Open Access Article
Open Access ArticleCovalently functionalized poly(etheretherketone) implants with osteogenic growth peptide (OGP) to improve osteogenesis activity†
Maihemuti Yakufu ab,
Zongliang Wang
ab,
Zongliang Wang b,
Yu Wangb,
Zixue Jiaob,
Min Guob,
Jianguo Liu
b,
Yu Wangb,
Zixue Jiaob,
Min Guob,
Jianguo Liu *a and
Peibiao Zhang
*a and
Peibiao Zhang *b
*b
aDepartment of Orthopaedics, The First Hospital of Jilin University, Changchun, Jilin 130021, China. E-mail: jianguoliujlu@yeah.net
bKey Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China. E-mail: zhangpb@ciac.ac.cn
First published on 6th March 2020
Abstract
Polyetheretherketone (PEEK), as the most promising implant material for orthopedics and dental applications, has bone-like stiffness, excellent fatigue resistance, X-ray transparency, and near absence of immune toxicity. However, due to biological inertness, its bone conduction and bone ingrowth performance is limited. The surface modification of PEEK is an option to overcome these shortcomings and retain most of its favorable properties, especially when excellent osseointegration is desired. In this study, a simple reaction procedure was employed to bind the osteogenic growth peptide (OGP) on the surface of PEEK materials by covalent chemical grafting to construct a bioactive interface. The PEEK surface was activated by N,N′-disuccinimidyl carbonate (DSC) after hydroxylation, and then OGP was covalently grafted with amino groups. The functionalized surface of PEEK samples were characterized by X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FT-IR), water contact angle measurement and biological evaluation in vitro. OGP-functionalized PEEK surface significantly promoted the attachment, proliferation, alkaline phosphatase (ALP) activity and mineralization of pre-osteoblast cells (MC3T3-E1). The in vivo rat tibia implantation model is adopted and micro-CT analyses demonstrated that the OGP coating significantly promoted new bone formation around the samples. The in vitro and in vivo results reveal that the modification by covalent chemical functionalization with OGP on PEEK surface can augment new bone formation surrounding implants compared to bare PEEK and PEEK implant modified by covalently attached OGP is promising in orthopedic and dental applications.
1. Introduction
The prevalence of orthopaedic or bone implants has greatly increased worldwide.1 For several decades, traditional metallic implants made of titanium alloys have been widely used as orthopaedic implants owing to their excellent corrosion resistance, high mechanical strength, and biocompatibility.2 However, the release of harmful metal ions and stress shielding effect caused by the mismatch of elastic modulus between metal and cortical bone may eventually lead to implant failure,3 which is the defect of the metal implant itself. Polymeric materials are not generally strong enough to support repetitive loading without plastic deformation,4 The exception is poly(etheretherketone) (PEEK), which has bone-like stiffness, excellent fatigue resistance, high chemical resistance and near absence of immune toxicity. Moreover, the radiolucency of PEEK helps clinicians clearly assess osseointegration.5,6 The bioinert nature of PEEK strongly limit its clinical applications to biomedical devices in situations where osseointegration is critical.7 Currently, in order to overcome the bio-inertness of PEEK, a large number of researches have been carried out and multiple strategies were found.7–10 It has been demonstrated that the surface chemistry and structures are prime factors governing cell adhesion and growth.11 One of the most important methods is the biofunctionalization of PEEK by surface modification with bioactive molecules, peptide or growth factors.9,10,12The osteogenic growth peptide (OGP, isoelectric point = 11.4, Mw = 1.5 kDa, Ala-Leu-Lys-Arg-Gln-Gly-Arg-Thr-Leu-Tyr-Gly-Phe-Gly-Gly) is a naturally occurring tetradecapeptide identical to the C-terminal amino acid sequence 89–102 of histone H4 (H4).13 OGP in high abundance occurs physiologically in human and rodent serum and in serum-free medium of osteoblastic and fibroblastic cells.14,15 Several studies have reported that, OGP and its active fragment acted similarly as soluble OGP in vitro, when immobilized on surfaces.16 To translate these findings to biomaterial applications, OGP has been immobilized into polymer scaffolds17–19 and on titanium substrates20–22 to observe its effects on osteoblast cell lines. Previous studies have indicated that immobilization of OGP could accelerate the osseointegration process. Hence, as shown in Scheme 1, in this study, we attempted to enhance cell adhesion, spreading, proliferation and osteogenic differentiation of mouse pre-osteoblasts (MC3T3-E1) on the modified PEEK films with covalently grafted OGP.
