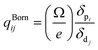Open Access Article
Open Access ArticleTellurium–oxygen group enhanced birefringence in tellurium phosphates: a first-principles investigation†
Pengyun Jina,
Xuerui Shia,
Xiuhua Cui *a,
Yi Jianga,
Qun Jing
*a,
Yi Jianga,
Qun Jing a,
Ming-Hsien Leeb,
Mengqiu Long
a,
Ming-Hsien Leeb,
Mengqiu Long ac,
Haibin Caod and
Hanqin Ding*a
ac,
Haibin Caod and
Hanqin Ding*a
aInstitute of Low-dimensional Quantum Materials and Devices, School of Physical Science and Technology, Xinjiang University, 666 Shengli Road, Urumqi 830046, China. E-mail: xjcxh0991@xju.edu.cn; dinghq@xju.edu.cn
bDepartment of Physics, Tamkang University, New Taipei City 25137, Taiwan
cHunan Key Laboratory of Super Micro-structure and Ultrafast Process, Central South University, Changsha 410083, China
dDepartment of Physics, College of Sciences, Shihezi University, Shihezi 832000, China
First published on 24th January 2020
Abstract
Phosphates possess a relatively large UV/DUV cutoff edge, but these compounds usually have very small birefringence. Recently the Te2P2O9 crystal was synthesized and its birefringence was reported to be as large as 0.106 at 1013.98 nm. Herein, we investigated the electronic structure and optical properties of Te2P2O9 using the first-principles method. The obtained results are in good agreement with the experimental values. The Born effective charges and SHG density of Te2P2O9 show that the contribution to the birefringence and SHG response mainly originates from the TeO5 group. The electronic structures and optical response of Ba2TeO(PO4)2 and Te3O3(PO4)2 were also investigated for comparison. The results show that these two tellurium phosphates also possess a large birefringence similar to Te2P2O9. Also, the birefringence originates from the TeOx polyhedrons, which was confirmed by the real-space atom-cutting results and distortion indices.
1. Introduction
Nowadays, nonlinear optical (NLO) materials have attracted widespread attention because they can be used to obtain wide-band and tunable laser sources via the second order NLO effect.1–5 During the past decades, numerous inorganic NLO materials have been obtained, including infrared, visible and ultraviolet (UV) materials.6–10 Also, borates, phosphates and carbonates are thought to be good candidates for UV/deep-UV NLO compounds, such as BBO,11,12 LiB3O5 (LBO),13–15 CsLiB6O10 (CLBO),16–18 CsB3O5 (CBO),19,20 and KBe2BO3F2 (KBBF).21–24 It has been proven that the introduction of fluorine into borates is beneficial to obtain short UV cutoff edges and suitable birefringence and large NLO responses,25,26 and new deep-UV fluorooxoborates with good performances were obtained such as Li2B6O9F2,25 AB4O6F (A = NH4, K, Rb, and Cs),27,28 and SrB5O7F3.29Recently, phosphates have been reported to be good candidates as UV and deep-UV NLO crystal materials because of their short UV cut-off edge,30,31 including Rb2Ba3(P2O7)3 (<200 nm),32 Ba5P6O20 (about 167 nm),33 and KLa(PO3)4 (162 nm).34 However, phosphates also have drawbacks. Most of the reported phosphates have a relatively small birefringence, which has relationship with the regular tetrahedron of the PO4 units. First principles investigations show that the anisotropic polarization of the regular PO4 unit is relatively small. Thus, to overcome this inherent drawback, different types of d0 transition metal cations (Ti4+, Mo6+, etc.)35 and lone pair electrons (Bi3+, Te4+, Pb2+, etc.) are introduced to phosphates. Wang et al. at Shandong University36 obtained large-size Te2P2O9 crystals via the Czochralski method. The crystals had the acentric polar space group Cc, and their basic building units were the PO4 tetrahedron and TeO5 square pyramid. Their linear and nonlinear optical properties were also investigated.36 The SHG response and birefringence of Te2P2O9 are relatively large, which is 1.3 × KDP, and 0.13786–0.10615 at 404.66–1013.98 nm, respectively. Curiously, how can it possess such a large birefringence in comparison with other phosphates? Also, what is the origination of this large birefringence in phosphates?
