Open Access Article
Open Access ArticleAnalysis and optimization of process parameters for in vitro biomineralization of CaCO3 by Klebsiella pneumoniae, isolated from a stalactite from the Sahastradhara cave†
Rachna Rautelaa and
Seema Rawat *ab
*ab
aMicrobial Diversity Lab, Department of Botany and Microbiology, School of Life Sciences, HNB Garhwal University, Srinagar-246174, Pauri Garhwal, Uttarakhand, India. E-mail: seema.rawat@cug.ac.in
bMicrobiology Lab, School of Life Sciences, Central University of Gujarat, Gandhinaagr-38210, Gandhinagar, Gujarat, India
First published on 27th February 2020
Abstract
Stalactite is a speleothem which is usually made up of calcium carbonate crystals. In the present study the bacterial isolates, recovered from a stalactite from the Sahastradhara cave, were screened for their ability to precipitate calcium carbonate in order to understand whether mineralization in caves is a biogenic process or not. Five bacterial isolates were found to precipitate calcium carbonate via urease. The most potent bacterial isolate was identified as Klebsiella pneumoniae (accession number MG946801) based on 16S rDNA sequencing. The optimized conditions, for calcium carbonate precipitation, determined by response surface methodology using CCD were found to be: 1.5625% urea, 19.98% inoculum level, 6.98 pH and 38 h 24 min. The morphology and crystalline structure of the precipitated mineral were revealed by SEM. EDX analysis confirmed the presence of carbon, oxygen and calcium in a precipitated crystal. XRD analysis confirmed the crystalline structure of a mineral with rhombohedral shape and 166 Å crystal size. This bacterium can serve as a promising candidate for producing bioconcrete.
1. Introduction
Calcium carbonate is the most important mineral on earth as it constitutes 4% of total earth crust. Calcite, aragonite and vaterite are pure forms of calcium carbonate which differ in structure.4 Calcite is a most stable form of CaCO3 with rhombohedral shape. The hydrated forms of minerals comprise about 60% of biogenic minerals such as the two forms of carbonate monohydrocalcite and one amorphous form containing water. Calcification is a general phenomenon among the bacteria but cave dwelling bacteria which lack photosynthetic activity, play a crucial role in calcium carbonate precipitation. However, there are still ambiguities regarding the origin of speleothems in caves. There are reports indicating that the formation of speleothems, such as stalactites and stalagmites through the precipitation of calcite, is an abiogenic process10,29 while many workers have reported the crucial role of microorganisms in calcium carbonate precipitation during speleogenesis.7,8,23,25,26,32The role of microorganisms in the cave environment can not be underestimated as studies have shown that microbial metabolism is responsible for mineral precipitation and dissolution of cave walls.22 However, the existing knowledge about the life-forms and biogeochemical processes contained within them is meager primarily due to difficulties in approaching this habitat. A variety of precipitation processes in caves result in the deposition of carbonate speleothems, silicates, iron and manganese oxides, sulfur compounds and nitrates. Among all the speleothems, stalactites are the most frequent and most common and resemble carrots hanging from cave ceilings. In cave ecosystems, the varied heterotrophic microbial communities in stalactite are well documented viz., Bacillus and Streptomyces from stalactite of Grotta dei Cervi,30 Kocuria sp. from stalactite of Cervo cave,12 Bacillus pumilis and Bacillus thuringiensis from stalactite of Sahastradhara cave,8,9 Bacillus, Burkholderia, and Pasteurella spp. from Gypsum cave of grave grubbo,13 Arthrobacter and Rhodococcus sp. from stalactite of Pristine Karstic Herrenberg cave.35
Stalactites are usually composed of calcite but may consist of other minerals. The calcium carbonate mineralization ability of cave bacteria has been widely reported viz., Rhodococcus sp. from Grotta dei Cervi,21 Bacillus pumilis and B. thuringiensis from Sahastradhara cave,8 Bacillus sp. from cave of central China,39 Arthrobacter and Rhodococcus sp. from Pristine Karstic Herrenberg cave,35 Lysinibacillus sp. and Bacillus sp. from caves of Meghalaya5 and Bacillus subtilis and Cupriavidus sp. from Rani cave.16 Several bacteria of Enterobacteriaceae family have been reported to form a crystalline structure containing calcium.28 The microorganisms precipitate calcium carbonate through various processes viz., photosynthesis, ammonification, denitrification, carbonic anhydrase production, urease enzyme production. Calcium precipitation through urease enzyme activity has been widely studied because it generates carbonate more easily than other reactions.1–3,18,19,27,36
The present study was carried out to determine the in vitro potential of bacteria recovered from stalactite of Sahastradhara cave to understand the origin of stalactite in the cave. Sahastradhara cave is located along the bank of river Baldi in Dehradun valley, Uttarakhand, India. The various factors which can affect the calcium carbonate precipitation were optimized using Response Surface Methodology (RSM) as it is a powerful and efficient mathematical approach which has been widely applied in the optimization processes.17,31,33 It helps in the understanding of the interaction between the factors affecting the response in fewer experimental trials which is not possible with the traditional one-factor-at-a-time approach.
