc)2/Et3N,4h Me3SiI/PhI(OAc)2,4i CuI/TBAB/Et3N,4j TBAI/Oxone,4k KI/TBHP,4l TBAI/PhI(OAc)2,4m chloramine-B/KI,4n KI/Cu2SO4/BPDS,4o ZnI2/TBN,4p NIS/[Au(AIPr)(NEt3)][HF2],4q HMBMIBDCI/DBU,4r NaI/MeOH/divided cell,4s and N-iodomorphonine/CuI,4t have been reported. However, some of these approaches might suffer from expensive metal catalysts, hazardous or toxic reagents, harsh reaction conditions, or generation of wastes that bring about environmental problems, thereby restricting their practical applications. Though considerable progress has been achieved, the development of efficient and environmentally benign protocols is still required.
It is well recognized that solids can play a vital role in developing new cleaner technologies via their capacities to serve as catalysts or support reagents and influence product selectivity, and some books have documented the applications of solids in organic synthesis.9–11 Aluminum oxide (Al2O3) is an inert, odorless and white amorphous material, and has been used as catalyst in various industrial processes for many years.12,13 But the utilization of aluminum oxide in synthetic organic chemistry especially for halogenation of aromatic compounds and alkynes is very little.14–18 γ-Aluminum oxide is one of the three common crystal forms of aluminum oxide. Pagni and Kabalka described γ-Al2O3 mediated iodination of alkynes and aromatic substrates with I2 to afford E-diiodoalkenes and iodinated aromatic compounds, respectively.14 Those iodinations did not occur without the activation of γ-Al2O3.
N-Iodosuccinimide (NIS) is a well-known iodinating agent and widely used in organic synthesis. Iodination with N-iodosuccinimide (NIS) often needs activating reagents. However, any accompanying reagents used along with iodinating reagents should be easily available to exploit more simple and efficient iodination procedure. Inspired by the indispensable role of γ-Al2O3 in the iodination of alkynes and aromatic compounds,14 we envisioned that γ-Al2O3 could activate the N-iodosuccinimide to generate 1-iodoalkynes from terminal alkynes. Herein, we report the γ-Al2O3 mediated direct iodination of terminal alkynes for the synthesis of 1-iodoalkynes under mild reaction condition.
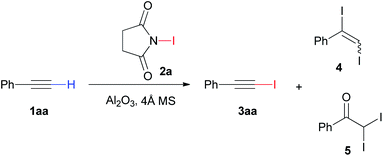
As shown in Table 1, the initial experiment was carried out by adding N-iodosuccinimide (NIS) 2a (2.2 mmol) to a solution of phenyl acetylene 1aa (2.0 mmol) and neutral γ-Al2O3 (2.6 mmol) in CH3CN. Surprisingly, the reaction afforded the desired product 1-(iodoethynyl)benzene 3aa in 90% yield after stirred at 80 °C for 1 h (Table 1, entry 1). To our delight, the reaction could provide 98% yield with the aid of 4 Å MS (entry 2). At the same time, the product 3aa could be obtained in 95% yield without the help of 4 Å MS when the amount of CH3CN used in the reaction was reduced from 10 mL to 2 mL (entry 2). Markedly, we obtained a mixture of 1aa, 3aa, 1,2-diiodovinylbenzene 4 and 2,2-diiodo-1-phenylethanone 5 in the absent of 4 Å MS and Al2O3 (entry 3). In addition, the 3aa was obtained in 41% yield only in the presence of 4 Å MS (entry 3). Therefore, the Al2O3 was indispensable to this iodination reaction (Table S1†). Similarly, high yields of 1-iodoalkyne 3aa were also obtained when basic and acidic Al2O3 were used instead of neutral Al2O3 (entry 4–5). Hence, the reaction was further examined in the presence of neutral Al2O3. The effort to decrease the reaction temperature to 25 °C only led to low yield (entry 6). Subsequently, we probed different solvents including DMF, THF, EA, MeOH and hexane, but those solvents resulted in poor yields of desired product (entry 7–11). Moreover, the reaction was investigated by varying the amount of Al2O3 and NIS to enhance the performance of the reaction (Table 1, entry 12–15). With 1.0 equivalents of NIS, the reaction generated 81% yield of 1-iodoalkyne 3aa (entry 12). However, when higher or lower amount of Al2O3 was used, low yields of the desired product were observed (entry 13–15). Finally, the best conditions to obtain the 1-iodoalkyne 3aa were treatment of 1aa with 1.1 equivalents of NIS, 1.3 equivalents of neutral γ-Al2O3 and 4 Å MS in CH3CN at 80 °C for 1 h. With the optimized conditions in hand, we also studied the halogenation of phenyl acetylene 1aa and used various halogenated reagents, including N-bromosuccinimide, 1,3-dibromo-5,5-dimethylhydantoin, pyridinium tribromide and N-chlorosuccinimide. These attempts did not provide satisfied results (Scheme S1†).