Herein, we calculated the electronic structure and optical properties of Te2P2O9. The obtained refractive indices, birefringence and SHG tensor agree well with the experimental values. Utilizing its electronic structures, Born effective charges, and the SHG density method, the atomic contribution to the birefringence and SHG tensors of Te2P2O9 are investigated. The results show that the TeO5 polyhedrons give the main contribution to the optical anisotropic birefringence and SHG response. Furthermore the optical response of the other tellurium phosphates Ba2TeO(PO4)2 (ref. 37) and Te3O3(PO4)2 (ref. 38) were also investigated for comparison. These two compounds possess similar basic building units as Te2P2O9, which are an isolated PO4 tetrahedron and TeOx polyhedrons (more details can be found in Table S1 in the ESI†). These two compounds also have very large birefringence like Te2P2O9. The large birefringence in these tellurium phosphates originate from the TeOx polyhedrons, which was further confirmed by the real-space atom-cutting results and the distortion indices.
2. Computational details
To better understand the relationship between the structure and optical properties, the electronic structure and Born effective charges were investigated using the first-principles method implemented in the CASTEP package.39,40 During the calculation, the exchange-correlation functional with the Perdew–Burke–Ernzerhof (PBE)41,42 functional and the norm-conserving pseudopotentials (NCP) was adopted. The kinetic energy cutoffs were set as 830 eV for Te2P2O9, 830 eV for Te3O3(PO4)2, and 830 eV for Ba2TeO(PO4)2. The k-point mesh in the Monkhorst–Pack was set as 5 × 5 × 3 (Te2P2O9), 2 × 3 × 3 (Te3O3(PO4)2), and 4 × 4 × 3 (Ba2TeO(PO4)2). After the electronic structures were obtained, the refractive indices and the birefringence were further calculated via the OPTADOS code.43,44 The nonlinear optical tensors of Te2P2O9 were further investigated using the method described in ref. 45–47. For comparison, the electronic structures and optical properties of Te2P2O9 were also investigated using the LDA(CA-PZ), GGA(PW91) and GGA(RPBE) functionals (shown in Table S2 in the ESI†). The results show that the GGA-PBE functional gives more reliable results in comparison with the experimental values. Hence, herein, we only discuss the GGA-PBE results.3. Results and discussions
3.1 The electronic structures of Te2P2O9
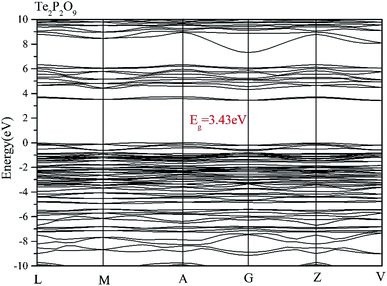
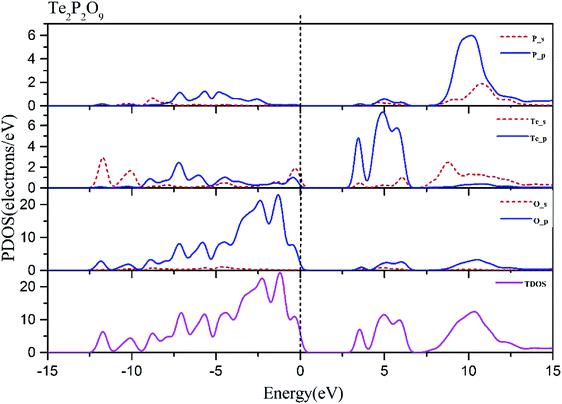
Using the method described above, the band structures and the projected density of states of Te2P2O9 were obtained. It can be seen clearly from Fig. 1 that Te2P2O9 is an indirect bandgap compound with the bandgap of 3.43 eV. The obtained bandgap is smaller than the experimental value (about 4.30 eV).36 This underestimation of the bandgap may be related with the derivative discontinuity of the exchange-correlation energy.48The projected density of states (PDOS) of Te2P2O9 is shown in Fig. 2. For the Te2P2O9 compound, the states of the valence bands (VB) from −10 eV to the Fermi level are mainly the Te sp, P sp and O sp states. The states at the bottom of the conduction bands (CB) from 3 eV to 6.5 eV are dominated by the Te 5p and O 2p orbitals. From the states at the valence band and the conduction band, the hybrid states of the P–O and Te–O chemical bonds can be determined. According to the revised model,10,49–53 the lone pair states from the Te–O chemical bond can be deduced. The optical properties of the material are closely related to the electronic transition between the top of the valence band and the bottom of the conduction band. Hence, we believe that the Te–O and P–O chemical bonds play an important role in determining the optical properties of Te2P2O9.