2. Experimental
2.1 Sampling
Stalactite samples were collected aseptically from the cave and carried to the laboratory in the ice bucket. The samples were air dried for 48 h and crushed into a fine powder using sterile mortar and pestle.2.2 Isolation of calcium precipitating bacteria
Calcium precipitating bacteria of stalactite samples were recovered by enrichment culturing on B4 medium (2.5 g l−1 calcium acetate, 10 g l−1 glucose, 4 g l−1 yeast extract) at 30 °C for 10 days.2.3 Screening for calcium precipitation
| Millimoles of EDTA = ml EDTA × molarity EDTA = millimoles of calcium |
| Mass of calcium = millimoles of calcium × molar mass of calcium (40.08) |
2.4 Optimization of calcium carbonate precipitation
The precipitation of calcium carbonate by the best isolate was optimized through response surface methodology using Design Expert 10 software (Stat-Ease Inc., Minneapolis, USA). Central Composite Design was used to generate a quadratic response with four factors, viz., urea percentage (X1), inoculum percentage (X2), pH (X3), time (X4). The coded and non-coded (actual) level of the variables is given in Table 1. The central composite rotatable design for experimental runs and combinations for biomineralization is given in Table 2. A total of 35 runs were performed in triplicate.| Independent variable | Coded levels | −2 | −1 | 0 | 1 | 2 |
|---|---|---|---|---|---|---|
| Urea percentage (%) | X1 | 0.5 | 1 | 1.5 | 2 | 2.5 |
| Inoculum percentage (%) | X2 | 10 | 15 | 20 | 25 | 30 |
| pH | X3 | 6 | 6.5 | 7 | 7.5 | 8 |
| Time (h) | X4 | 12 | 24 | 36 | 48 | 60 |
| Run | Coded variables | Uncoded variables | Response (%) of CaCO3 precipitated | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Urea concentration (%) | Inoculum level (%) | pH | Time (h) | ||
| 1 | 2 | 0 | 0 | 0 | 2.5 | 20 | 7 | 36 | 97.453 |
| 2 | 2 | 0 | 0 | 0 | 2.5 | 20 | 7 | 36 | 97.562 |
| 3 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.765 |
| 4 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.632 |
| 5 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.542 |
| 6 | −1 | 1 | 1 | −1 | 1 | 25 | 7.5 | 24 | 96.763 |
| 7 | −1 | −1 | −1 | −1 | 1 | 15 | 6.5 | 24 | 96.342 |
| 8 | −1 | −1 | 1 | −1 | 1 | 15 | 7.5 | 24 | 97.021 |
| 9 | −1 | 1 | −1 | −1 | 1 | 25 | 6.5 | 24 | 95.923 |
| 10 | −1 | −1 | −1 | 1 | 1 | 15 | 6.5 | 48 | 97.145 |
| 11 | −1 | 1 | 1 | 1 | 1 | 25 | 7.5 | 48 | 96.231 |
| 12 | −1 | −1 | 1 | 1 | 1 | 15 | 7.5 | 48 | 96.873 |
| 13 | −1 | 1 | −1 | 1 | 1 | 25 | 6.5 | 48 | 96.321 |
| 14 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.365 |
| 15 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.674 |
| 16 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.567 |
| 17 | 1 | 1 | −1 | −1 | 2 | 25 | 6.5 | 24 | 93.563 |
| 18 | 1 | −1 | 1 | −1 | 2 | 15 | 7.5 | 24 | 93.432 |
| 19 | 1 | −1 | −1 | 1 | 2 | 15 | 6.5 | 48 | 93.564 |
| 20 | 1 | −1 | 1 | 1 | 2 | 15 | 7.5 | 48 | 93.564 |
| 21 | 1 | 1 | 1 | 1 | 2 | 25 | 7.5 | 48 | 93.763 |
| 22 | 1 | 1 | 1 | −1 | 2 | 25 | 7.5 | 24 | 93.932 |
| 23 | 1 | 1 | −1 | 1 | 2 | 25 | 6.5 | 48 | 94.231 |
| 24 | 1 | −1 | −1 | −1 | 2 | 15 | 6.5 | 24 | 91.674 |
| 25 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.598 |
| 26 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.819 |
| 27 | 0 | 0 | 0 | 0 | 1.5 | 20 | 7 | 36 | 99.879 |
| 28 | −2 | 0 | 0 | 0 | 0.5 | 20 | 7 | 36 | 91.876 |
| 29 | −2 | 0 | 0 | 0 | 0.5 | 20 | 7 | 36 | 91.743 |
| 30 | 0 | 0 | 2 | 0 | 1.5 | 20 | 8 | 36 | 92.543 |
| 31 | 0 | 2 | 0 | 0 | 1.5 | 30 | 7 | 36 | 92.986 |
| 32 | 0 | 0 | 0 | −2 | 1.5 | 20 | 7 | 12 | 92.764 |
| 33 | 0 | 0 | 0 | 2 | 1.5 | 20 | 7 | 60 | 94.682 |
| 34 | 0 | 0 | −2 | 0 | 1.5 | 20 | 6 | 36 | 94.419 |
| 35 | 0 | −2 | 0 | 0 | 1.5 | 10 | 7 | 36 | 94.862 |
2.5 Morphological and elemental analysis