| Entry | Solvent | 2a (equiv.) | T (°C) | Al2O3 (equiv.) | Yieldb (%) |
|---|---|---|---|---|---|
a Unless noted otherwise, all reaction were conducted using phenylacetylene 1aa (2.0 mmol), N-iodosuccinimide 2a (2.2 mmol), 200 mg 4 Å MS, 265.0 mg neutral Al2O3 in 10 mL CH3CN at 80 °C for 1 h.b Isolated yield.c No 4 Å MS.d No Al2O3 and 4 Å MS, HPLC analysis of the reaction mixture after reacting at 80 °C for 1 h showed the molar ratio of 1aa to 1-iodoalkyne 3aa to 1,2-diiodovinylbenzene 4 to 2,2-diiodo-1-phenylethanone 5 was 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45.e No Al2O3.f Basic Al2O3 was used.g Acidic Al2O3 was used.h The reaction was conducted in 2 mL CH3CN. EA = ethyl acetate. 45.e No Al2O3.f Basic Al2O3 was used.g Acidic Al2O3 was used.h The reaction was conducted in 2 mL CH3CN. EA = ethyl acetate. |
|||||
| 1 | CH3CN | 1.1 | 80 | 1.3 | 90c |
| 2 | CH3CN | 1.1 | 80 | 1.3 | 98(95h) |
| 3 | CH3CN | 1.1 | 80 | — | —d(41e) |
| 4 | CH3CN | 1.1 | 80 | 1.3f | 96 |
| 5 | CH3CN | 1.1 | 80 | 1.3g | 97 |
| 6 | CH3CN | 1.1 | 25 | 1.3 | 42 |
| 7 | DMF | 1.1 | 80 | 1.3 | 63 |
| 8 | THF | 1.1 | 80 | 1.3 | 78 |
| 9 | EA | 1.1 | 80 | 1.3 | 86 |
| 10 | MeOH | 1.1 | 80 | 1.3 | 79 |
| 11 | Hexane | 1.1 | 80 | 1.3 | 62 |
| 12 | CH3CN | 1.0 | 80 | 1.3 | 81 |
| 13 | CH3CN | 1.1 | 80 | 1.0 | 84 |
| 14 | CH3CN | 1.1 | 80 | 0.1 | 70 |
| 15 | CH3CN | 1.1 | 80 | 2.0 | 91 |
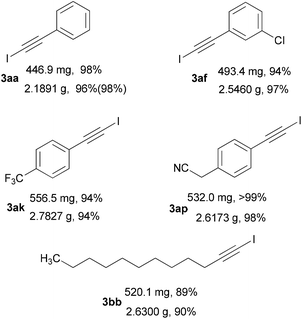
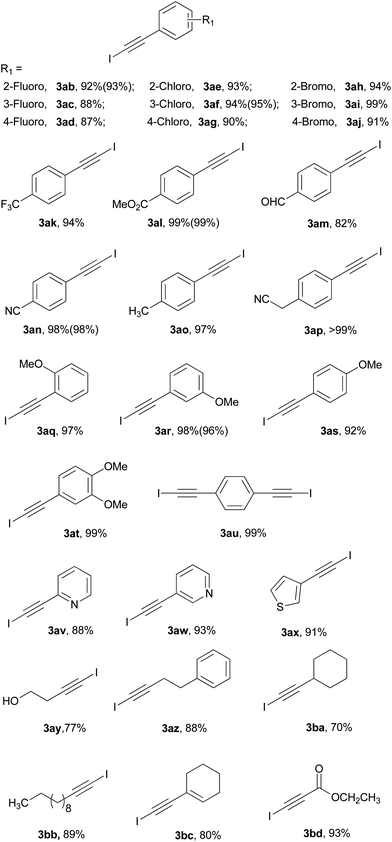
Based on the optimized reaction conditions, the generality of the direct iodination of various terminal alkynes was investigated (Table 2). Firstly, a wide variety of aromatic alkynes were firstly evaluated and could react with NIS to afford the corresponding 1-iodoalkynes 3ab–3au with good to excellent yields. Substrates having both electron-donating (e.g. Me, OMe, CH2CN) and electron-withdrawing (e.g. Cl, Br, CF3, CO2Me, CN) groups were effectively furnished the desired products. The halogen substituted aromatic alkynes gave the respective 1-iodoalkynes 3ab–3aj in excellent yields in relation to the position of the halogen on the phenyl ring and the electronegativity of the halogen. Among them, the meta bromo substituted alkyne 1ai gave the best yield (99%). The para substituted aromatic alkynes 1ak–1ap afforded the corresponding products 3ak–3ap in good to excellent yields. Although the para formyl substituted alkyne 1am yielded the 1-iodoalkyne 3am in 82% yield, the alkyne 1al could provide the corresponding product in 99% yield. Notably, the para cyanomethyl substituted alkyne 1ap afforded the desired product in almost quantitative yield (>99%). The dimethoxy substituted alkyne 1at provided