3.2 The refractive indices and Born effective charges of Te2P2O9
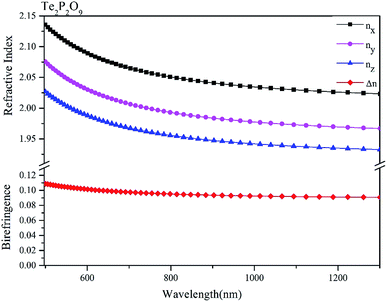
The obtained refractive indices and birefringence of Te2P2O9 are shown in Fig. 3. From the data shown in Fig. 3, it can be determined that the calculated refractive indices follow the order of nx > ny > nz. The calculated birefringence is 0.12496–0.09236 in the wavelength range of 404.65–1013.61 nm. It is interesting to note that the reported experimental birefringence of Te2P2O9 is 0.13786–0.10615 in the wavelength range of 404.66–1013.98 nm. Thus, it is obvious that the calculated value matches well with the experimental value.To better understand the atomic contribution to the optical anisotropic birefringence, the Born effective charges54–56 were also investigated in this work. Because the optical anisotropic birefringence is closely related with the difference in the macroscopic polarizability along different optical axes, we focused on the anisotropic polarization.57–59 The Born effective charges are defined as:
| Compound | Atom | qxx | qyy | qzz | Δq(Born) |
|---|---|---|---|---|---|
| Te2P2O9 | Te1 | 4.34265 | 4.65936 | 6.63644 | −2.29379 |
| Te2 | 6.4218 | 5.38842 | 3.37218 | 3.04962 | |
| Te3 | 4.34265 | 4.65936 | 6.63644 | −2.29379 | |
| Te4 | 6.4218 | 5.38842 | 3.37218 | 3.04962 | |
| P1 | 4.40285 | 3.49299 | 4.36297 | 0.03988 | |
| P2 | 4.70919 | 4.86415 | 3.77507 | 0.93412 | |
| P3 | 4.40285 | 3.49299 | 4.36297 | 0.03988 | |
| P4 | 4.70919 | 4.86415 | 3.77507 | 0.93412 | |
| O1 | −1.78151 | −1.84853 | −2.74356 | 0.96205 | |
| O2 | −3.2844 | −2.12701 | −1.33367 | −1.95073 | |
| O3 | −2.85789 | −1.94646 | −1.32288 | −1.53501 | |
| O4 | −1.40561 | −0.89945 | −3.71391 | 2.3083 | |
| O5 | −1.30274 | −2.25599 | −1.88828 | 0.58554 | |
| O6 | −1.56074 | −3.25254 | −1.86712 | 0.30638 | |
| O7 | −1.54143 | −1.92023 | −2.76231 | 1.22088 | |
| O8 | −3.00828 | −2.10674 | −1.13507 | −1.87321 | |
| O9 | −3.13389 | −2.04796 | −1.37986 | −1.75403 | |
| O10 | −1.78151 | −1.84853 | −2.74356 | 0.96205 | |
| O11 | −3.2844 | −2.12701 | −1.33367 | −1.95073 | |
| O12 | −2.85789 | −1.94646 | −1.32288 | −1.53501 | |
| O13 | −1.40561 | −0.89945 | −3.71391 | 2.3083 | |
| O14 | −1.30274 | −2.25599 | −1.88828 | 0.58554 | |
| O15 | −1.56074 | −3.25254 | −1.86712 | 0.30638 | |
| O16 | −1.54143 | −1.92023 | −2.76231 | 1.22088 | |
| O17 | −3.00828 | −2.10674 | −1.13507 | −1.87321 | |
| O18 | −3.13389 | −2.04796 | −1.37986 | −1.75403 |
3.3 The contribution from TeOx polyhedrons in tellurium phosphate
To better understand the contribution from the TeOx polyhedrons to the birefringence, the electronic structures and the optical response of the other tellurium phosphates Ba2TeO(PO4)2 and Te3O3(PO4)2 were also investigated. The obtained band structures and projected density of states (PDOS) of Ba2TeO(PO4)2 and Te3O3(PO4)2 are shown in Fig. S1–S4 in the ESI.† As shown in Fig. S1 and S3,† the obtained bandgap of Ba2TeO(PO4)2 and Te3O3(PO4)2 are 4.11 and 3.63 eV, respectively. To overcome the underestimation of the bandgap, HSE06 calculations62 were performed using the PWmat code.63,64 The obtained HSE06 bandgap of Ba2TeO(PO4)2 and Te3O3(PO4)2 is 4.47, and 4.18 eV, respectively. As shown in Fig. S2 and S4,† the states at the top of the valence band and at the bottom of the conduction band are mainly the Te-sp states, O-sp states, and P-sp states. Also, the chemical bonds of Te–O and P–O can also be found at the top of the valence band. Hence, it can be deduced that the TeOx polyhedron and PO4 tetrahedron may play an important role in determining the optical response of these tellurite phosphates.The refractive indices and birefringence of Ba2TeO(PO4)2 and Te3O3(PO4)2 were also obtained using the method described above, and the results are shown in Fig. S5 and S6,† respectively, and Table 2. As shown in Fig. S5 and S6,† the Ba2TeO(PO4)2 and Te3O3(PO4)2 possess a relatively large birefringence of 0.110 and 0.121 (at 1064 nm), respectively. The birefringence of these compounds follow the sequence of Δn(Te3O3(PO4)2) > Δn(Ba2TeO(PO4)2) > Δn(Te2P2O9).
