Open Access Article
Open Access ArticleNon-cytotoxic, temperature-responsive and antibacterial POEGMA based nanocomposite coatings with silver nanoparticles†
Svyatoslav Nastyshyna,
Joanna Raczkowska*a,
Yurij Stetsyshyn *b,
Barbara Orzechowskac,
Andrzej Bernasik
*b,
Barbara Orzechowskac,
Andrzej Bernasik d,
Yana Shymborskab,
Monika Brzychczy-Włoche,
Tomasz Gosiewskie,
Ostap Lishchynskyib,
Halyna Oharb,
Dorota Ochońskae,
Kamil Awsiuka and
Andrzej Budkowskia
d,
Yana Shymborskab,
Monika Brzychczy-Włoche,
Tomasz Gosiewskie,
Ostap Lishchynskyib,
Halyna Oharb,
Dorota Ochońskae,
Kamil Awsiuka and
Andrzej Budkowskia
aSmoluchowski Institute of Physics, Jagiellonian University, Łojasiewicza 11, 30-348 Kraków, Poland. E-mail: joanna.raczkowska@uj.edu.pl
bLviv Polytechnic National University, St. George's Square 2, 79013 Lviv, Ukraine. E-mail: yrstecushun@ukr.net
cInstitute of Nuclear Physics Polish Academy of Sciences, Radzikowskiego 152, 31-342 Kraków, Poland
dFaculty of Physics and Applied Computer Science, Academic Centre for Materials and Nanotechnology, AGH University of Science and Technology, Al. Mickiewicza 30, 30-049 Kraków, Poland
eChair of Microbiology, Department of Molecular Medical Microbiology, Faculty of Medicine, Jagiellonian University Medical College, Czysta 18, 31-121 Kraków, Poland
First published on 10th March 2020
Abstract
Non-cytotoxic, temperature-responsive and antibacterial poly(di(ethylene glycol)methyl ether methacrylate) – POEGMA188 based nanocomposite coatings attached to a glass surface were successfully prepared using ATRP polymerization. The thickness, morphology and wettability of the resulting coatings were analyzed using ellipsometry, AFM and contact angle measurements, respectively. The strong impact of the thicknesses of the POEGMA188 grafted brush coatings and content of AgNPs on the morphology and temperature-induced wettability changes of the nanocomposite was demonstrated. In addition to the strong temperature-dependent antibacterial activity, the proposed nanocomposite coatings have no significant cytotoxic effect towards normal cells. Moreover, the slight anti-cancer effect of AgNPs may be suggested.
1. Introduction
Silver nanoparticles (AgNPs) are one of the most famous antibacterial agents and demonstrate their properties even against multidrug-resistant bacteria. Due to remarkable optical, electrical and antimicrobial properties, AgNPs have promising applications in biotechnology and life sciences.1–3 Over the last years the development of modern technologies, which allow for the production of AgNPs of different sizes and shapes, is of great importance4–6 as it provides an easy way to modify their properties. In addition, the interactions between mammalian cells and Ag-NPs7,8 are of crucial importance, and strong dependence of the cytotoxicity and geno-toxicity on Ag-NPs functionalities and the type of cells have been demonstrated in recent studies.7,8Composite materials including AgNPs were fabricated using matrix polymers, thin polymer films, gels and microgels, capsules and grafted coatings.9–21 Remarkable work by R. Fakhrullin's group reported silver nanoparticle-coated “cyborg” microorganisms, which can be used not only to deliver nanoparticles into organisms, but also for the fabrication of smart antibacterial coatings.22 In turn, the remote activation of capsules containing Ag nanoparticles and infrared dye by laser light was described by Skirtach and co-workers.23 Special interest is paid to smart antibacterial surfaces because they prevent bacterial attachment and biofilm formation especially using a promising “kill-release” strategy. In this approach, smart materials are able to kill bacteria attached to their surface and then undergo an on-demand release of the dead bacteria and other biological components to reveal a clean surface under an appropriate stimulus, thereby maintaining effective long-term antibacterial activity.24–26 Silver-polymer nanocomposites with antimicrobial and non-fouling properties were presented by Hu and coworkers27 and Yang and co-workers.28 In turn, Ag-nanoparticle-loaded hydrogel coatings with thermoresponsive antibacterial properties were described by He and co-workers,24 where AgNPs are embedded in cross-linked N-isopropylacrylamide and sodium acrylate coating grafted to the substrate.
In our previous works we have described a new method of fabrication of the “command” antibacterial surfaces with temperature-switched killing of the bacteria.29,30 The coatings were based on silver nanoparticles (AgNPs) embedded in temperature-responsive poly(di(ethylene glycol)methyl ether methacrylate) – (POEGMA188) as well as in poly(4-vinylpyridine) – (P4VP) coatings attached to a glass surface. Detailed analysis of their properties, performed using numerous surface-sensitive techniques, showed strong impact of the chemical nature of the grafted polymer brushes on the shape of the silver nanoparticles. Moreover, the very effective temperature-switched killing of the bacteria was demonstrated for Escherichia coli and Staphylococcus aureus.
Motivated by the strong progress within research in the field of smart antibacterial polymeric materials,23–28 we decided to conduct systematic studies aimed at finding the main regularities between the experimental conditions of the fabrication of the POEGMA188 based nanocomposite coatings with AgNPs and their properties. For this purpose, the AgNPs embedded in POEGMA188 coatings with different thicknesses attached to a glass surface were prepared using ATRP polymerization. AgNPs were synthesized using Ag ions adsorbed into the polymer coatings and reduced to AgNPs by sodium borohydride. Morphology and wettability of the resulting coatings were studied in details using atomic force microscopy (AFM) and measurements of wetting contact angles (CA), respectively. One of the main issues studied in this work was an influence of the fabrication condition of the nanocomposite coatings with AgNPs on their temperature-responsive properties. Obtained results imply that above some threshold values of the silver abundance temperature-responsive properties of the POEGMA188 coatings are no longer preserved. X-ray photoelectron spectroscopy (XPS) analysis implies prolonged release of silver ions from the POEGMA188 coatings caused by the bonding of the AgNPs to the polymer brushes and limiting therefore their possibility to relocate to the human body and making them very prospective materials for human-related applications.