better yield than the single methoxy substituted alkynes (1aq–1as). The 1,4-diethynylbenzene 1au could also be iodinated to generate the desired bisiodinated product in 99% yield. Secondly, the hetero aromatic and aliphatic alkynes were examined under the optimized conditions. The hetero aromatic alkynes delivered the iodination products 3av, 3aw and 3ax in 88%, 93% and 91% yield, respectively. The iodination reaction of aliphatic alkynes underwent smoothly to afford the corresponding products 3ay–3bb in good yields. The conjugated enyne 1bc could also react with NIS and gave the anticipated product 3bc in 80% yield. In addition, the iodination of more reactive alkyne 1bd was also evaluated and gave the synthetically useful 3bd in 93% yield. Finally, we also chose five terminal alkynes (1ab, 1af, 1al, 1an, 1ar) to examine the direct iodination requiring a small amount of solvent. The yields of solvent-reduced reaction (values in parentheses) performed in 2 mL CH3CN were comparable to the yields of the reaction conducted in 10 mL CH3CN. These results further confirmed that the 4 Å MS was not essential for the Al2O3 mediated iodination (Table S1†).
| a Reaction conditions: 1 (2.0 mmol), 2a (2.2 mmol), 4 Å MS (200 mg), Al2O3 (2.6 mmol), CH3CN (10 mL), 80 °C, 1 h. Isolated yields are given. The values in parentheses are the yields of reaction conducted in 2 mL CH3CN in the absent of 4 Å MS. |
|---|
 |
To further explore the potential of this protocol, we conducted the Al2O3 mediated direct iodination system for larger-scale synthesis (Scheme 2). The iodination reaction with (10 mmol, 1.0213 g) of phenylacetylene 1aa in 50 mL CH3CN afforded 96% yield. Delightfully, when the reaction was performed in 4 mL CH3CN, the yield could reach to 98% (value in parentheses). Moreover, the iodination of 1af, 1ak and 1ap proceeded smoothly in CH3CN, producing the corresponding 1-iodoalkynes in 97%, 94% and 98% yield, respectively. Finally, the larger scale reaction of aliphatic alkyne 1bb, could also generate 3bb in 90% yield. These excellent results indicated the promise of this direct iodination system for larger-scale preparation of 1-iodoalkynes from terminal alkynes.
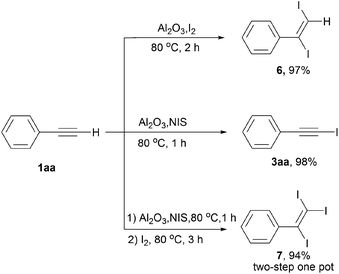
As presented in Scheme 3, we also tried to construct mono-, di-, and tri-iodination of terminal alkynes based on direct iodination mediated by γ-Al2O3 using phenyl acetylene 1aa as the model substrate. As depicted in the literature,14b the di-iodination product 6 could be obtained in 97% yield after stirred at 80 °C for 2 h in the presence of I2. Combining the NIS and I2 system in one pot provided the corresponding tri-iodination product 7 in 94% yield.