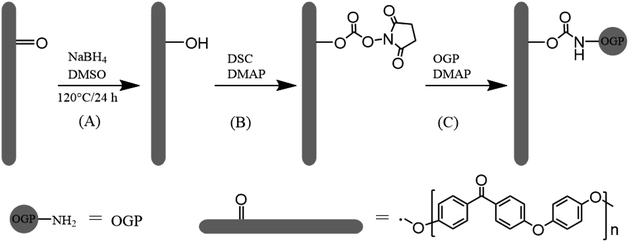 | ||
| Scheme 1 Schematic representation of (A) keto group reduction on the PEEK surface, (B) activation of hydroxyl group with N,N′-disuccinimidyl carbonate and (C) immobilization of OGP. | ||
2. Experimental part
2.1. Materials
The PEEK samples were purchased from Victrex (England). It was cut into disk sample with a diameter of 15 mm and a thickness of 0.5 mm for modification, characterization and in vitro assessment in 24-well tissue culture plates. In order to obtain pristine surface, the PEEK sheets were immersed in refluxing acetone for 48 h, rinsed twice with acetone, and dried under vacuum at 60 °C for 3 h. The OGP peptide (ALKRQGRTLYGFGG, 95% purity) was obtained from GL Biochem (Shanghai) Ltd. (China). Alizarin red-S, calcein-AM, and CCK-8 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium borohydride, N,N′-disuccinimidyl carbonate (DSC), sodium phosphate, ethanolamine hydrochloride, and 4-dimethylaminopyridine (DMAP) were obtained from Aladdin Reagent Co., Ltd (Shanghai, China). DMSO was purified with distillation from calcium hydride before use. All other chemicals and solvents were of analytical grade or higher and used as received. MC3T3-E1 cells were purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.2.2. Hydroxylation on PEEK surface (PEEK–OH)
A protocol reported by Noiset23 et al. was used herein to selectively reduce PEEK surface carbonyl groups to hydroxyl groups (Scheme 1). The hydroxylation of PEEK films was achieved according to the protocol reported by the literature.24 In brief, DMSO had been distilled to remove water and oxygen prior to use. DMSO (70 mL) and sodium borohydride (140 mg) were introduced in a 150 mL round bottomed flask equipped with reflux condenser and heated at 120 °C under stirring until dissolution occurred. PEEK sheets were immersed in the reaction mixture at 120 °C for 24 h under nitrogen, then removed from reaction solution and successively rinsed in stirred methanol for 15 min, distilled water for 10 min, 0.5 M HCl for 10 min, deionized water for 10 min, and ethanol for 10 min, respectively. The sheets were subsequently dried at 60 °C under vacuum for 3 h and stored under N2. The PEEK sheet with surface hydroxyl groups was abbreviated as PEEK–OH.2.3. Immobilization of OGP peptide on PEEK surface (PEEK–OGP)
N,N′-Disuccinimidyl carbonate was used for activation of hydroxyl group of PEEK–OH. The procedure was performed as previously described.25 In brief, PEEK–OH sheets were immersed in 20 mL of anhydrous DMSO and N,N′-disuccinimidyl carbonate (7 mmol dissolved in 10 mL anhydrous DMSO) was added. 4-Dimethylaminopyridine (7 mmol in 5 mL anhydrous DMSO) was added slowly under magnetic stirring. Activation proceeded for 6 h at room temperature. The activated PEEK–NHS (Scheme 1) was then transferred to a new beaker followed by washing thrice with anhydrous DMSO.Then, the samples were immersed in 10 mL deionized water containing 1 mg OGP (Scheme 1), and stirred at room temperature for 24 h. PEEK–OGP specimens were then rinsed in ethanolamine hydrochloride solution (0.05 M in deionized water) at room temperature for 4 h in order to eliminate the unreacted NHS groups. The prepared substrates were thoroughly washed with PBS, ultrasonically treated with 1 M sodium chloride for 10 min each to remove weakly bounded peptides.26 And then it was rinsed with distilled water and acetone for 10 min, respectively. The sheets were subsequently dried under vacuum for 3 h and stored under N2. The finally obtained PEEK sheet was designated as PEEK–OGP.