| Crystal | Contribution | nx | ny | nz | Δn |
|---|---|---|---|---|---|
| Te2P2O9 | Cut-PO | 1.484 | 1.458 | 1.405 | 0.079 |
| Cut-TeO | 1.232 | 1.226 | 1.203 | 0.029 | |
| Origin | 2.031 | 1.974 | 1.939 | 0.092 | |
| Ba2TeO(PO4)2 | Cut-BaO | 1.408 | 1.332 | 1.323 | 0.085 |
| Cut-PO | 1.555 | 1.486 | 1.475 | 0.080 | |
| Cut-TeO | 1.600 | 1.588 | 1.563 | 0.037 | |
| Origin | 1.877 | 1.860 | 1.767 | 0.110 | |
| Te3O3(PO4)2 | Cut-PO | 1.622 | 1.585 | 1.570 | 0.052 |
| Cut-TeO | 1.243 | 1.229 | 1.211 | 0.032 | |
| Origin | 2.027 | 1.998 | 1.906 | 0.121 |
The contribution from different polyhedrons was further investigated using the real-space atom-cutting method.45 During the calculation, the atom-cutting radius of O, P, Te, and Ba was set as 1.10, 0.95, 0.96, and 1.74 Å, respectively. After the real-space atom-cutting method was performed, the refractive indices and birefringence of Te2P2O9, Ba2TeO(PO4)2, and Te3O3(PO4)2 were obtained, which are shown in Table 2, and Fig. S7–S9 in the ESI,† respectively. As shown in Table 2, in comparison with the PO4 tetrahedron and BaOx polyhedrons, the TeOx polyhedrons give the main contribution to the total birefringence. Taking Te2P2O9 as example, the its birefringence is about 0.092 at 1064 nm. However, after the TeO5 polyhedron was removed, the obtained birefringence of the other part (marked as cut-TeO) is only about 0.029, implying that the TeO5 polyhedron may give a contribution of about (0.092 − 0.029 = 0.063) to the total birefringence of Te2P2O9. Thus, in comparison with the PO4 tetrahedron, the TeO5 polyhedron gives main contribution to the total birefringence. A similar conclusion was also found for the other tellurium phosphates, Ba2TeO(PO4)2 and Te3O3(PO4)2 (as shown in Table 2). It is interesting to note that the conclusion obtained from the real-space atom-cutting is consistent with the results obtained from the Born-effective charges analysis (as described above).
The optical anisotropic birefringence is related with the anisotropic optical response from the different polyhedrons. The relatively small anisotropic polarization of the regular PO4 unit may be related with the relatively small distortion with the regular PO4 unit. Herein, the distortion indices of the different polyhedrons in these tellurium phosphates were also investigated. The distortion indices, defined by Baur as 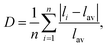 65 was calculated using the VESTA software.66 The obtained distortion indices of the different polyhedrons in these tellurium phosphates are shown in Table 3. As described above, the TeOx polyhedrons give the main contribution to the total birefringence, and hence was focused on the distortion indices of the TeOx polyhedron. As shown in Table 3, the distortion indices of the TeOx polyhedrons follow the sequence of D(Te3O3(PO4)2) > D(Ba2TeO(PO4)2) > D(Te2P2O9), which is consistent with the sequence of the birefringence of Δn(Te3O3(PO4)2) > Δn(Ba2TeO(PO4)2) > Δn(Te2P2O9).