Temperature-depended antibacteriality was demonstrated here for model bacterial strains (S. aureus and E. coli), confirming our previous results.30 Additionally, the impact of nanocomposite coatings on mammalian cells was examined. For this purpose proliferation of healthy and cancerous cells was analyzed for two cell lines. They were in vitro spontaneously transformed keratinocytes from histologically normal skin (HaCaT) and WM35 cell line derived from the primary melanoma site of the patient's skin diagnosed with radial growth phase (RGP) melanoma. The results indicate that the impact of AgNPs is highly cell-dependent. The anti-cancer impact of AgNPs, postulated by the literature data, reporting their higher cytotoxicity against cancer cells over normal cells31,32 cannot be proven by the obtained data. However, our research shows that AgNPs embedded in polymer brushes do not have any cytotoxic effect towards normal cells for short exposure times and start to affect them for longer times. This effect might be related with potential long-term release of AgNPs from the grafted brushes and their subsequent accumulation in cells.7,8,31,33,34
2. Experimental section
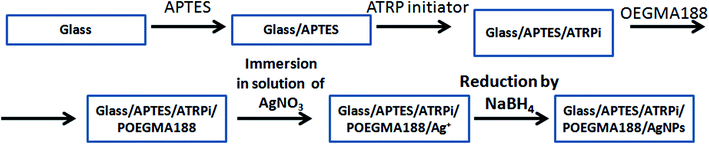
2.1. Materials
2-Bromoisobutyryl bromide – (ATRP initiator), (3-aminopropyl)triethoxysilane – (APTES), (di(ethylene glycol)methyl ether methacrylate) – (OEGMA188), CuBr2, 2,2′-dipyridyl (bpy), triethylamine (Et3N), sodium L-ascorbate, silver nitrate (AgNO3), sodium borohydride (NaBH4) and solvents were supplied by Sigma-Aldrich.2.2. Fabrication of nanocomposite coatings
After annealing, substrates can be reacted directly with 2-bromoisobutyryl bromide. For this, 10 mL anhydrous tetrahydrofuran was mixed with 2-bromoisobutyryl bromide (0.26 mL, 2.10 mmol) and anhydrous triethylamine (0.30 mL, 2.10 mmol) and were then added to a amino functionalized substrate.
2.3. Characterization of nanocomposite coatings
| n(λ) = n0 + n1λ−2 + n2λ−4 |
The typical thickness of the grafted APTES, measured by ellipsometry for the conditions used for glass functionalization, was equal to 0.5 nm.36,37 The thickness of ATRP films did not exceed 1 nm.
2.4. Antibacterial test
2.5. Cell culture
Two cell lines were used in this study, keratinocytes from histologically normal skin (HaCaT) and WM35 cell line from the primary melanoma site of the patient's skin diagnosed with radial growth phase (RGP) melanoma. HaCaT cells were cultured in the EMEM (Eagle's Minimum Essential Medium, LGC Standards) and WM35 cells were cultured in the RPMI-1640 (cell culture medium developed at Roswell Park Memorial Institute in 1966 by Moore and his co-workers), both supplemented with a 10% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich) and 1% antibiotics (Penicillin–Streptomycin–Neomycin Solution Stabilized, Sigma) in culture flasks, in a CO2 incubator providing 95% air/5% CO2 atmosphere. The POEGMA188 grafted brush coatings with and without AgNPs embedded were put into the Petri dish (35 mm in diameter) and sterilized for 3 minutes in 99.8% ethanol (POCH, Gliwice). After sterilization, a solution with cells (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL in the culture medium) was placed over the coatings. Next, the Petri dish was moved into the CO2 incubator for 1, 3 and 6 days. The experiments were repeated at least twice for each cell line and a time-point.
000 cells per mL in the culture medium) was placed over the coatings. Next, the Petri dish was moved into the CO2 incubator for 1, 3 and 6 days. The experiments were repeated at least twice for each cell line and a time-point.
3. Results and discussion
In the presented work the “smart” nanocomposite brush coating based on AgNPs embedded in temperature-responsive POEGMA188 grafted polymer brush were fabricated (Scheme 1). First, grafted temperature-responsive polymer brushes with different thicknesses were fabricated in a three-step process, for the glass surface previously functionalized by APTES and then ATRP molecules. The thicknesses of grafted coatings were tuned by the time of the ATR polymerization of POEGMA188. Subsequently, Ag ions were adsorbed into the polymer coating and reduced to AgNPs by sodium borohydride.29,30 It is important to note, that the “freshness” of the sodium borohydride is of a crucial impact on the amount of the synthesized AgNPs. Therefore, only freshly opened reagent was used here.The thickness, morphology and wettability of the resulting coatings were analyzed using ellipsometry, AFM and CA measurements, respectively. In addition, the impact of the thickness of the POEGMA188 grafted brush coatings and content of AgNPs on the morphology and temperature-induced wettability were examined and described in details in Section 3.1. In turn, the antibacterial properties and cytotoxic influence of the POEGMA188 coatings with embedded AgNPs on healthy keratinocytes (HaCaT) and cancerous WM35 cell lines are presented in Section 3.2.
3.1. Fabrication and characterization of the POEGMA188 based nanocomposite brush coatings with AgNPs: morphology and temperature-responsive properties
POEGMA188 based nanocomposite brush coatings with silver AgNPs were fabricated using different experimental conditions, listed in Table 1. Resulting POEGMA188 coatings with different thickness, tuned by adjustment of polymerization time (Table 1), were used as templates for synthesis of the AgNPs. In addition, glass decorated with APTES was used as a reference. By changing the time of the immersion of the POEGMA188 coatings in AgNO3 solution as well as the concentration of the AgNO3 we were able to modify significantly the properties of the nanocomposite brush coatings.| Samples of the POEGMA188 brush coatings with different polymerization times | Concentration of the AgNO3 in solution and time of the immersion | |||
|---|---|---|---|---|
| 3 mM | 5 mM | |||
| 15 min | 15 min | 30 min | 60 min | |
| APTES | + | |||
| POEGMA 2 hours | + | |||
| POEGMA 5 hours | + | + | + | |
| POEGMA 16 hours | + | + | + | + |
| POEGMA 26 hours | + | |||
Thickness and refractive index of grafted coatings were examined using ellipsometry. Typical thickness of APTES, measured by ellipsometry was equal to 0.5–1.5 nm (in accord with literature36,37). In turn, the thickness of the ATRP coating used as a substrate for POEGMA188 polymerization was equal to approximately 1 nm. The average thickness of the POEGMA188 grafted brush coatings in a dry state at room temperature (20 °C) depends strongly on polymerization time and is in the range from 0 to 86 nm (Fig. S1†). In turn, refractive index of the “pure” POEGMA188 coatings is in the range of 1.50–1.54. The incorporation of AgNPs into the POEGMA188 polymer matrix has very weak impact on the thickness of POEGMA188 coatings, in contrary to the refractive indexes which increases significantly and approach the values varying from 1.59 to 1.63.