The morphology of stalactite samples collected from both caves and precipitated calcium carbonate crystal was done by scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis. The elemental composition was determined by Energy Dispersive X-ray (EDX) analysis.2.6 Identification of calcium precipitating bacteria
The phylogenetic analysis of the most potent calcium precipitating bacterial isolate was done by 16S rDNA sequencing using PCR amplification primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-CGGTTACCTTGTTACGACTT-3′). The sequencing was done using primers 907R (5′-CCGTCAATTCCTTTRAGTTT-3′) and 785F (5′-GGATTAGATACCCTGGTA-3′) and BDT v3.1 cycle sequencing kit on ABI 3730xl genetic analyzer. The obtained sequences were used to carry out BLAST analysis with the NCBI gene bank database. Based on the maximum identity score, first ten sequences were selected and aligned using Clustal W. Phylogenetic tree was constructed using MEGA 7.0. The sequence was then submitted to NCBI database for procurement of accession number.3. Results and discussion
3.1 Analysis of stalactite
The crystalline structure of stalactite of Sahastradhara cave (Fig. 1) matched with calcium magnesium carbonate belonging to rhombohedral crystal system (reference code: 00-060-0473; Fig. 2). The stalactite sample of Sahastradhara cave was found to contain 29.41% carbon, 46.89% oxygen, 23.25% calcium and 0.45% magnesium (Fig. 3). The percentage of carbon was found to be quite high as compared to that reported in stalactite of Lake cave (4.9 ± 1.7%) and Mammoth cave (5.4 ± 1.5%) as reported by Dhami et al.40 They reported the presence of many other elements like N, Si, K, Na, S, Mg, Al, Fe and P which were not detected in stalactite of Sahastradhara cave except magnesium.3.2 Estimation of calcium carbonate precipitation
A total of 6 bacterial isolates were found to produce urease (Fig. S1†). None of the isolate produced carbonic anhydrase. Urea hydrolysis generates carbonate most easily than any other reactions. If the urease producing microorganism is present in calcium-rich environment, then the precipitation of calcium carbonate occurs.B4SSt3 was found to carry out the maximum precipitation (97%) of calcium carbonate raising the pH of medium to 9.0 ± 0.05 (Table 3 and Fig. S2†). The rise in pH of medium can be due to higher urease activity as reported by Cabrera et al.11 and Okyay and Rodrigues.33 No other bacterial isolate could match with B4SSt3. As there was only a marginal increase in calcium precipitation from 48 h to 60 h, therefore further experiment was not carried out.
| Name of bacterial isolate | Initial pH | 12 h | 24 h | 36 h | 48 h | 60 h | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | CaCO3 (%) | pH | CaCO3 (%) | pH | CaCO3 (%) | pH | CaCO3 (%) | pH | CaCO3 (%) | ||
| a Each value is expressed as mean value. B4 – B4 medium; SSt – Sahastradhara stalactite. | |||||||||||
| B4SSt1 | 6.00 ± 0.00 | 6.00 ± 0.02 | 12.13 ± 0.05 | 6.00 ± 0.01 | 13.50 ± 0.05 | 6.00 ± 0.01 | 14.32 ± 0.05 | 6.00 ± 0.05 | 15.89 ± 0.08 | 6.00 ± 0.02 | 16.32 ± 0.09 |
| B4SSt2 | 6.00 ± 0.00 | 6.00 ± 0.01 | 10.67 ± 0.03 | 8.00 ± 0.04 | 19.00 ± 0.02 | 8.00 ± 0.10 | 24.08 ± 0.12 | 8.00 ± 0.10 | 26.32 ± 0.10 | 8.00 ± 0.04 | 26.63 ± 0.10 |
| B4SSt3 | 6.00 ± 0.00 | 6.00 ± 0.01 | 78.00 ± 0.02 | 9.00 ± 0.01 | 87.00 ± 0.01 | 9.00 ± 0.11 | 92.00 ± 0.23 | 9.00 ± 0.11 | 96.00 ± 0.21 | 9.00 ± 0.05 | 97.00 ± 0.23 |
| B4SSt4 | 6.00 ± 0.00 | 6.00 ± 0.05 | 15.38 ± 0.15 | 6.50 ± 0.02 | 18.45 ± 0.05 | 7.00 ± 0.08 | 25.64 ± 0.12 | 7.00 ± 0.11 | 26.43 ± 0.08 | 7.00 ± 0.10 | 27.87 ± 0.12 |
| B4SSt5 | 6.00 ± 0.00 | 6.00 ± 0.02 | 12.43 ± 0.60 | 6.00 ± 0.01 | 14.32 ± 0.02 | 7.00 ± 0.10 | 20.32 ± 0.14 | 7.00 ± 0.08 | 21.03 ± 0.10 | 7.00 ± 0.12 | 22.47 ± 0.10 |