Finally, we studied the recyclability of Al2O3 and 4 Å MS. Al2O3 and 4 Å MS could be used as a recyclable catalyst for the direct iodination of phenyl acetylene 1aa (10 mmol) as it provided 96%, 93% and 88% yield at the first, second and third run, respectively (Fig. S1†). The severe decrease of the yield of the iodination was probably because the active sites of Al2O3 were blocked by unknown compounds and the unavoidable loss of solid catalyst during recovery and washing.
Although the detailed mechanism for the γ-Al2O3 mediated iodination remains unclear, we proposed that the dehydrated surface of γ-Al2O3, which contains partly exposed Al3+ and O2−, could serve as an effective medium for electrophilic iodination and greatly increase the chemoselectivity and rate of the reaction. Investigation of the detail mechanism and further applications of this methodology are toward in our laboratory.
Conclusions
In conclusion, we have developed an efficient approach for the synthesis of 1-iodoalkynes via γ-Al2O3 mediated direct iodination of terminal alkynes in the presence of NIS. Firstly, this protocol has excellent reactivity, high chemoselectivity and good functional group tolerance, and can be used for large-scale preparation of various 1-iodoalkynes. Secondly, with the aid of Al2O3 the mono-, di-, and tri-iodination products could be obtained in high yield. Thirdly, the Al2O3 and 4 Å MS could be reused for the direct iodination of terminal alkynes at least once without severe decrease of the yield.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Hubei Provincial Department of Education (T201719) for generous financial support.References
- For reviews, see: (a) A. Haque, R. A. Al-Balushi, I. J. Al-Busaidi, M. S. Khan and P. R. Raithby, Chem. Rev., 2018, 118, 8474–8597 CrossRef CAS; (b) W. Wu and H. Jiang, Acc. Chem. Res., 2014, 47, 82483–82504 Search PubMed; (c) W. Shi and A. Lei, Tetrahedron Lett., 2014, 55, 2763–2772 CrossRef CAS; (d) J. P. Brand and J. Waser, Chem. Soc. Rev., 2012, 41, 4165–4179 RSC.
- For selected recent examples, see: (a) M. E. de Orbe, M. Zanini, O. Quinonero and A. M. Echavarren, ACS Catal., 2019, 9, 7817–7822 CrossRef CAS; (b) X. Zeng, B. Chen, Z. Lu, G. B. Hammond and B. Xu, Org. Lett., 2019, 21, 2772–2776 CrossRef CAS PubMed; (c) S. M. Khake, S. Jain, U. N. Patel, R. G. Gonnade, K. Vanka and B. Punji, Organometallics, 2018, 37, 2037–2045 CrossRef CAS; (d) X. Zeng, S. Liu, G. B. Hammond and B. Xu, ACS Catal., 2018, 8, 904–909 CrossRef CAS PubMed; (e) G. Jiang, J. Li, C. Zhu, W. Wu and H. Jiang, Org. Lett., 2017, 19, 4440–4443 CrossRef CAS PubMed; (f) R. Chung, A. Vo and J. E. Hein, ACS Catal., 2017, 7, 2505–2510 CrossRef CAS; (g) P. J. Gonzalez-Liste, J. Francos, S. E. Garcia-Garrido and V. Cadierno, J. Org. Chem., 2017, 82, 1507–1516 CrossRef CAS PubMed; (h) X. Zeng, S. Liu, Z. Shi and B. Xu, Org. Lett., 2016, 18, 4770–4773 CrossRef CAS PubMed; (i) L. Huang, A. M. Olivares and D. J. Weix, Angew. Chem., Int. Ed., 2017, 56, 11901–11905 CrossRef CAS PubMed; (j) G. Jiang, C. Zhu, J. Li, W. Wu and H. Jiang, Adv. Synth. Catal., 2017, 359, 1208–1212 CrossRef CAS; (k) J. Li, W. Hu, C. Li, S. Yang, W. Wu and H. Jiang, Org. Chem. Front., 2017, 4, 373–376 RSC; (l) M. Ramanathan and S. Liu, Tetrahedron, 2017, 73, 4317–4322 CrossRef CAS; (m) V. K. Singh, A. Upadhyay and R. K. P. Singh, Tetrahedron Lett., 2017, 58, 156–158 CrossRef CAS; (n) P. Szuroczki, B. Boros and L. Kollar, Tetrahedron, 2018, 74, 6129–6136 CrossRef CAS; (o) M. Ye, Y. Wen, H. Li, Y. Fu and Q. Wang, Tetrahedron Lett., 2016, 57, 4983–4986 CrossRef CAS; (p) C. R. Reddy, S. A. Panda and A. Ramaraju, J. Org. Chem., 2017, 82, 944–949 CrossRef CAS; (q) R. M. Chowdhury and J. D. Wilden, Org. Biomol. Chem., 2015, 13, 5859–5861 RSC; (r) S. Mader, L. Molinari, M. Rudolph, F. Rominger and S. K. Hashmi, Chem.–Eur. J., 2015, 21, 3910–3913 CrossRef CAS.