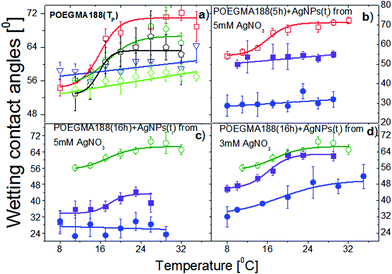
POEGMA188 brushes are able to change their properties sharply upon relatively small temperature changes due to the coil–globule transition in aqueous solutions at the low critical solution temperature. To investigate temperature-induced switching of wetting properties and the impact of the AgNPs on this process, the contact angles of sessile water droplets on the grafted brush coatings were measured between 5 and 34 °C for POEGMA188 (Fig. 1a). Except for very thin POEGMA layers, corresponding to the weak modification (polymerization time – 2 hours and thickness nearly 10 nm, blue symbols), all other POEGMA188 coatings exhibit well expressed transitions of wetting properties described by Boltzmann's curves, observed for temperatures between 16–19 °C. In contrast, no sharp transition in contact angles recorded for surface functionalized by APTES and ATRP initiator (Fig. 1a, green open diamantes) was observed. Fig. 1b shows temperature dependences of water contact angles determined for “pure” POEGMA188 coatings fabricated for the polymerization time equal to 5 hours (thickness nearly 30 nm, red open squares) as well as with AgNPs embedded into polymer matrix after 15 min (violet solid squares) and 30 min (blue solid circles) of the silver immobilization at 5 mM AgNO3 concentration in solution and followed by reduction in 0.2 M NaBH4 solution for 12 h. Recorded results show, that nanocomposite coatings with embedded AgNPs, especially the ones prepared after 30 min immobilization in AgNO3 solution, are more hydrophilic than the “pure” POEGMA188 coating. For both coatings with embedded AgNPs only a slight increase in hydrophobicity with rising temperature was described as a linear relation. Analogical studies were performed for POEGMA188 coatings fabricated with polymerization time equal to 16 hours (thickness nearly 60 nm), where for nanocomposite coatings with AgNPs synthesized after 15 min of the silver immobilization (Fig. 1c, olive open circles) a sharp transition of wetting properties at 18 °C was recorded. In contrary, for POEGMA188 coatings with AgNPs fabricated for silver immobilization time equal to 30 min, no transition was observed (Fig. 1c, blue solid circles). Although the curves of the temperature dependences of water contact angles determined for the POEGMA188 grafted brush coatings with embedded AgNPs fabricated after 15 min of the silver immobilization as well as the “pure” ones have a similar character, POEGMA188 grafted brush coatings with embedded AgNPs are significantly more hydrophilic than “pure” POEGMA188 coatings. A similar effect was described by Kasraei and co-workers38 where the incorporation of silver nanoparticles into composite resins was studied. Metallic nanoparticles have a large surface energy. The nano-sized silver particles have a higher surface energy than micro-sized ones, resulting in an increase of the surface energy of the grafted polymer brush coatings and consequently decreasing the water contact angle.38 Moreover, a strong dependence between concentration of the embedded AgNPs and temperature-responsive properties of POEGMA188 grafted brush coatings is demonstrated.
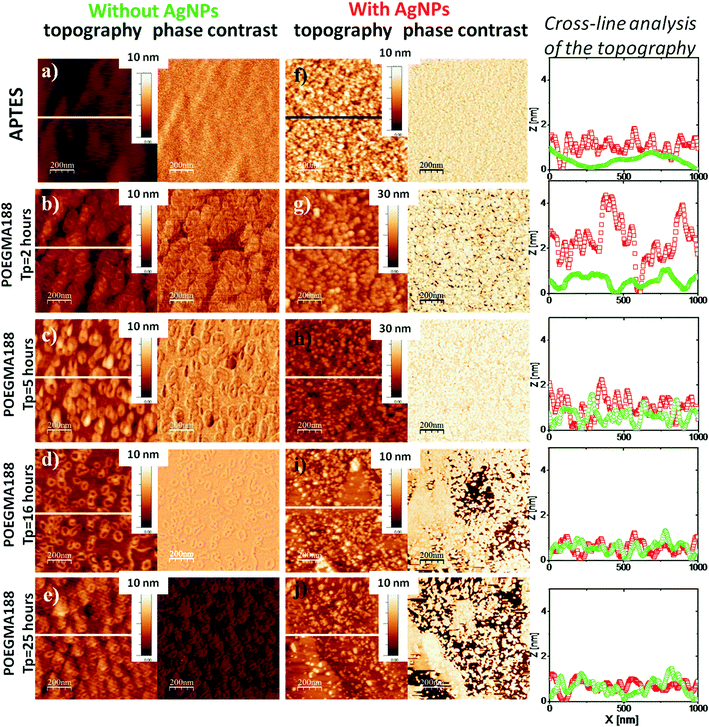
To study the influence of the silver nanoparticles on the morphology of the coatings comprehensive AFM analysis was performed for APTES and POEGMA188 coatings with different grafting polymerization time (or different ellipsometry thickness, 0–86 nm). Morphology of the APTES is smooth, with uniform silane coverage (Fig. 2a). In turn, AFM morphologies recorded for all analyzed types of “pure” POEGMA188 coatings without AgNPs (Fig. 2b–e) depict relatively smooth surfaces with small patch structures. Root mean square (RMS) values calculated for the POEGMA188 coatings (Table 2) grow almost four times with polymerization time up to 5 hours (thickness ∼ 30 nm) and remain almost constant for longer fabrication times. Moreover, analyzed surfaces do not show any phase contrast.