2.4. Surface characterization
FT-IR (Bio-Rad Win-IR spectrometer, UK) was used to detect chemical groups. The FT-IR spectra were carried out in the wavelength range between 600 and 4000 cm−1 with a resolution of 2 cm−1. X-ray photoelectron spectroscopy (XPS) (Thermo) was used to detect the surface chemical constituents and to confirm the presence of OGP on PEEK surfaces. Surface hydrophilicity of the specimens was assessed using the sessile drop method on a contact angle system (VCA 2000, AST) by placing 2 μL of distilled water on the PEEK surface. Each different substrate was measured at three separate points and the final values were an average of these measurements with the standard deviation.2.5. Biological response
Cell spreading morphology was also visualized by scanning electron microscope (SEM). After cultured for 24 h, the medium was removed, and the samples were rinsed with PBS and then fixed in 4% PFA solution for 30 min. The PFA solution was then removed, and the samples were washed thrice with PBS and dehydrated using a graded series of ethanol aqueous solutions from 50% to 100% v/v. The samples were kept in each solution for 30 min. Finally, the critically dried samples were sputtered with gold and examined under SEM.
2.6. In vivo studies
2.7. Statistical analysis
All experiments were performed at least in triplicate. Quantitative data are expressed as the mean ± standard deviation (SD). Statistically significant differences (p) among groups were measured using one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison analysis using SPSS 19.0 software. Paired t-test for in vivo evaluation was used to determine the statistical significance of observed differences. p < 0.05 was considered statistically significant.3. Results and discussion
3.1. Carbonyl reduction on PEEK surface
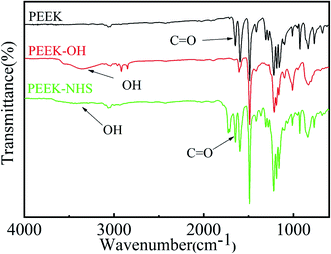
Fig. 1 shows the ATR-FTIR spectra of PEEK and PEEK–OH. Compared to PEEK, the broad stretching band for hydroxyl groups was observed around 3400 cm−1 and a diminution in the intensity of the carbonyl band at 1648 cm−1 was clearly visible in the PEEK–OH spectra corroborated the conversion from carbonyl groups to hydroxyl groups.28 In order to quantitatively track the reduction process, the ratio between the absorbance of the carbonyl peak at 1648 cm−1 and the absorbance of the reference band (almost unchanging) at 1490 cm−1 due to the aromatic rings was calculated for PEEK and PEEK–OH. The value decreased from 0.306 for PEEK to 0.002 for PEEK–OH suggesting that the carbonyl group was successfully reduced.The static water contact angles (CAwater) were evaluated to analyse the hydrophilicity of the modified surface. The measured water contact angles are summarized as a histogram in Fig. 2, and the images of the water droplets on the samples are presented in the top right of each column in Fig. 2. Pure PEEK surfaces exhibited water contact angles of 71.22° ± 5.17°. Furthermore, after hydroxylation of PEEK, the existence of surface hydroxyl groups improved the surface wettability, and then the contact angle of the surface decreased to 49.78° ± 2.43° (Fig. 2). On the basis of these observations, it can be safely concluded that surface hydroxylation of PEEK sheet was successfully achieved through selective carbonyl reduction.10
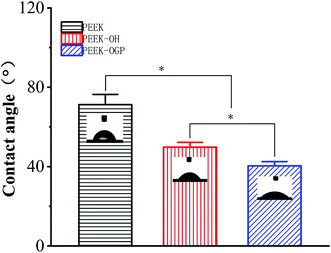 | ||
| Fig. 2 Contact angle of PEEK after surface treatment. Statistical significance is indicated by *p < 0.05. | ||
3.2. Immobilization of OGP on PEEK surface
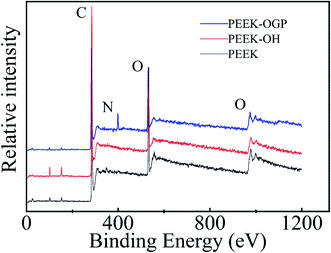
The immobilization of OGP accompanied by the degradation of NHS active groups which was used to activate PEEK surfaces reacting with the amino group of OGP as depicted in Fig. 1. After OGP modification, the water contact angle of PEEK–OGP was significantly decreased to 40.39° ± 2.15° (Fig. 2). These results could be attributed to the OGP peptide molecule layer and the introduction of OGP can elevate the hydrophilicity of the modified PEEK.XPS analysis was carried out to explore the chemical element changes in the samples at each step of the modification, and the results are presented in (Fig. 3). N peaks were identified on the PEEK–OGP spectra, indicating that OGP was successfully immobilized on the surface of PEEK.