65 was calculated using the VESTA software.66 The obtained distortion indices of the different polyhedrons in these tellurium phosphates are shown in Table 3. As described above, the TeOx polyhedrons give the main contribution to the total birefringence, and hence was focused on the distortion indices of the TeOx polyhedron. As shown in Table 3, the distortion indices of the TeOx polyhedrons follow the sequence of D(Te3O3(PO4)2) > D(Ba2TeO(PO4)2) > D(Te2P2O9), which is consistent with the sequence of the birefringence of Δn(Te3O3(PO4)2) > Δn(Ba2TeO(PO4)2) > Δn(Te2P2O9).
| Te2P2O9 | Ba2TeO(PO4)2 | Te3O3(PO4)2 | |
|---|---|---|---|
| PO4 | 0.005 | 0.020 | 0.013 |
| TeOx | 0.034 | 0.039 | 0.051 |
| BaOx | 0.034 |
3.4 The atomic contribution to the SHG response of Te2P2O9
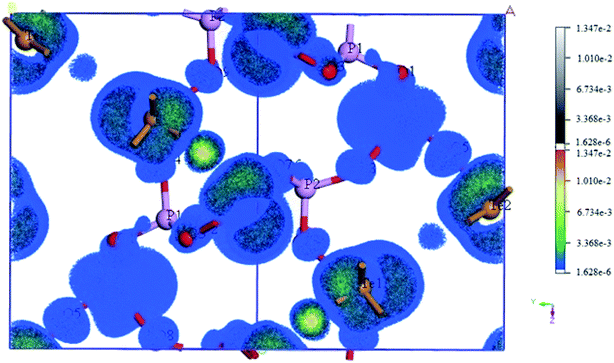
The SHG tensors of Te2P2O9 were also obtained (shown in Table 4). For that crystallized in the Cc space group, there are six independent nonzero SHG tensors, d11, d12, d13, d15, d24 and d33. As shown in Table 4, for Te2P2O9, the maximum is d33 = 1.01 pm V−1, which is about 2.6 times that of d36(KDP). The obtained SHG tensors are comparable with the experimental powder second-harmonic generation (PSHG) intensity (about 1.3 × KDP). The experimental values agree well with the calculated values, indicating that the method selected in this work is appropriate. To deeply investigate the atomic contribution to the SHG tensors, the spatial distribution of the atomic SHG density was also calculated. The SHG density method is a normalized weighting coefficient via the use of the effective SHG response of each occupied and unoccupied band.59,60 Using this method, the states irrelevant to the SHG response will not be shown, and the distribution of the SHG density represents the origin of the SHG response. Herein, we only show the SHG density obtained from the virtual electron process (labeled as veocc) and the virtual hole process (labeled as vhunocc). The obtained SHG density of Te2P2O9 is shown in Fig. 4, where the color part and white–black part represent the SHG density of the veocc and vhunocc process, respectively. As shown in Fig. 4, there is no SHG distribution around the P atoms, implying that the P atoms give a relatively small contribution to the total SHG response. It is shown that the SHG density is distributed mainly on the Te and O atoms, indicating that the TeO5 polyhedrons give the main contribution to the total SHG response.| Crystal | Space group | Calculated SHG tensors (pm V−1) | PSHG intensity |
|---|---|---|---|
| Te2P2O9 | Cc | d11 = −0.17, d15 = −0.53, d12 = 0.59, d13 = −0.56, d24 = −0.95, d33 = 1.01 | 1.3 × KDP |
4. Conclusions
In this work, the refractive indices, birefringence, and the SHG coefficient of Te2P2O9 were obtained using the first-principles method. The obtained results are in good agreement with the experimental values. The calculated birefringence of Te2P2O9 is 0.12496–0.09236 in the wavelength range of 404.65–1013.61 nm. The maximum SHG tensor d33 is 1.01 pm V−1, which is about 2.6 times that of d36 (KDP). The atomic contribution to the birefringence and SHG response was also investigated using the projected density of states, Born effective charges, and real-space SHG density method. The results show that the TeO5 groups play an important role in determining the birefringence and SHG response of Te2P2O9. The electronic structures and optical response of Ba2TeO(PO4)2 and Te3O3(PO4)2 were also investigated for comparison. The results show these two tellurium phosphates also possess a large birefringence like Te2P2O9. Also, the birefringence originates from the TeOx polyhedrons, which was confirmed by the real-space atom-cutting results and distortion indices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 11664037, 11864040), the Natural Science Foundation of Xinjiang Uygur Autonomous Region of China (Grant No. 2018D01C079, 2018D01C072), the Science and Technology Research Program for Colleges and Universities in the Department of Education in Xinjiang Uygur Autonomous Region of China (Grant No. XJEDU2017M006). The author Haibin Cao acknowledges support from Foundation for High-level Talents in Shihezi University (RCZX201511), and Applied Basic Research Foundation of Science and Technology in Shihezi University (2015ZRKXYQ07).References
- C. T. Chen, G. L. Wang, X. Y. Wang and Z. Y. Xu, Deep-UV nonlinear optical crystal KBe2BO3F2 – discovery, growth, optical properties and applications, Appl. Phys. B, 2009, 97, 9–25 CrossRef CAS.