| Samples | RMS [nm] | |||
|---|---|---|---|---|
| Without AgNPs | With AgNPs | |||
| Time of the immersion | ||||
| 15 min | 30 min | 60 min | ||
| APTES | 0.3 ± 0.1 | 1.8 ± 0.2 | ||
| POEGMA 2 hours | 0.7 ± 0.1 | 3.4 ± 0.1 | ||
| POEGMA 5 hours | 1.3 ± 0.2 | 2.6 ± 0.2 | 2.2 ± 0.2 | 1.5 ± 0.2 |
| POEGMA 16 hours | 1.1 ± 0.2 | 1.5 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.2 |
| POEGMA 26 hours | 1.1 ± 0.1 | 1.4 ± 0.1 | ||
In contrast, for the samples with incorporated AgNPs (with 15 min immersion in AgNO3 solution) (Fig. 2f–j) well developed, patch structures are clearly visible and the RMS values, increase considerably except the POEGMA188 nanocomposite coatings with polymerization times of 16 and 25 hours where only relatively slight increase in RMS values are shown (see Table 2). RMS value calculated for the APTES coating with embedded AgNPs increased six times, whereas for POEGMA188 coatings after 2, 5, 16 and 26 hours of the polymerization an increase by 4.8, 2, 1.4 and 1.3 times, respectively was observed. The cross-line analysis of the topography for APTES and POEGMA188 coatings with 2 and 5 hours of the polymerization time demonstrates significantly better developed surface for coating with AgNPs (red line) suggesting that nanoparticles are at least partially located at the top of the polymer matrix. In contrast, the cross-line analysis of the topography of POEGMA188 coatings with polymerization time of 16 and 26 hours demonstrates higher degree of the topographical homogeneity. However, the phase contrast inhomogeneity visible in this case implies the existence of nanocomposite material where AgNPs are incorporated in the polymer matrix.
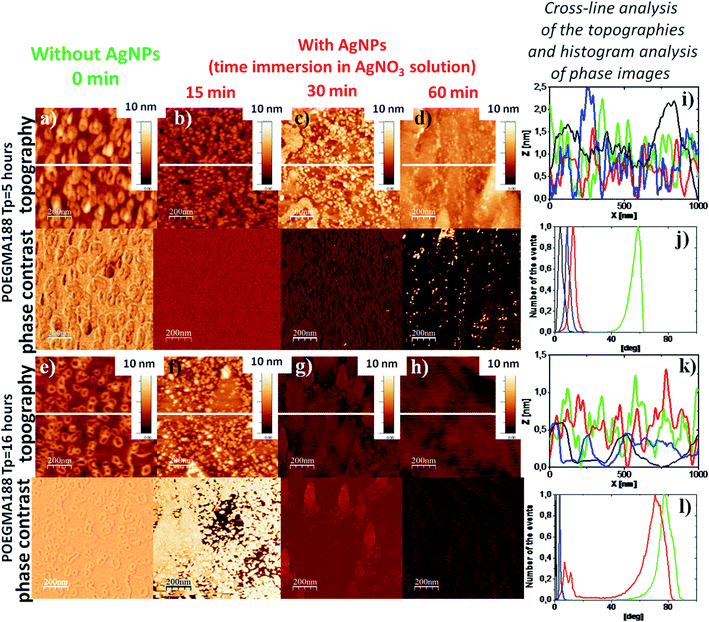
Another important factor enabling modification of nanocomposite properties is the time of brush coating immersion in AgNO3 solution. POEGMA188 coatings fabricated after 5 and 16 hours of the polymerization (with thicknesses 31 and 59 nm, respectively) were used to study in detail an impact of the time of immersion in AgNO3 solution (ranging from 0 to 60 min).
The RMS values determined for the POEGMA188 coatings fabricated using 5 hours of polymerization with incorporated AgNPs after 15 min of immersion in AgNO3 solution (Fig. 3b) is doubled as compared to the native coatings (Fig. 3a) (Table 2). In turn, an increase in immersion time from 15 to 30 and 60 min is followed by slight decrease of RMS values. The cross-line analysis of the topographies of these coatings shows similar surface structuring (Fig. 3i). In contrast, histogram analysis of phase images shows essential difference between the POEGMA188 coatings (Fig. 3j, green line) and POEGMA188 brushes with AgNPs incorporated after different times of the immersion in AgNO3 solution (Fig. 3j, red, blue and black lines). This suggests only one phase for nanocomposites with AgNPs and strong metallization of the coatings. Phase imaging allows recognizing regions with different elasticity39–41 or other structural heterogeneities41 by recording offsets and phase angles of input signal variations with respect to those of the oscillating cantilever.
For the POEGMA188 coatings with polymerization time equal to 16 hours (Fig. 3e) and AgNPs synthesized after 15 min immersion (Fig. 3f), only slightly increased RMS value is recorded, as compared with the “native” POEGMA188 coatings (Table 2). In contrast, for nanocomposites obtained after 30 and 60 min of immersion in AgNO3 solution (Fig. 3g and h) the RMS values are sharply reduced by almost four times (Table 2). The topographies of these two samples are more homogeneous, which is confirmed also by the cross-line analysis of the topography (Fig. 3k, blue and black lines). Histogram analysis of the phase images demonstrates the properties of the nanocomposite surfaces changing with the amount of silver immobilized from AgNO3 solution (Fig. 3l). “Bare” POEGMA188 coating exhibits one peak on the phase histogram centered around 80 degree (Fig. 3l, green line). The same coating incorporating AgNPs synthesized after 15 min immersion in AgNO3 solution reveals two well expressed peaks (Fig. 3l, red line), one centered around the phase value characteristic for ‘bare’ POEGMA188 brush (Fig. 3l, green line) and the second peak located close to those characteristic for the nanocomposites with higher amount with immobilized silver (Fig. 3l, blue and black lines, for 30 min and 60 min immersion in AgNO3 solution, respectively). The number of histogram peaks corresponds to the number of separated phases on the surface of the coating, suggesting the two-phase system for the POEGMA188 with AgNPs synthesized after 15 min long immersion in AgNO3 solution, where temperature-responsive properties of the coatings are preserved (Fig. 1c, red line, squares).