3.3. Biological response
As shown in Fig. 4, after cultured for 6 and 24 h, PEEK with the immobilization of OGP (Fig. 4c and f) showed significantly higher cell density compared to the PEEK without OGP immobilization (Fig. 4a and d). Besides, as shown in Fig. 4b, the hydroxylated PEEK surface also improved cell adhesion to some extent. Although the pre-osteoblasts on the samples exhibited spindle-shaped or rounded morphologies after incubation for 6 h, the attached pre-osteoblasts on the PEEK–OH and PEEK–OGP were elongated and the rounded pre-osteoblasts on the PEEK–OGP had more pseudopods extensions than that on the PEEK and PEEK–OH (Fig. 4). In addition, more better cell spreading was observed on the PEEK substrates with immobilization of OGP instead of that on the untreated PEEK which remained as isolated single cells.
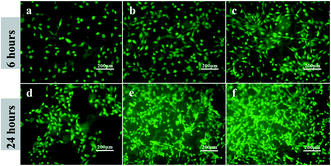 | ||
| Fig. 4 Adhesion of MC3T3-E1 cells on various samples after 6 h and 24 h incubation. PEEK (a and d), PEEK–OH (b and e), PEEK–OGP (c and f). | ||
As shown in Fig. 5, the cell spreading morphology differed greatly on various sample surfaces. The MC3T3-E1 cells attached on pure PEEK showed a spheroid shape and cells on the PEEK–OH samples were found to be transformed from spheroid shape to thread spindles, the MC3T3-E1 spreading on PEEK–OGP stretched more and exhibited more filopodia than in the PEEK control.
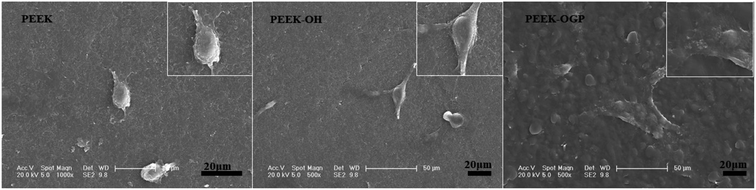 | ||
| Fig. 5 Cell spreading morphologies of MC3T3-E1 detected by SEM at low and high magnifications after 24 hours of culture on different samples. | ||
Thus, these results suggested that the immobilization of OGP on the PEEK films would be more favourable for cell adhesion and spreading.
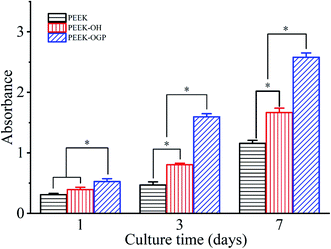 | ||
| Fig. 6 Cell proliferation of MC3T3-E1 pre-osteoblasts cultured on the PEEK, PEEK–OH and PEEK–OGP for 1, 3 and 7 days. Statistical significance is indicated by *p < 0.05. | ||
Cell adhesion is correlated with the cellular ability to survive and initiate proliferation on the substrate surface, with consequently increased cell spreading, increased cell survival, and cell cycling. Cell proliferation is closely correlated with the amount of new bone formation. Hence, better cell adhesion, spreading, and proliferation probably results in a larger amount of bone tissue around the implants and robust bone-implant bonding in vivo.