3.3 Identification of calcium precipitating bacteria
The isolate B4SSt3 was identified to be Klebsiella pneumoniae based on 16S rDNA sequence analysis (Fig. S3†). The sequence was submitted to NCBI nucleotide database with accession number MG946801.The presence of Klebsiella pneumoniae in the cave has been observed by few researchers. Campbell et al.14 reported Klebsiella ozaenae, K. oxytoca and K. pneumoniae in Pettyjohns cave. Seman et al.38 reported Klebsiella ornithinolytica and K. oxytoca from Domica and Gombasecka cave, Klebsiella ozaenae from Domica and Milada cave and Klebsiella pneumoniae from Krasnohorska cave. The exploration of ammonia oxidation (amoA) and nitrogen fixation gene in Lava caves of Terceira, Azores and Portugal revealed that the nitrogen fixation community was dominated by Klebsiella pneumoniae-like sequences.24 Urease production from Klebsiella pneumoniae has also been well studied.20 However, no report of biomineralization potential of Klebsiella pneumoniae has been reported from caves. Till date, the calcium carbonate precipitation studies of cave have been limited to Bacillus, Cupriavidus sp., Lysinibacillus sp. and actinomycetes only.5,8,9,16,37 Baskar et al.9 identified Bacillus anthracis, B. cereus, B. circulans, B. lentus, B. pumilis, B. sphaericus and Actinomycetes as calcium precipitating isolates from moonmilk and stalactite sample of Sahastradhara cave.
3.4 Optimization of calcium carbonate precipitation by Klebsiella pneumoniae
Central composite rotatable design (CCRD) was employed to determine the correlation between the independent variables and the response (Table 4).| Y = +9.98 + 0.073X1 − 0.024X2 − 0.024X3 + 0.025X4 + 0.017X1X2 + 1.71X1X3 + 6.515X1X4 − 4.935X2X3 − 7.460X2X4 − 0.014X3X4 − 0.064X12 − 0.073X22 − 0.078X32 − 0.075X42 − 7.121X1X2X3 − 2.449X1X2X4 − 2.428X1X3X4 + 3.071X2X3X4 + 0.028X12X2 + 0.033X12X3 − 0.015X12X4 − 0.15X1X22 + 2.51X1X2X3X4 |
| Source | Term degree of freedom | Error degree of freedom | F | Prob > F |
|---|---|---|---|---|
| a X1: concentration of urea; X2 – inoculum level; X3 – pH; X4 – time. | ||||
| Whole-plot | 4 | 1.09 | 294.141 | 0.03454 |
| X1 | 1 | 2.9 | 1067 | <0.0001 |
| X12 | 1 | 2.53 | 2075 | <0.001 |
| X1X22 | 1 | 1.37 | 1358.43 | 0.00505 |
| X12X22 | 1 | 1.19 | 108.425 | 0.0407 |
| Subplot | 20 | 4.62 | 192.09 | <0.0001 |
| X2 | 1 | 4.62 | 111.53 | <0.0001 |
| X3 | 1 | 8 | 112.06 | <0.0001 |
| X4 | 1 | 8 | 116.83 | <0.0001 |
| X1X2 | 1 | 8 | 114.77 | <0.0001 |
| X1X3 | 1 | 8 | 1.11 | 0.322 |
| X1X4 | 1 | 8 | 16.17 | 0.0038 |
| X2X3 | 1 | 8 | 9.28 | 0.0159 |
| X2X4 | 1 | 8 | 21.2 | 0.0017 |
| X3X4 | 1 | 8 | 78.91 | <0.0001 |
| X22 | 1 | 8 | 1653.44 | <0.0001 |
| X32 | 1 | 2.67 | 1923.6 | <0.0001 |
| X42 | 1 | 2.67 | 1773.47 | <0.0001 |
| X1X2X3 | 1 | 2.67 | 19.32 | 0.0023 |
| X1X2X4 | 1 | 8 | 2.29 | 0.169 |
| X1X3X4 | 1 | 8 | 2.25 | 0.1723 |
| X2X3X4 | 1 | 8 | 3.59 | 0.0946 |
| X12X2 | 1 | 8 | 99.02 | <0.0001 |
| X12X3 | 1 | 8 | 141.07 | <0.0001 |
| X12X4 | 1 | 8 | 28.32 | 0.0007 |
| X1X2X3X4 | 1 | 8 | 3.32 | <0.0001 |
The analysis of variance (ANOVA) of suggested quadratic regression model demonstrated that the model was significant as indicated by F-value with a very low probability (Table 4). The R2 value of 1 for response suggested a very less difference or negligible difference between the calculated and observed value and thus indicating the well fitness of regression models for predicting the precipitation results. All the p-values were found to be less than 0.05 which indicated that these variables had a significant effect upon the calcium carbonate precipitation. This implied that the linear as well as quadratic effects of all four factors were highly significant (p < 0.0001) at the 5% significance level.