3.2. Release of the silver with POEGMA188 based nanocomposite coatings with embedded AgNPs
The applicability of materials containing AgNPs is strongly restricted by their potential cytotoxicity as well as by the tendency to accumulate in human organs. However, this risk should be minimalized for the proposed command surfaces as the AgNPs are bonded to the polymer brushes so their possibility to relocate to the human body should be strongly limited. To verify this issue, we have performed systematic XPS studies of silver release from produced coatings into the aqueous environment. For this purpose, each sample was measured ‘as prepared’, and then after immersion in water at room temperature for times ranging up to 55 days. To eliminate the influence of potential statistical variations between the samples, the ratio between the Ag content measured for immersed coating and the initial Ag abundance Ag0, recorded for ‘as prepared’ sample was calculated. The results, presented in Fig. S2† show the monotonic decrease of Ag amount in POEGMA-grafted brushes, down to 50% for first 15 days of incubation in water and significant suppression of this effect for longer incubation times. The abundance of Ag does not drop below 35% of initial value even after 55 days of incubation. This effect may be related to a very good isolation of the AgNPs in polymer matrix observed for some critical sizes of AgNPs, not allowing for further release of the silver ions. Moreover, for small sizes of the AgNPs the strong affinity of oxygen from ether groups of the POEGMA188 with silver clusters can be predicted.42,43Observed prolonged release of silver ions from the POEGMA188 coatings suggests that they may be very prospective materials for human-related applications, as they strongly minimalize the risks of negative effects towards human health. However, further studies on Ag release in more physiological conditions, e.g. in phosphate saline buffer (PBS) at 37 °C are required.
3.3. Antibacteriality and cytotoxicity of POEGMA188 based nanocomposite coatings with embedded AgNPs
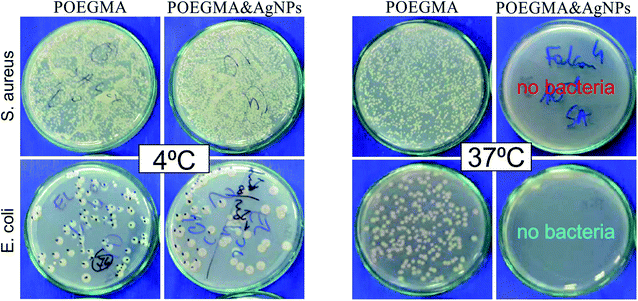
In our previous work30 we have demonstrated the temperature-switched killing of the bacteria on POEGMA188 and P4VP based coatings where no significant difference was observed at 4 °C between the amounts of bacteria counted on the nanocomposite coatings with AgNPs and on the “bare” coating control sample. In contrast, at 37 °C almost no bacteria were visible for temperature-responsive coating with AgNPs, whereas the growth of bacteria remained unperturbed for “pure” coating, indicating well-expressed temperature-dependent antibacterial properties of AgNPs integrated into brushes. Similar results were obtained also for the coatings examined in the present study fabricated with optimized conditions (16 hours of polymerization, 15 min of silver immobilization from 5 mM AgNO3 solution). Fig. 4 demonstrates cultivation of E. coli and S. aureus model bacterial strains on the Petri plates after incubation with the POEGMA188-based nanocomposites as well as POEGMA188 control samples at 4 °C (left panel) and 37 °C (right panel) that confirms our previous results.30Although AgNPs have astounding pharmacological properties, the possibility of their application is strongly limited by their potential cytotoxicity and genotoxicity, activated by various mechanisms, in particular the production of excess reactive oxygen species (ROS), which results in oxidative stress, reduced levels of glutathione, elevated lipid peroxidation, inflammation, DNA damage, altered cell cycle and proliferation capacity, and apoptosis and necrosis.33,44–46 AgNPs have also the tendency to accumulate in human organs, like liver, spleen, lungs, and kidney7,8,31 and they are able to can cross the blood–brain barrier (BBB) and accumulate in different regions of the brain.34 These effects depend strongly on the shape, size and concentration of AgNPs, as well as on the cell type.45,47,48 Although the cytotoxic and differentiation-inducing properties are generally considered as negative, they result in a great anti-cancer impact of AgNPs, since they show higher cytotoxicity against cancer cells in comparison with normal cells.31,32 Numerous research show, that in cancer chemotherapeutic regimes, AgNPs can promote apoptosis and tumor cell differentiation.49,50 To minimalize the risks of negative effects towards human health, the materials with prolonged release of silver ions from permanently bonded AgNPs are highly demanded. XPS results (Fig. S2†) indicate that proposed coatings fulfill this requirement. Moreover, according to the literature51,52 potential hazardous effects of AgNPs depend also on their location in the coating. Based on these criteria, materials may be grouped into three categories: ‘expected to cause exposure’ for NPs suspended in liquids and airborne NPs, ‘may cause exposure’ for surface-bound NPs and ‘no expected exposure’ for NPs suspended in solids.53 Due to the conformation of polymer brushes, the AgNPs in the studied coatings are dispersed uniformly in the whole brush and not only on the surface, therefore they should fall rather into the third category. What is more, the thermo-responsivity of the coatings limits strongly the possible interactions between AgNPs and the external environment, as they are permitted only at temperatures above LCST.
To study the impact of AgNPs embedded into the POEGMA188 nanocomposite coatings (16 h of polymerization time, 15 min of silver immobilization from 5 mM AgNO3 solution) on proliferation of healthy and cancerous cells, two cell lines were chosen. They were in vitro spontaneously transformed keratinocytes from histologically normal skin (HaCaT) and WM35 cell line derived from the primary melanoma site of the patient's skin diagnosed with radial growth phase (RGP) melanoma. Cells were seeded on all substrates using the same initial solution to avoid discrepancies linked with various cellular densities.