- Y. Ning, C. Tu, X. Long and M. Hong, Recent Advances in Crystal Growth in China: Laser, Nonlinear Optical, and Ferroelectric Crystals, Cryst. Growth Des., 2010, 10, 4672–4681 CrossRef.
- W. Zhang, H. Yu, H. Wu and P. S. Halasyamani, Phase-Matching in Nonlinear Optical Compounds: A Materials Perspective, Chem. Mater., 2017, 29, 2655–2668 CrossRef CAS.
- Z. Hu, T. Higashiyama, M. Yoshimura, Y. Yap, Y. Mori and T. Sasaki, A New Nonlinear Optical Borate Crystal K2Al2B2O7 (KAB), Jpn. J. Appl. Phys., 1998, 37, L1093–L1094 CrossRef CAS.
- H. P. Wu, H. W. Yu, Z. H. Yang, X. L. Hou, S. L. Pan, X. Su, K. R. Poeppelmeier and J. M. Rondinelli, Designing a deep ultraviolet nonlinear optical material with large second harmonic generation response, J. Am. Chem. Soc., 2013, 135, 4215–4219 CrossRef CAS PubMed.
- X. L. Chen, B. B. Zhang, F. F. Zhang, Y. Wang, M. Zhang, Z. H. Yang, K. R. Poeppelmeier and S. L. Pan, Designing an excellent deep-ultraviolet birefringent material for light polarization, J. Am. Chem. Soc., 2018, 140, 16311–16319 CrossRef CAS PubMed.
- Y. Wang, B. Zhang, Z. Yang and S. Pan, Cation-Tuned Synthesis of Fluorooxoborates: Approaching the Optimal Deep-Ultraviolet Nonlinear Optical Materials, Angew. Chem., Int. Ed., 2018, 57, 2150–2154 CrossRef CAS PubMed.
- G. Q. Shi, Y. Wang, F. F. Zhan, B. B. Zhang, R. H. Yang, X. L. Hou, S. L. Pan and K. R. Poeppelmeier, Finding the Next Deep-Ultraviolet Nonlinear Optical Material: NH4B4O6F, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef CAS PubMed.
- B. H. Lei, Z. Yang and S. Pan, Enhancing optical anisotropy of crystals by optimizing bonding electron distribution in anionic groups, Chem. Commun., 2017, 53, 2818–2821 RSC.
- X. Dong, Q. Jing, Y. Shi, Z. Yang, S. Pan, K. R. Poeppelmeier, J. Young and J. M. Rondinelli, Pb2Ba3(BO3)3Cl: A Material with Large SHG Enhancement Activated by Pb-Chelated BO3Groups, J. Am. Chem. Soc., 2015, 46, 9417–9422 CrossRef PubMed.
- G. Qu, Z. Hu, Y. Wang, Q. Yang and L. Tong, Synthesis of Optical-Quality Single-Crystal β-BaB2O4 Microwires and Nanowires, Adv. Funct. Mater., 2013, 23, 1232–1237 CrossRef CAS.
- Y. Xu, W. Y. Ching and R. H. French, Electronic structure and interatomic bonding of crystalline β-BaB2O4 with comparison to LiB3O5, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 17695–17702 CrossRef CAS PubMed.
- C. Chen, Y. Wu, A. Jiang, B. Wu, G. You, R. Li and S. Lin, New nonlinear-optical crystal: LiB3O5, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef CAS.
- A. Borsutzky, R. Brünger, C. Huang and R. Wallenstein, Harmonic and sum-frequency generation of pulsed laser radiation in BBO, LBO, and KD*P, Appl. Phys. B: Photophys. Laser Chem., 1991, 52, 55–62 CrossRef.