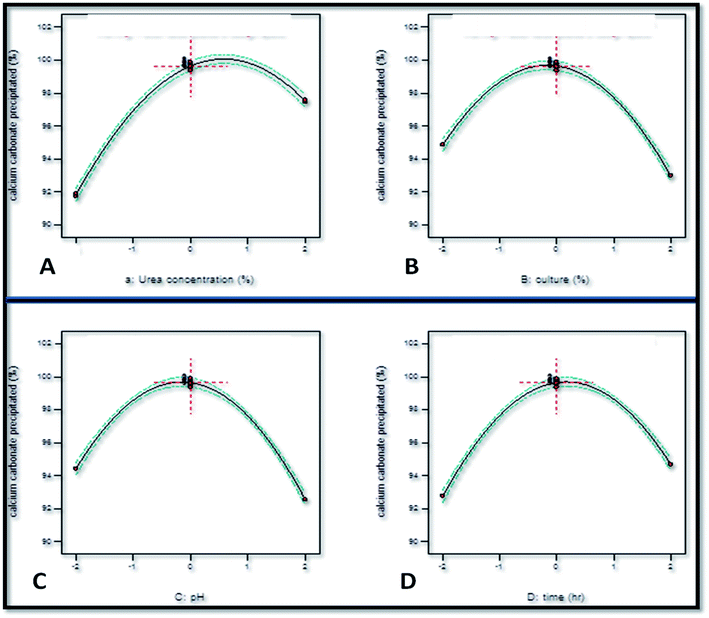
The individual effect of increasing the concentration of urea was an augmentation in the calcium carbonate precipitation as the coefficient value for X1 obtained was +0.073 (Fig. 4). However, increasing the initial concentration of urea beyond a limit exerted a negative effect on calcium carbonate precipitation as the coefficient of X12 obtained was −0.064. This observation corroborated well with the study of Okyay and Rodrigues33 who reported the positive effect of urea concentration on calcium carbonate precipitation by Sporosarcina pasteurii using response surface methodology as the value of X1 = +0.106. However, beyond a limit, the increase in the initial concentration of urea exerted a negative effect on calcium carbonate precipitation as the value of X12 = −0.016. The linear coefficient value of −0.024 as well squared coefficient value of −0.073 of inoculum level indicated that the rate of calcium carbonate precipitation decreased with the increase in the inoculum level. pH was also found to have a negative effect on calcium carbonate precipitation as the linear coefficient value obtained was X3 = −0.024. Time was found to have a positive effect as the coefficient value obtained was +0.025.
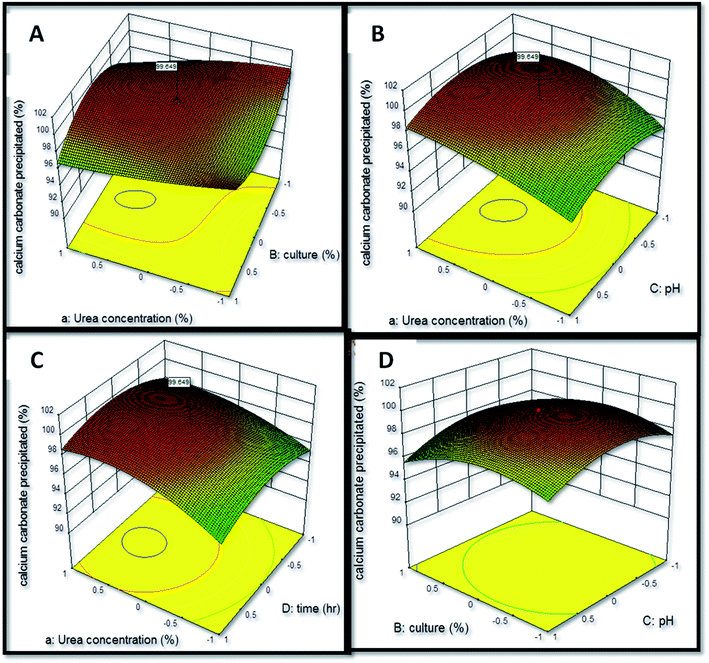
The interactive effect of factors on calcium carbonate precipitation was analyzed by the construction of three-dimensional (3-d) graphical response (Fig. 5) in which two factors were varied while keeping third factor constant. The interaction of urea concentration and inoculum level was found to exert a positive effect upon the calcium carbonate precipitation as the coefficient value of this interaction obtained was X1X2 = +0.017. The interaction of urea concentration and pH was also found to have a positive influence upon calcium carbonate precipitation (X1X3 = +1.71). The most significant positive effect was found to be of interaction of urea concentration and time (X1X4 = +6.515). The interaction of inoculum level and pH was found to exert a negative effect (X2X3 = −4.935). The study of interaction between the variables can be extrapolated for understanding the effect of variables on calcium carbonate precipitation and thus formation of speleothems in caves. Similar observations have been reported by Li et al.31 and Daskalakis et al.17 who studied calcium carbonate precipitation ability of a mutant strain of Sporosarcina pasteurii and Cupriavidus metallidurans, respectively.