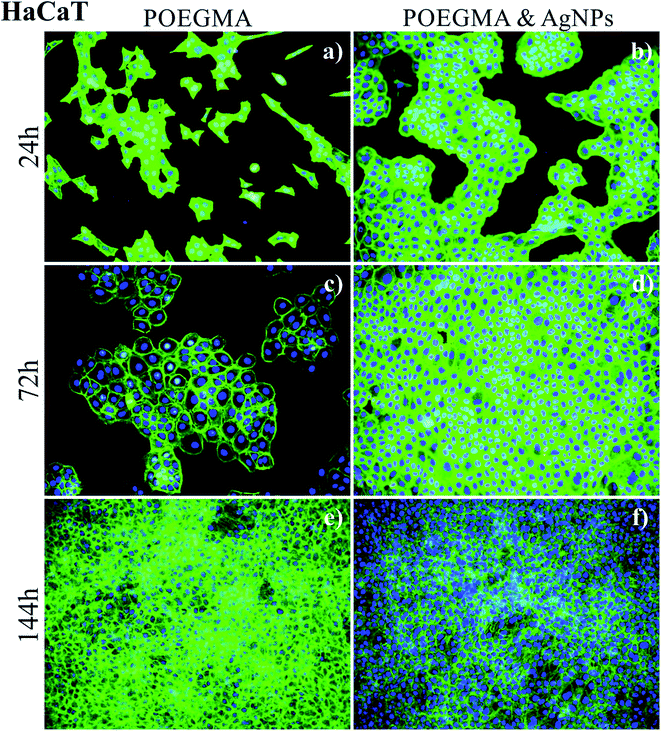
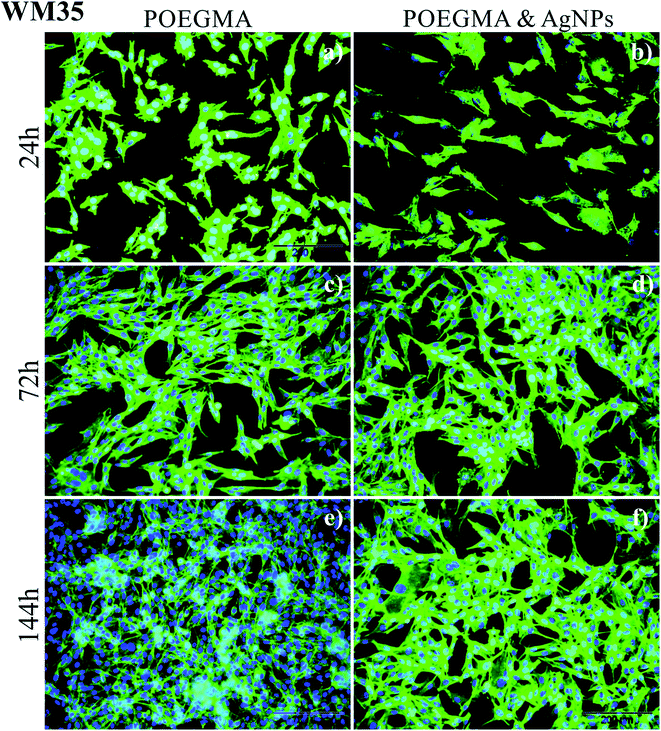
Representative fluorescence images recorded after 24, 72 and 144 h culture time, are presented in Fig. 5 for keratinocytes and Fig. 6 for melanoma cells.
The comparison of the growth of healthy (keratinocyte) HaCaT cells on “pure” POEGMA coating (Fig. 5, left panel) and on POEGMA coating with AgNPs (Fig. 5, right panel) suggests that cells grow faster on the polymer brush with nanoparticles, where they form confluent layer already after 72 h of incubation. For the coating without AgNPs, formation of such layer is observed first after 144 h culture time.
In turn, for WM35 cancerous (melanoma) cells (Fig. 6) rather an opposite effect was noticed. The amount of cells is slightly smaller on the POEGMA brushes with AgNPs and after 144 h incubation time they only start to converge. In contrast, on the “pure” polymer coating the monocellular layer is already formed after the same incubation time. These results indicate that AgNPs embedded in polymer brush do not have any significant cytotoxic effect towards normal cells and suggest a slight anti-cancer impact of AgNPs.
To confirm these statements, quantitative analysis was performed, with number of cells as well as proliferation index determined (Fig. 7), the latter defined as the ratio between the number of cells after given incubation time and the number of cells after 24 hours of culture. The number of cells presents on the surface after a given culture time depends on two factors, i.e. the number of initially adhered cells and their further ability to proliferate. The proliferation index provides information about the ability of adhered cells to proliferate on the examined surfaces independently of their adhesive properties. For cancerous WM35 cell line, both, the number of cells and proliferation index, are comparable and do not show any noticeable differences independently of the substrate used for the study. In contrast, for healthy keratinocytes (HaCaT), significant, time-dependent impact of AgNPs can be observed. For short incubation times, up to 72 h, the number of HaCaT cells counted for the POEGMA188 coating with embedded AgNPs is approximately 5 times larger for POEGMA coating with embedded AgNPs and at the same time proliferation index is almost doubled in comparison with the “pure” polymer coating.
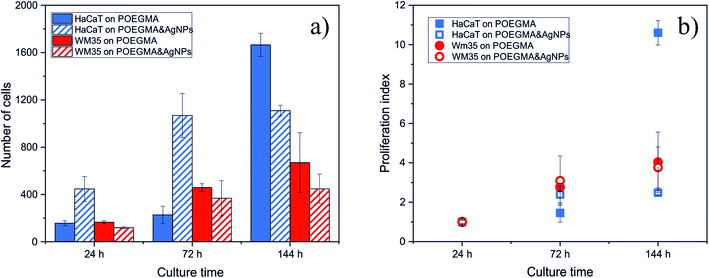 | ||
| Fig. 7 Impact of AgNPs embedded in POEGMA188 grafted brush coatings on the amount (a) and proliferation index (b) of HaCaT and WM35 cells. | ||
However, this situation changes for longer incubation time (144 h) such that the number of cells on POEGMA188 coating starts to exceed the number of cells on polymer brush with embedded AgNPs, by 25%. This effect is particularly manifested in the value of proliferation index, increasing sharply for cells incubated on POEGMA-grafted brush and remaining almost unchanged for the substrate with AgNPs.
These results indicate that the impact of nanocomposites with AgNPs is highly cell-dependent. The slight decrease of the number of cancerous cells do not allow to confirm an anti-cancer impact of AgNPs, postulated by the literature data, reporting their higher cytotoxicity against cancer cells over normal cells.31,32 In turn, our research shows that AgNPs embedded in polymer brush do not have any cytotoxic effect towards the normal cells for short exposure times and start to affect them for longer times. This effect might be related with potential long-term release of AgNPs from the grafted brush and their subsequent accumulation in cells.7,8,31,33,34
4. Conclusion
In the present work, the non-cytotoxic, temperature-responsive and antibacterial POEGMA188 based nanocomposite coatings attached to a glass surface were successfully prepared using ATRP polymerization. The nanocomposite coatings were fabricated in an easy process, for the glass surface previously functionalized by APTES and subsequently with ATRP molecules. In the next step, POEGMA188 grafted brushes were fabricated and then Ag ions were adsorbed into the coatings and reduced to AgNPs by sodium borohydride.29,30The thickness, morphology and wettability of the resulting coatings were analyzed using ellipsometry, AFM and CA measurements, respectively. The strong impact of the thicknesses of the POEGMA188 grafted brush coatings and the amount of AgNPs on the morphology and temperature-induced wettability of the nanocomposite was demonstrated. Above some threshold concentration of the AgNPs in POEGMA188 coatings, temperature-responsive properties are absent. Based on our previous work30 and presented here results it can be stated that temperature-responsive POEGMA188 based nanocomposite coatings with silver nanoparticles can be synthesized only for brush thickness larger than 45 nm in a dry state, and for moderate AgNPs concentrations equivalent to Ag+ immobilization in 5 mM AgNO3 solution no longer than 15 min.