- Q. H. Xue, Q. Zheng, Y. K. Bu, F. Q. Jia and L. S. Qian, High-power efficient diode-pumped Nd : YVO4/LiB3O5 457 nm blue laser with 4.6 W of output power, Opt. Lett., 2006, 31, 1070–1072 CrossRef CAS PubMed.
- J. Sakuma, Y. Asakawa and M. Obara, Generation of 5-W deep-UV continuous-wave radiation at 266 nm by an external cavity with a CsLiB6O10 crystal, Opt. Lett., 2004, 29, 92–94 CrossRef CAS PubMed.
- N. Umemura and K. Kato, Ultraviolet generation tunable to 0.185 microm in CsLiB6O10, Appl. Opt., 1997, 36, 6794–6796 CrossRef CAS PubMed.
- D. Xue, K. Betzler and H. Hesse, Chemical-bond analysis of the nonlinear optical properties of the borate crystals LiB3O5, CsLiB6O10, and CsB3O5, Appl. Phys. A: Mater. Sci. Process., 2002, 74, 779–782 CrossRef CAS.
- Y. Wu, et al., CsB3O5: A new nonlinear optical crystal, Appl. Phys. Lett., 1993, 62, 2614–2615 CrossRef CAS.
- H. Kitano, et al., Efficient 355-nm generation in CsB3O5 crystal, Opt. Lett., 2003, 28, 263–265 CrossRef CAS PubMed.
- C. Chen, Z. Xu, D. Deng, J. Zhang, G. Wong, B. Wu, N. Ye and D. Tang, The vacuum ultraviolet phase matching characteristics of nonlinear optical KBe2BO3F2 crystal, Appl. Phys. Lett., 1998, 68, 2930–2932 CrossRef.
- C. Chen, J. Lu, G. Wang, Z. Xu, J. Wang, C. Zhang and Y. Liu, Deep Ultraviolet Harmonic Generation with KBe2BO3F2 Crystal, Chin. Phys. Lett., 2001, 18, 1081 CrossRef.
- C. Chen, J. Lu, T. Togashi, T. Suganuma, T. Sekikawa, S. Watanabe, Z. Y. Xu and J. Wang, Second-harmonic generation from a KBe2BO3F2 crystal in the deep ultraviolet, Opt. Lett., 2002, 27, 637–639 CrossRef CAS PubMed.
- T. Togashi, T. Kanai, T. Sekikawa, S. Watanabe, C. Chen, C. Zhang, Z. Xu and J. Wang, Generation of vacuum-ultraviolet light by an optically contacted, prism-coupled KBe2BO3F2 crystal, Opt. Lett., 2003, 28, 254 CrossRef CAS PubMed.
- B. B. Zhang, G. Q. Shi, Z. H. Yang, F. F. Zhang and S. L. Pan, Fluorooxoborates: Beryllium-Free Deep-Ultraviolet Nonlinear Optical Materials without Layered Growth, Angew. Chem., Int. Ed., 2017, 56, 3916–3919 CrossRef CAS PubMed.
- Z. Yang, B.-H. Lei, W. Zhang and S. Pan, Module-Analysis-Assisted Design of Deep Ultraviolet Fluorooxoborates with Extremely Large Gap and High Structural Stability, Chem. Mater., 2019, 31, 2807–2813 CrossRef CAS.
- X. Wang, Y. Wang, B. Zhang, F. Zhang, Z. Yang and S. Pan, CsB4O6F: A Congruent-Melting Deep-Ultraviolet Nonlinear Optical Material by Combining Superior Functional Units, Angew. Chem., Int. Ed., 2017, 56, 14119–14123 CrossRef CAS PubMed.
- Z. Zhang, Y. Wang, B. Zhang, Z. Yang and S. Pan, Polar Fluorooxoborate, NaB4O6F: A Promising Material for Ionic Conduction and Nonlinear Optics, Angew. Chem., Int. Ed., 2018, 57, 6577–6581 CrossRef CAS PubMed.
- M. Mutailipu, M. Zhang, B. Zhang, L. Wang, Z. Yang, X. Zhou and S. Pan, SrB5O7F3 Functionalized with [B5O9F3]6− Chromophores: Accelerating the Rational Design of Deep-ultraviolet Nonlinear Optical Materials, Angew. Chem., Int. Ed., 2018, 57, 6095–6099 CrossRef CAS PubMed.