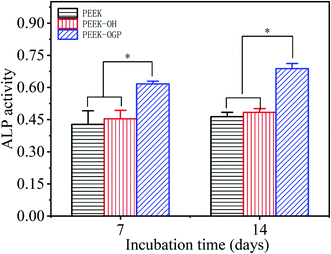
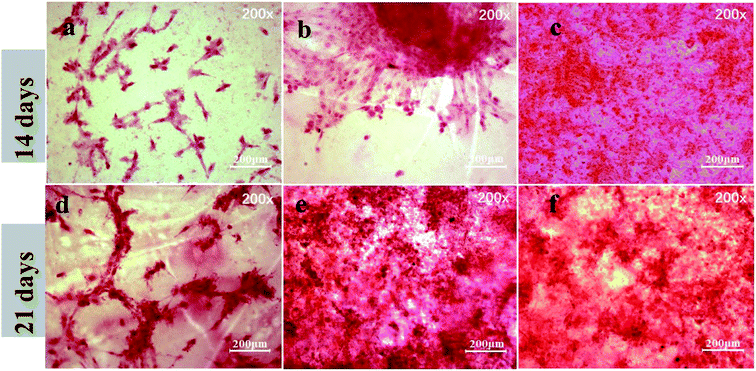
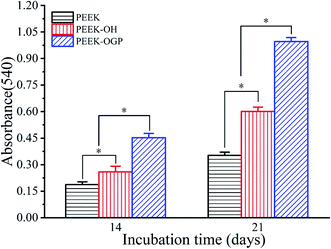
ARS staining was also performed after 21 day incubation, and the microscopic images are displayed in Fig. 8(d–f). These showed more significant differences in the calcium minerals between each group after 21 day incubation. Among all the groups, the most enhanced mineral nodules were observed for the PEEK–OGP, which was corresponded with the 14 day analysis. The results demonstrated that the OGP could significantly enhance cell differentiation compared to that observed with the PEEK–OH. Moreover, Fig. 9 indicates that the quantity of calcium minerals on PEEK–OH and PEEK–OGP was higher than that on PEEK, which corresponded with the results of our ALP activity study. The samples modified with OGP showed significantly higher calcium deposition than those of PEEK and PEEK–OH as shown in Fig. 9, indicating that immobilization of OGP on the PEEK surface could enhance osteodifferentiation of MC3T3-E1 cells.
It is widely accepted that the initial interactions between the cells and implant surface are crucial to clinical success and improvement can lead to faster bone formation.33 In this investigation, the results show that cell adhesion and proliferation are significantly enhanced on PEEK–OGP compared to the PEEK and no cytotoxic effects can be found from the MC3T3-E1 cells. Therefore, PEEK modified with OGP on its surface may produce more bone tissues around the implants and more robust bone-implant integration is also expected in vivo.
3.4. Osteogenic properties in vivo
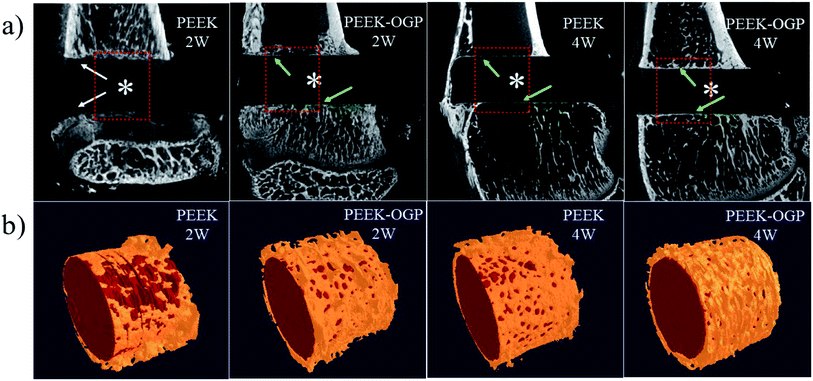
At 2 weeks and 4 weeks post-implantation, the new bone formations are detected by micro-CT. The 2D and 3D reconstructed micro-CT images of the two groups are displayed in Fig. 10. Fig. 10a shows representative 2D micro-CT images of transverse sections of rat tibial metaphysis implanted with different samples. The white asterisks mark the position of the implanted samples. As observed generally, two weeks after implantation, obvious gap could be seen around PEEK (white arrows), but the newly formed bone was obviously surrounding the latter.More new bone can be observed around PEEK–OGP implants compared to bare PEEK, indicating that PEEK–OGP possessed superior osteoconduction in vivo. Fig. 10b shows the 3D reconstruction of different samples (red) and their surrounding new bone (yellow). There was negligible bone around PEEK, but a lot of bone were observed around the PEEK–OGP. The bone volume fraction (bone volume/total volume, BV/TV) of the new bone were respectively shown in Fig. 11, and the values of PEEK–OGP were obviously higher at all the different time points.