| Variable | Optimization condition | % of calcium carbonate precipitated | ||||
|---|---|---|---|---|---|---|
| Before | Predicted | Actual | Before | Predicted | Actual | |
| a Value represent mean ± SD. | ||||||
| Urea concentration (%) | 1.00 | 1.50 | 1.5625 | 97 ± 0.23 | 99.614 | 99.879 ± 0.04 |
| Inoculum level (%) | 10.00 | 20.00 | 19.98 | |||
| pH | 6.00 | 7.00 | 6.98 | |||
| Time (h) | 60 h | 36 h | 38 h 24 min | |||
3.5 Analysis of precipitated calcium carbonate crystals
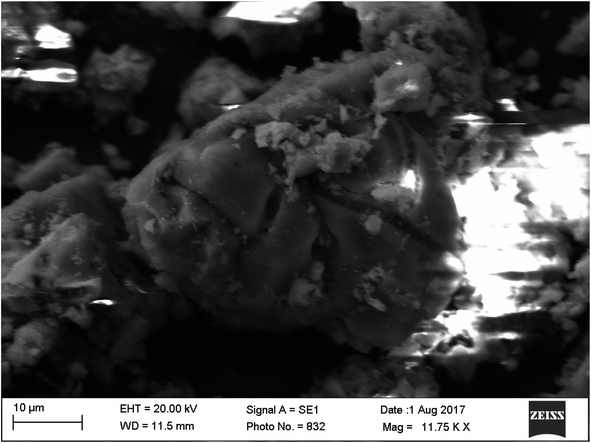
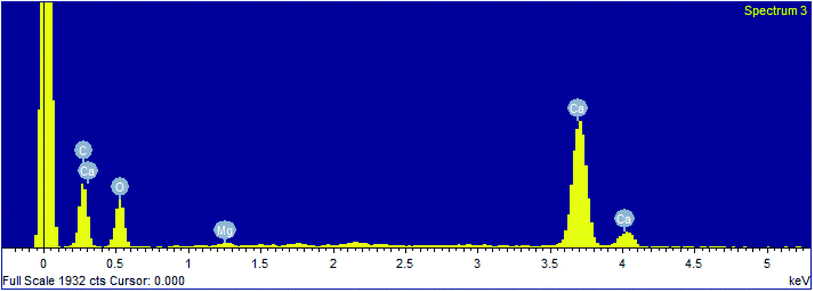
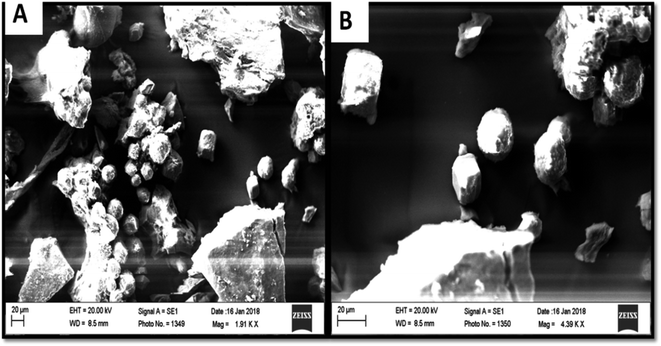
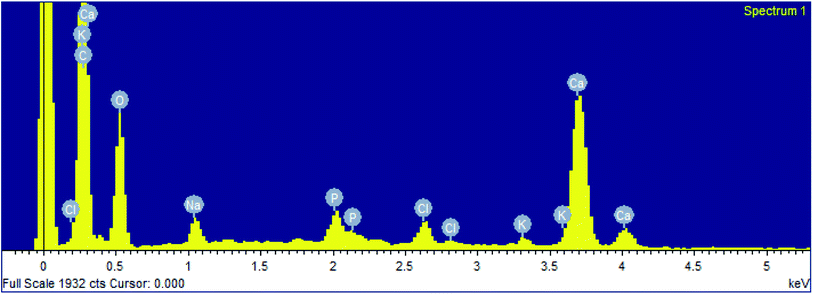
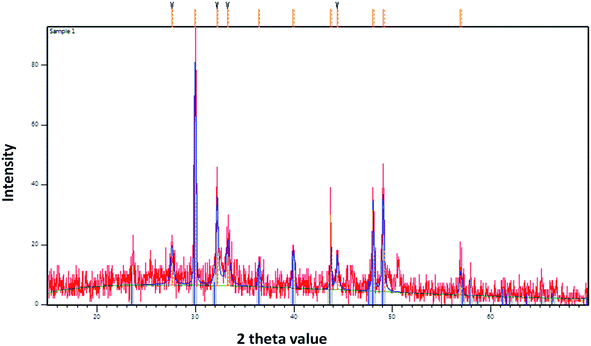
The precipitation of calcium carbonate crystal (Fig. S4†) was proved by SEM-EDX and XRD analysis. The round and cubical shaped particle clearly visible in the SEM image indicated the production of the crystal (Fig. 6). EDX analysis revealed that the major elements in the crystal were carbon (50.97%), oxygen (37.14%) and calcium (8.24%) (Table 6 and Fig. 7). XRD analysis confirmed the crystalline structure and revealed the size of precipitated sample (Fig. 8). The prominent peak matched with the ICSD reference code 01-072-4582 calcium carbonate with 2θ value = 29.938. The displacement value (2θ value) of crystal was obtained 0.397 and the size of crystal size was found to be 166 Å. The shape of crystal shape was found to be rhombohedral.| Elements | Weight (%) |
|---|---|
| C | 50.97 |
| O | 37.14 |
| Na | 1.41 |
| P | 0.92 |
| Cl | 0.96 |
| K | 0.36 |
| Ca | 8.24 |
4. Conclusions
The present study confirmed the precipitation of calcium carbonate by bacteria isolated from stalactite of Sahastradhara cave indicating the biogenic origin of speleothems in the cave. Klebsiella pneumoniae was able to precipitate calcium carbonate in 38 h 24 minute which is the shortest time reported till date by cave microbiota. Calcium precipitating ability of cave microorganisms can be exploited in construction industries as the microorganisms can repair the cracks in concrete by producing calcite crystals. Klebsiella pneumoniae can serve as potential bacterium for formation of bioconcrete.Conflicts of interest