- (a) G. W. Kabalka and A. R. Mereddy, Tetrahedron, 2004, 45, 1417–1419 CrossRef CAS; (b) J. E. Luithle and J. Pietruszka, Eur. J. Org. Chem., 2000, 2557–2562 CrossRef CAS; (c) I. J. Blackmore, A. N. Boa, E. J. Murray, M. Dennis and S. Woodward, Tetrahedron Lett., 1999, 40, 6671–6672 CrossRef CAS; (d) T. Mandai, T. Matsumoto, M. Kawada and J. Tsuji, J. Org. Chem., 1992, 57, 6090–6094 CrossRef CAS.
- (a) D. L. Uasnov and H. Yamamoto, J. Am. Chem. Soc., 2011, 133, 1286–1289 CrossRef PubMed; (b) M. L. N. Rao and M. Periasamy, Synth. Commun., 1995, 25, 2295–2299 CrossRef; (c) L. Meng, P. Cai, Q. Guo and S. Xue, Synth. Commun., 2008, 38, 225–231 CrossRef CAS; (d) S. Mader, L. Molinari, M. Rudolph, F. Rominger and S. K. Hashmi, Chem.–Eur. J., 2015, 21, 3910–3913 CrossRef CAS PubMed; (e) B. Wang, J. Zhang, X. Wang, N. Liu, W. Chen and Y. Hu, J. Org. Chem., 2013, 78, 10519–10523 CrossRef CAS; (f) M. Li, Y. Li, B. Zhao, F. Liang and L. Jin, RSC Adv., 2014, 4, 30046–30049 RSC; (g) W. Shi, Z. Guan, P. Cai and H. Chen, J. Catal., 2017, 353, 199–204 CrossRef CAS; (h) J. Yan, J. Li and D. Cheng, Synlett, 2007, 2442–2444 CrossRef CAS; (i) D. S. Rao, T. R. Reddy and S. Kashyap, Org. Biomol. Chem., 2018, 16, 1508–1518 RSC; (j) S. Chen, T. Hung, T. Lin and F. Tsai, J. Chin. Chem. Soc., 2009, 56, 1078–1081 CrossRef CAS; (k) S. Kodumuri, S. Peraka, N. Mameda, D. Chevella, R. Banothu, K. S. Gajula, S. Thokali and N. Nama, ChemistrySelect, 2017, 2, 748–752 CrossRef; (l) K. R. Reddy, M. Venkateshwar, C. U. Maheswari and P. S. Kumar, Tetrahedron Lett., 2010, 51, 2170–2173 CrossRef; (m) Y. Liu, D. Huang, J. Huang and K. Maruoka, J. Org. Chem., 2017, 82, 11865–11871 CrossRef CAS PubMed; (n) X. Liu, G. Chen, C. Li and P. Liu, Synlett, 2018, 2051–2055 CrossRef CAS; (o) T. Ferris, L. Carroll, R. C. Mease, A. C. Spivey and E. O. Aboagye, Tetrahedron Lett., 2019, 60, 936–939 CrossRef CAS PubMed; (p) S. Chen, X. Zhang, H. Zhao, X. Guo and X. Hu, Chin. J. Inorg. Chem., 2018, 38, 1172–1176 CrossRef CAS; (q) A. Gomez-Herrera, F. Nahra, M. Brill, S. P. Nolan and C. S. J. Cazin, ChemCatChem, 2016, 8, 3381–3388 CrossRef; (r) M. Nouzarian, R. Hosseinzadeh and H. Golchoubian, Synth. Commun., 2013, 43, 2913–2925 CrossRef CAS; (s) I. Nishiguchi, O. Kanbe, K. Itoh and H. Maekawa, Synlett, 2000, 89–91 CAS; (t) J. E. Hein, J. C. Tripp, L. B. Krasnova, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2009, 48, 8018–8021 CrossRef CAS.