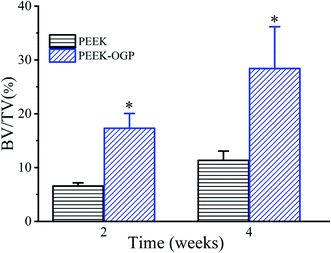 | ||
| Fig. 11 Corresponding values of new bone volume/total volume (BV/TV) for various samples, respectively; *p < 0.05. | ||
Previous studies have shown that osseointegration direct osteoblast colonization on an implant surface, synthesizing extracellular bone matrix and finally forming new bone.12,18,34 The results above indicate that PEEK with the OGP coating significantly enhanced new bone formation compared with the pristine PEEK control, which corresponded to the proliferation and osteogenic differentiation of MC3T3-E1 in vitro. Previous studies reported that OGP modification of nanoparticles or polymers yielded higher osteo-related protein expression and calcified bone formation.17,18 Hence, functionalized PEEK, after modification with OGP, enhanced new bone formation around the implant and further promoted ossification between the implant and the bone. These findings confirm that the OGP-decorated PEEK favoured the improvement of in vivo osteoconduction.
4. Conclusions
OGP was successfully immobilized on the PEEK surface by covalent bond. As the in vitro results shown, the samples with immobilized OGP exhibited excellent osteogenic activity. In addition, the in vitro results show that the samples immobilized with OGP can promote the bone related proteins expression. The in vivo results agree well with the in vitro results, the newly formed bone around the samples loaded with OGP is more than the pristine substrate in the rat tibias. Revealing that PEEK sample with the covalently bonded OGP could have huge potential for orthopaedic and dental applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Projects. 51473164 and 51673186), the joint funded program of Chinese Academy of Sciences and Japan Society for the Promotion of Science (GJHZ1519), Achievement Transformation Fund of the First Hospital of Jilin University (JDYYZH-1902043), Jilin Medical and Health Talents Special project (JLSCZD2019-023), and the Special Fund for Industrialization of Science and Technology Cooperation between Jilin Province and Chinese Academy of Sciences (2017SYHZ0021).Notes and references
- C. Stewart, B. Akhavan, S. G. Wise and M. M. M. Bilek, Prog. Mater. Sci., 2019, 106, 40 CrossRef.
- X. Liu, P. K. Chu and C. Ding, Mater. Sci. Eng., R, 2004, 47, 49–121 CrossRef.
- Q. Chen and G. A. Thouas, Mater. Sci. Eng., R, 2015, 87, 1–57 CrossRef.
- L. Pruitt and J. Furmanski, Jom, 2009, 61, 14–20 CrossRef CAS.
- S. M. Kurtz and J. N. Devine, Biomaterials, 2007, 28, 4845–4869 CrossRef CAS.
- J. M. Toth, M. Wang, B. T. Estes, J. L. Scifert, H. B. Seim and A. S. Turner, Biomaterials, 2006, 27, 324–334 CrossRef CAS PubMed.
- Y. Ren, P. Sikder, B. Lin and S. B. Bhaduri, Mater. Sci. Eng. C, 2018, 85, 107–113 CrossRef CAS PubMed.
- J.-L. Sui, M.-S. Li, Y.-P. Lü, L.-W. Yin and Y.-J. Song, Surf. Coat. Technol., 2004, 176, 188–192 CrossRef CAS.
- T. Wan, L. Li, M. Guo, Z. Jiao, Z. Wang, Y. Ito, Y. Wan, P. Zhang and Q. Liu, J. Mater. Sci., 2019, 54, 11179–11196 CrossRef CAS.
- Y. Zheng, C. Xiong, X. Li and L. Zhang, Appl. Surf. Sci., 2014, 320, 93–101 CrossRef CAS.
- X. Zhu, J. Chen, L. Scheideler, R. Reichl and J. Geis-Gerstorfer, Biomaterials, 2004, 25, 4087–4103 CrossRef CAS PubMed.
- Y. Zheng, L. Liu, L. Xiao, Q. Zhang and Y. Liu, Colloids Surf., B, 2019, 173, 591–598 CrossRef CAS.
- I. Bab, D. Gazit, M. Chorev, A. Muhlrad, A. Shteyer, Z. Greenberg, M. Namdar and A. Kahn, EMBO J., 1992, 11, 1867–1873 CrossRef CAS.
- Z. Greenberg, M. Chorev, A. Muhlrad, A. Shteyer, M. Namdar-Attar, N. Casap, A. Tartakovsky, M. Vidson and I. Bab, J. Clin. Endocrinol. Metab., 1995, 80, 2330–2335 CAS.