Strong temperature-responsive antibacterial effect of POEGMA188 based nanocomposite was demonstrated here, confirming our previous work.30 The possibility of application of materials containing AgNPs is strongly restricted, due to their potential cytotoxicity as well as their tendency to accumulate in human organs. However, this risk should be minimalized for the proposed nanocomposite coatings where the AgNPs are bonded to the polymer brushes so their possibility to relocate to the human body should be strongly limited. XPS results have shown prolonged release of silver from the POEGMA188 coatings suggesting release of silver ions and not whole Ag nanoparticles, however abundance of Ag does not drop below 35% of initial value even after 55 days of incubation. Moreover, POEGMA188 nanocomposite coatings have no significant cytotoxic effect towards normal cells examined here and only a slight anti-cancer impact, which confirms our hypothesis that only silver ions are released.
New POEGMA-based nanocomposite grafted brush coatings have at least three advantages. First, the temperature-dependent antibacterial properties have a great potential for applications, from medical laboratories to food packaging, where the intensive growth of microorganisms at elevated temperatures is a natural but undesired process. Second, AgNPs embedded in the polymer brush do not have any cytotoxic effect towards the normal cells for short exposure times. Third, the release of silver ions from the POEGMA188 nanocomposite coatings in water has prolonged character (at least some weeks).
Notes
A patent applications P.424734 (PL, 2018-03-02) and PCT/PL2019/05001 (Patent Cooperation Treaty, 2019-09-06) related to this work have been filed.Conflicts of interest
All authors have no conflict of interest.Acknowledgements
This work was partially supported by the Dean of the Faculty of Physics, Astronomy and Applied Computer Science at Jagiellonian University. The authors thank K. Małek-Ziętek from the Centre for Technology Transfer CITTRU at Jagiellonian University for her support during process of legal protection and commercialization of intellectual property. The research was carried out with equipment purchased with financial support from the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract no. POIG.02.01.00-12-023/08). Svyatoslav Nastyshyn thanks for financial support from DSC2019 #N17/MNS/000031 and Descartes Project in Jagiellonian University. Yana Shymborska thanks for financial support from Queen Jadwiga Fund.References
- J. Kim, E. Kuk, K. Yu, J. Kim, S. Park, H. Lee, S. Kim, Y. Park, Y. Park, C. Hwang, Y. Kim, Y. Lee, D. Jeong and M. Cho, Nanomedicine, 2007, 3, 95 CrossRef CAS PubMed.
- D. Evanoff and G. Chumanov, ChemPhysChem, 2005, 6, 1221 CrossRef CAS PubMed.
- D. Chen, X. Qiao, X. Qiu and J. Chen, J. Mater. Sci., 2009, 44, 1076 CrossRef CAS.
- B. Rybak, M. Ornatska, K. Bergman, K. Genson and V. Tsukruk, Langmuir, 2006, 22, 1027 CrossRef CAS PubMed.
- Z. Shervani, Y. Ikushima, M. Sato, H. Kawanami, Y. Hakuta, T. Yokoyama, T. Nagase, H. Kuneida and K. Aramaki, Colloid Polym. Sci., 2008, 286, 403 CrossRef CAS.
- M. Moffitt and A. Eisenberg, Chem. Mater., 1995, 7, 1178 CrossRef CAS.
- I. Sur, M. Altunbek, M. Kahraman and M. Culha, Nanotechnology, 2012, 23, 375102 CrossRef PubMed.
- I. Sur, D. Cam, M. Kahraman, A. Baysal and M. Culha, Nanotechnology, 2010, 21, 175104 CrossRef PubMed.
- U. Chatterjee, S. Jewrajka and S. Guha, Polym. Compos., 2009, 30, 827 CrossRef CAS.
- M. Carbone, D. Donia, G. Sabbatella and R. Antiochia, J. King Saud Univ., Sci., 2016, 28, 273 CrossRef.
- S. Lee, H. Kim, R. Patel, S. Im, J. Kim and B. Min, Polym. Adv. Technol., 2007, 18, 562 CrossRef CAS.
- A. Pich, A. Karak, Y. Lu, A. Ghosh and H. Adler, Macromol. Rapid Commun., 2006, 27, 344 CrossRef CAS.
- Y. Dong, Y. Ma, T. Zhai, F. Shen, Y. Zeng, H. Fu and J. Yao, Macromol. Rapid Commun., 2007, 28, 2339 CrossRef CAS.
- G. V. Ramesh, B. Sreedhar and T. P. Radhakrishnan, Phys. Chem. Chem. Phys., 2009, 11, 10059 RSC.
- T. Wu, Z. Ge and S. Liu, Chem. Mater., 2011, 23, 2370 CrossRef CAS.
- V. Skorokhoda, Y. Melnyk, N. Semenyuk, N. Ortynska and O. Suberlyak, Chem. Chem. Technol., 2017, 11, 171 CrossRef CAS.
- V. Skorokhoda, Y. Melnyk, V. Shalata, T. Skorokhoda and S. Suberliak, East.-Eur. J. Enterp. Technol., 2017, 1, 50 CrossRef.
- V. Skorokhoda, N. Semenyuk, I. Dziaman and O. Suberlyak, Chem. Chem. Technol., 2016, 10, 187 CrossRef CAS.
- E. Benetti, X. Sui, S. Zapotoczny and G. Vancso, Adv. Funct. Mater., 2010, 20, 939 CrossRef CAS.
- S. Gupta, P. Uhlmann, M. Agrawal, S. Chapuis, U. Oertel and M. Stamm, Macromolecules, 2008, 41, 2874 CrossRef CAS.
- S. Gupta, M. Agrawal, M. Conrad, N. Hutter, P. Olk, F. Simon, L. Eng, M. Stamm and R. Jordan, Adv. Funct. Mater., 2010, 20, 1756 CrossRef CAS.