- (a) T. Nishikawa, S. Shibuya, S. Hosokawa and M. Isobe, Synlett, 1994, 485–486 CrossRef CAS; (b) P. Starkov, F. Rota, J. M. D'Oyley and T. D. Sheppard, Adv. Synth. Catal., 2012, 354, 3217–3224 CrossRef CAS.
- (a) J. P. Das and S. Roy, J. Org. Chem., 2002, 67, 7861–7864 CrossRef CAS; (b) D. Naskar and S. Roy, J. Org. Chem., 1999, 64, 4896–6897 CrossRef.
- G. Pelletier, S. Lie, J. J. Mousseau and A. B. Charette, Org. Lett., 2012, 14, 5464–5467 CrossRef CAS.
- (a) Z. Wang, S. Campagna, K. Yang, G. Xu, M. E. Pierce, J. M. Fortunak and P. N. Confalone, J. Org. Chem., 2000, 65, 1889–1891 CrossRef CAS PubMed; (b) P. Michel and A. Rassat, Tetrahedron Lett., 1999, 40, 8579–8581 CrossRef CAS; (c) G. J. Hollingworth, A. M. E. Richecoeur and J. Sweeney, J. Chem. Soc., Perkin Trans. 1, 1996, 2833–2836 RSC; (d) G. J. Hollingworth and J. Sweeney, Synlett, 1993, 463–465 CrossRef CAS; (e) B. Bonnet, Y. Le Gallic, G. Ple and L. Duhamel, Synthesis, 1993, 1071–1073 CrossRef CAS; (f) E. J. Corey and P. L. Fuchs, Tetrahedron Lett., 1972, 13, 3769–3772 CrossRef.
- K. Smith, Solids Supports and Catalysts in Organic Synthesis, Ellis Horwood, Chichester, UK, 1992 Search PubMed.
- P. Laszlo, Preparative Chemistry Using Supported Reagents, Academic Press, London, UK, 1987 Search PubMed.
- H. van Bekkum, E. M. Flanigen and J. C. Jansen, Introduction to Zeolite Science and Practice, Studies in Surface Science and Catalysis, 1991, vol. 58 Search PubMed.
- H. Pines and W. O. Haag, J. Am. Chem. Soc., 1960, 82, 2471–2483 CrossRef CAS.
- A. Corado, A. Kiss, H. Knozinger and H. D. Muller, J. Catal., 1975, 37, 68–80 CrossRef CAS.
- (a) R. Boothe, C. Dial, R. Conaway, R. M. Pagni and G. W. Kabalka, Tetrahedron Lett., 1986, 27, 2207–2210 CrossRef CAS; (b) S. Larson, T. Luidhardt, G. W. Kabalka and R. M. Pagni, Tetrahedron Lett., 1988, 29, 35–36 CrossRef CAS; (c) G. Hondrogiannis, L. C. Lee, G. W. Abalka and R. M. Pagni, Tetrahedron Lett., 1989, 30, 2069–2070 CrossRef CAS.
- W. D. Bancroft and A. B. George, J. Phys. Chem., 1931, 35, 2943–2949 CrossRef CAS.
- W. S. Brey and K. A. Krieger, J. Am. Chem. Soc., 1949, 71, 3637–3641 CrossRef CAS.
- (a) M. Kodomari, N. Amanokura, K. Takeuchi and S. Yoshitomi, Bull. Chem. Soc. Jpn., 1992, 65, 306–308 CrossRef CAS; (b) M. Kodomari, H. Satoh and S. Yoshitomi, J. Org. Chem., 1988, 53, 2093–2094 CrossRef CAS; (c) M. Kodomari, H. Satoh and S. Yoshitomi, Ind. Chem. Libr., 1995, 7, 17–28 CAS.
- S. Stavber, M. Jereb and M. Zupan, Synthesis, 2008, 1487–1513 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00251h |
| This journal is © The Royal Society of Chemistry 2020 |