There are no conflicts of interest.Acknowledgements
The authors are thankful to UGC for providing fellowship to the first author. The authors would also like to thank the Department of Botany and Microbiology, HNBGU, Srinagar for providing all facilities for carrying out this research work. The authors would also like to acknowledge University Service Instrumentation Centre, HNBGU for providing SEM-EDX and XRD facilities.References
- V. Achal, A. Mukherjee, P. C. Basu and M. S. Reddy, J. Ind. Microbiol. Biotechnol., 2009, 36, 981–988 CrossRef CAS PubMed.
- V. Achal and X. Pan, Curr. Microbiol., 2011, 62(3), 894–902 CrossRef CAS PubMed.
- V. Achal and X. Pan, Appl. Biochem. Biotechnol., 2014, 173, 307–317 CrossRef CAS PubMed.
- L. Addadi, S. Raz and S. Weiner, Adv. Mater., 2003, 15, 959–970 CrossRef CAS.
- S. Banerjee and S. R. Joshi, J. Microsc. Ultrastruct., 2014, 2, 217–223 CrossRef.
- S. Baskar, R. Baskar and J. Routh, Geomicrobiol. J., 2011, 28, 252–265 CrossRef.
- S. Baskar, R. Baskar, L. Mauclaire and J. A. Mckenzie, Curr. Sci., 2005, 88, 1305–1308 CAS.
- S. Baskar, R. Baskar, L. Mauclaire and J. A. Mckenzie, Curr. Sci., 2006, 90(1), 58–64 CAS.
- S. Baskar, R. Baskar and J. Routh, Geomicrobiol. J., 2014, 31(8), 664–681 CrossRef.
- P. L. Broughton, Int. J. Speleol., 1983, 13, 31–41 CrossRef.
- M. L. Cabrera, D. E. Kissel and B. R. Brock, Soil Biol. Biochem., 1991, 23, 1121–1124 CrossRef CAS.
- P. Cacchio, R. Contento, C. Ercole, G. Cappuccio, M. Preit Martinez and A. Lepidi, Geomicrobiol. J., 2004, 21, 497–509 CrossRef CAS.
- P. Cacchio, C. Ercole, R. Contento, G. Cappuccio, M. P. Martinez and M. D. Gallo, J. Cave Karst Stud., 2010, 74(1), 7–18 CrossRef.
- J. W. Campbell, A. Watson, C. Watson, H. Ball and R. Pirkle, J. Cave Karst Stud., 2011, 73(2), 75–82 CrossRef.