- S. Konnova, A. Danilushkina, G. Fakhrullina, F. Akhatova, A. Badrutdinov and R. Fakhrullin, RSC Adv., 2015, 5, 13530 RSC.
- A. Skirtach, A. Antipov, D. Shchukin and G. Sukhorukov, Langmuir, 2004, 20, 6988 CrossRef CAS PubMed.
- M. He, Q. Wang, J. Zhang, W. Zhao and C. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 44782 CrossRef CAS PubMed.
- T. Wei, Z. Tang, Q. Yu and H. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 37511 CrossRef CAS PubMed.
- X. Wang, S. Yan, L. Song, H. Shi, H. Yang, S. Luan, Y. Huang, J. Yin, A. Khan and J. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 40930 CrossRef CAS PubMed.
- R. Hu, G. Li, Y. Jiang, Y. Zhang, J. Zou, L. Wang and X. Zhang, Langmuir, 2013, 29, 3773 CrossRef CAS PubMed.
- H. Yang, G. Li, J. Stansbury, X. Zhu, X. Wang and J. Nie, ACS Appl. Mater. Interfaces, 2016, 8, 28047 CrossRef CAS PubMed.
- Y. Stetsyshyn, K. Awsiuk, V. Kusnezh, J. Raczkowska, B. R. Jany, A. Kostruba, K. Harhay, H. Ohar, O. Lishchynskyi, Y. Shymborska, Y. Kryvenchuk, F. Krok and A. Budkowski, Appl. Surf. Sci., 2019, 463, 1124 CrossRef CAS.
- J. Raczkowska, Y. Stetsyshyn, K. Awsiuk, M. Brzychczy-Włoch, T. Gosiewski, B. Jany, O. Lishchynskyi, Y. Shymborska, S. Nastyshyn, A. Bernasik, H. Ohar, F. Krok, D. Ochońska, A. Kostruba and A. Budkowski, Mater. Sci. Eng., C, 2019, 103, 109806 CrossRef CAS PubMed.
- K. McNamara and S. Tofail, Adv. Phys.: X, 2017, 2, 54 CAS.
- M. Azizi, H. Ghourchian, F. Yazdian, S. Bagherifam, S. Bekhradnia and B. Nyström, Sci. Rep., 2017, 7, 5178 CrossRef PubMed.
- T. Zhang, L. Wang, Q. Chen and C. Chen, Yonsei Med. J., 2014, 55, 283 CrossRef CAS PubMed.
- F. Liu, M. Mahmood, Y. Xu, F. Watanabe, A. Biris, D. Hansen, A. Inselman, D. Casciano, T. Patterson, M. Paule, W. Slikker and C. Wang, Front. Neurosci., 2015, 9, 115 Search PubMed.
- S. Edmondson and B. Zhu, Mater. Sci., 2012, 12–14 Search PubMed.
- A. Kostruba, M. Ohar, B. Kulyk, O. Zolobko and Y. Stetsyshyn, Appl. Surf. Sci., 2013, 276, 340 CrossRef CAS.
- A. Kostruba, Y. Stetsyshyn and R. Vlokh, Appl. Opt., 2015, 54, 6208 CrossRef CAS PubMed.
- S. Kasraei and M. Azarsina, Braz. Oral Res., 2012, 26, 505 CrossRef PubMed.
- R. Jagtap and A. Ambre, Indian Journal of Materials Science, 2013, 13, 368 Search PubMed.
- K. Awsiuk, A. Bernasik, M. Kitsara, A. Budkowski, J. Rysz, J. Haberko, P. Petrou, K. Beltsios and J. Raczkowska, Colloids Surf., B, 2010, 80, 63 CrossRef CAS PubMed.
- M. Marrese, V. Guarino and L. Ambrosio, J. Funct. Biomater., 2017, 8, 7 CrossRef PubMed.
- S. Malynych, A. Luzinov and G. Chumanov, J. Phys. Chem., 2002, 106, 1280 CrossRef CAS.
- S. Nam, D. V. Parikh, B. D. Condon, Q. Zhao and M. Yoshioka-Tarver, J. Nanopart. Res., 2011, 13, 3755 CrossRef CAS.
- S. Gurunathan, M. Qasim, C. Park, H. Yoo, D. Choi, H. Song, C. Park, J.-H. Kim and K. Hong, Int. J. Mol. Sci., 2018, 19, 3618 CrossRef PubMed.
- M. Akter, M. T. Sikder, M. M. Rahman, A. K. M. A. Ullah, K. F. B. Hossain, S. Banik, T. Hosokawa, T. Saito and M. Kurasaki, J. Adv. Res., 2018, 9, 1 CrossRef CAS PubMed.
- W. Liu, Y. Wu, C. Wang, H. C. Li, T. Wang, C. Y. Liao, L. Cui, Q. F. Zhou, B. Yan and G. B. Jiang, Nanotoxicology, 2010, 4, 319 CrossRef CAS PubMed.
- P. Rajanahalli, C. J. Stucke and Y. Hong, Toxicol. Rep., 2015, 2, 758 CrossRef CAS PubMed.
- M. Milić, G. Leitinger, I. Pavičić, M. Zebić Avdičević, S. Dobrović, W. Goessler and I. Vinković Vrček, J. Appl. Toxicol., 2015, 35, 581–592 CrossRef PubMed.
- J. Han, S. Gurunathan, Y. Choi and J. Kim, Int. J. Nanomed., 2017, 12, 7529 CrossRef CAS PubMed.
- S. Gurunathan, J. W. Han, V. Eppakayala, M. Jeyaraj and J.-H. Kim, BioMed Res. Int., 2013, 2013, 535796 Search PubMed.
- S. F. Hansen, B. H. Larsen, S. I. Olsen and A. Baun, Nanotoxicology, 2007, 1(3), 243 CrossRef CAS.
- SCENIHR, Scientific Committee on Emerging and Newly Identified Health Risks, SCENIHR Opinion on Nanosilver: safety, health and environmental effects and role in antimicrobial resistance, 1. 06. 2014, ISSN: 831-4783 Search PubMed.
- S. F. Hansen, E. S. Michelson, A. Kamper, P. Borling, F. Stuer-Lauridsen and A. Baun, Ecotoxicology, 2008, 17, 438 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10874b |
| This journal is © The Royal Society of Chemistry 2020 |