- Z. Greenberg, H. Gavish, A. Muhlrad, M. Chorev, A. Shteyer, M. Attar-Namdar, A. Tartakovsky and I. Bab, J. Cell. Biochem., 1997, 65, 359–367 CrossRef CAS.
- Y. Gabet, R. Müller, E. Regev, J. Sela, A. Shteyer, K. Salisbury, M. Chorev and I. Bab, Bone, 2004, 35, 65–73 CrossRef CAS PubMed.
- S. C. Pigossi, G. J. P. L. de Oliveira, L. S. Finoti, R. Nepomuceno, L. C. Spolidorio, C. Rossa Jr, S. J. L. Ribeiro, S. Saska and R. M. Scarel-Caminaga, J. Biomed. Mater. Res., Part A, 2015, 103, 3397–3406 CrossRef CAS.
- G. M. Policastro, F. Lin, L. A. Smith Callahan, A. Esterle, M. Graham, K. Sloan Stakleff and M. L. Becker, Biomacromolecules, 2015, 16, 1358–1371 CrossRef CAS.
- K. S. Stakleff, F. Lin, L. A. Smith Callahan, M. B. Wade, A. Esterle, J. Miller, M. Graham and M. L. Becker, Acta Biomater., 2013, 9, 5132–5142 CrossRef CAS PubMed.
- C. Chen, X. Kong, S.-M. Zhang and I.-S. Lee, Appl. Surf. Sci., 2015, 334, 62–68 CrossRef CAS.
- C. Chen, H. Li, X. Kong, S.-M. Zhang and I.-S. Lee, Int. J. Nanomed., 2014, 10, 283–295 Search PubMed.
- W. Tang, G. M. Policastro, G. Hua, K. Guo, J. Zhou, C. Wesdemiotis, G. L. Doll and M. L. Becker, J. Am. Chem. Soc., 2014, 136, 16357–16367 CrossRef CAS.
- O. Noiset, C. Henneuse, Y.-J. Schneider and J. Marchand-Brynaert, Macromolecules, 1997, 30, 540–548 CrossRef CAS.
- J. Wu, L. Li, C. Fu, F. Yang, Z. Jiao, X. Shi, Y. Ito, Z. Wang, Q. Liu and P. Zhang, Colloids Surf., B, 2018, 169, 233–241 CrossRef CAS.
- T. Sawayama, M. Tsukamoto, T. Sasagawa, K. Nishimura, T. Deguchi, K. Takeyama and K. Hosoki, Chem. Pharm. Bull., 1990, 38, 110–115 CrossRef CAS PubMed.
- K. C. Dee, T. T. Andersen and R. Bizios, Tissue Eng., 1995, 1, 135–145 CrossRef CAS.
- R. Agarwal, C. González-García, B. Torstrick, R. E. Guldberg, M. Salmerón-Sánchez and A. J. García, Biomaterials, 2015, 63, 137–145 CrossRef CAS.
- A. M. Díez-Pascual, G. Martínez and M. A. Gómez, Macromolecules, 2009, 42, 6885–6892 CrossRef.
- C. A. Scotchford, M. Ball, M. Winkelmann, J. Vörös, C. Csucs, D. M. Brunette, G. Danuser and M. Textor, Biomaterials, 2003, 24, 1147–1158 CrossRef CAS.
- A. M. DeLise, L. Fischer and R. S. Tuan, Osteoarthr. Cartil., 2000, 8, 309–334 CrossRef CAS PubMed.
- X. Hu, K. G. Neoh, Z. Shi, E. T. Kang, C. Poh and W. Wang, Biomaterials, 2010, 31, 8854–8863 CrossRef CAS PubMed.
- P. Ni, J. Xie, C. Chen, Y. Jiang, Z. Zhao, Y. Zhang, Y. Lu and J. Yu, Microchim. Acta, 2019, 186, 320 CrossRef PubMed.
- Y. Zhao, H. M. Wong, W. Wang, P. Li, Z. Xu, E. Y. W. Chong, C. H. Yan, K. W. K. Yeung and P. K. Chu, Biomaterials, 2013, 34, 9264–9277 CrossRef CAS PubMed.
- Z. J. Sun, L. P. Ouyang, X. H. Ma, Y. Q. Qiao and X. Y. Liu, Colloids Surf., B, 2018, 171, 668–674 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00103a |
| This journal is © The Royal Society of Chemistry 2020 |