- J. G. Cappuccino and N. Sherman, Microbiology A Laboratory Manual, TATA Art Printers, India, 7th edn, 2007 Search PubMed.
- S. Chalia, S. Baskar, M. Prasad, R. Baskar and K. Ranjan, Geomicrobiol. J., 2016, 34(9), 737–752 CrossRef.
- M. I. Daskalakis, A. Magoulas, G. Kotoulas, I. Katsikis, A. Bakolas, A. P. Karageorgis, A. Mavridou, D. Doulia and F. Rigas, Appl. Microbiol. Biotechnol., 2014, 98, 6871–6883 CrossRef CAS PubMed.
- N. K. Dhami, M. S. Reddy and A. Mukherjee, J. Microbiol. Biotechnol., 2013, 23, 707–714 CrossRef CAS PubMed.
- N. K. Dhami, M. S. Reddy and A. Mukherjee, Appl. Biochem. Biotechnol., 2014, 172, 2552–2561 CrossRef CAS PubMed.
- G. Gerlach, S. Clegg and W. A. Nichols, FEMS Microbiol. Lett., 1988, 50(2–3), 131–135 CrossRef CAS.
- I. Groth, P. Schumann, L. Laiz, S. Sanchez-Moral, J. C. Cañveras and C. Saiz-Jimenez, Geomicrobiol. J., 2001, 18, 241–258 CrossRef CAS.
- C. Gurtner, C. Jimenez, G. Pinar, W. Lubitz and S. Rolleke, FEMS Microbiol. Ecol., 2004, 47, 235–247 CrossRef.
- F. Hammes and W. Verstraete, Rev. Environ. Sci. Bio/Technol., 2002, 1, 3–7 CrossRef CAS.
- J. J. M. Hathway, R. L. Sinsabaugh, M. D. Lurdes, N. E. Dapkevicius and D. E. Northup, Geomicrobiol. J., 2014, 31(3), 221–235 CrossRef PubMed.
- B. Jones, in Tufas and speleothems: unravelling the microbial and physical controls, ed. H. M. Pedley and M. Rogerson, Geological Society, London. Special Pub., 2010, vol. 336, pp. 7–30 Search PubMed.
- B. Jones and A. Motyka, Can. J. Earth Sci., 1987, 24, 1402–1411 CrossRef CAS.
- C. H. Kang, J. H. Choi, J. G. Noh, D. Y. Kwak, S. H. Han and J. S. So, Appl. Biochem. Biotechnol., 2014, 174, 2482–2491 CrossRef CAS PubMed.
- W. E. Keefe, Infect. Immun., 1976, 14, 590–592 CrossRef CAS PubMed.
- A. C. Kendall and P. L. Broughton, J. Sediment. Petrol., 1978, 48, 519–538 CAS.
- L. Laiz, I. Groth, P. Schumann, F. Zezza, A. Felske, B. Hermosin and C. Saiz-Jimenez, Int. Microbiol., 2000, 3, 25–30 CAS.
- W. Li, L. Li-Pong, Z. Peng-Peng, L. Cao, L.-J. Yu and J. Shi-Yun, Curr. Sci., 2011, 100(4), 502–508 CAS.
- D. E. Northup and K. H. Lavoie, Geomicrobiol. J., 2001, 18, 199–222 CrossRef CAS.
- T. O. Okyay and D. F. Rodrigues, Ecol. Eng., 2014, 62, 168–174 CrossRef.
- R. Ramanan, K. Kannan, S. D. Sivanesan, S. Mudliar, S. Kaur, A. K. Tripathi and T. Chakrabarti, World J. Microbiol. Biotechnol., 2009, 25, 981–987 CrossRef CAS.
- A. Rusznyak, D. M. Akob, S. Nietzsche, K. Eusterhues, K. U. Totsche, T. R. Neu, T. Frosch, J. Popp, R. Keiner and J. Geletneky, Appl. Environ. Microbiol., 2012, 78, 1157–1167 CrossRef CAS PubMed.
- D. Sarada, H. S. Choonia, D. D. Sarode and S. S. Lele, J. Ind. Microbiol. Biotechnol., 2009, 36, 1111–1115 CrossRef PubMed.
- M. Seifan, A. K. Samani and A. Berenjian, Appl. Microbiol. Biotechnol., 2016, 100, 9895–9960 CrossRef CAS PubMed.
- M. Seman, B. Gaalova, M. Cichova, M. Proksova, D. Haviarova and R. Flakova, Folia Microbiol., 2015, 60, 269–278 CrossRef CAS PubMed.
- H. Wang, C. Zeng, Q. Liu, D. Liu, X. Qui and L. Gong, Front. Earth Sci. China, 2010, 4(2), 148–151 CrossRef CAS.
- N. K. Dhami, A. Mukherjee and E. L. J. Watkin, Front. Microbiol., 2018, 9, 40 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00090f |
| This journal is © The Royal Society of Chemistry 2020 |