- Y. Shen, S. Zhao, B. Zhao, C. Ji, L. Li, Z. Sun, M. Hong and J. Luo, Strong Nonlinear-Optical Response in the Pyrophosphate CsLiCdP2O7 with a Short Cutoff Edge, Inorg. Chem., 2016, 55, 11626–11629 CrossRef CAS PubMed.
- M. Abudoureheman, S. Han, B. Lei, Z. Yang, X. Long and S. Pan, KPb2(PO3)5: a novel nonlinear optical lead polyphosphate with a short deep-UV cutoff edge, J. Mater. Chem. C, 2016, 4, 10630–10637 RSC.
- S. Zhao, P. Gong, S. Luo, L. Bai, Z. Lin, C. Ji, T. Chen, M. Hong and J. Luo, Deep-Ultraviolet Transparent Phosphates RbBa2(PO3)5 and Rb2Ba3(P2O7)2 Show Nonlinear Optical Activity from Condensation of [PO4]3− Units, J. Am. Chem. Soc., 2014, 136, 8560–8563 CrossRef CAS PubMed.
- S. Zhao, P. Gong, S. Luo, L. Bai, Z. Lin, Y. Tang, Y. Zhou, M. Hong and J. Luo, Tailored Synthesis of a Nonlinear Optical Phosphate with a Short Absorption Edge, Angew. Chem., Int. Ed. Engl., 2015, 127, 4291–4295 CrossRef.
- P. Shan, T. Sun, H. Chen, H. Liu, S. Chen, X. Liu, Y. Kong and J. Xu, Crystal growth and optical characteristics of beryllium-free polyphosphate, KLa(PO3)4, a possible deep-ultraviolet nonlinear optical crystal, Sci. Rep., 2016, 6, 25201 CrossRef CAS PubMed.
- V. G. Dmitriev, G. G. Gurzadyan and D. N. Nikogosyan, Properties of Nonlinear Optical Crystals, in Handbook of Nonlinear Optical Crystals. Springer Series in Optical Sciences, Springer, Berlin, Heidelberg, 1997, vol. 64 Search PubMed.
- W. Dong, Q. Yao, J. Zhang, L. Liu, J. Li and J. Wang, Czochralski Growth and Characterization of a Novel Nonlinear Optical Crystal Te2P2O9, Cryst. Growth Des., 2018, 18, 5919–5926 CrossRef CAS.
- K. Min Ok and P. S. Halasyamani, Synthesis, structure, and characterization of a new one-dimensional tellurite phosphate, Ba2TeO(PO4)2, ChemInform, 2006, 179(5), 1345–1350 Search PubMed.
- H. Mayer and M. Weil, Synthesis and crystal structure of Te3O3(PO4)2, a compound with 5-fold coordinate tellurium(IV), Cheminform, 2003, 629(6), 1068–1072 CAS.
- M. Segall, P. Lindan, M. Probert, C. Pickard, P. Hasnip, S. Clark and M. Payne, First-principles simulation: ideas, illustrations and the CASTEP code, J. Phys.: Condens. Matter, 2002, 14, 2717–2744 CrossRef CAS.
- S. Clark, M. Segall, C. Pickard, P. Hasnip, M. Probert, K. Refson and M. Payne, First principles methods using CASTEP, Z. Kristallogr., 2005, 220, 567–570 CAS.
- M. Ernzerhof and G. E. Scuseria, Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- B. Hammer, L. Hansen and J. Nørskov, Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 7413–7421 CrossRef.
- R. J. Nicholls, A. J. Morris, C. J. Pickard and J. R. Yates, OptaDOS - a new tool for EELS calculations, J. Phys.: Conf. Ser., 2012, 371, 012062 CrossRef.
- A. J. Morris, R. J. Nicholls, C. J. Pickard and J. R. Yates, OptaDOS: A tool for obtaining density of states, core-level and optical spectra from electronic structure codes, Comput. Phys. Commun., 2014, 185, 1477–1485 CrossRef CAS.
- Z. Lin, Z. Wang, C. Chen, S. Chen and M. Lee, Mechanism for linear and nonlinear optical effects in crystals of the Sr2Be2B2O7 family, J. Appl. Phys., 2003, 93, 9717–9723 CrossRef CAS.
- B. Zhang, M. Lee, Z. Yang, Q. Jing, S. Pan, M. Zhang, H. Wu, X. Su and C. Li, Simulated pressure-induced blue-shift of phase-matching region and nonlinear optical mechanism for K3B6O10X (X = Cl, Br), Appl. Phys. Lett., 2015, 106, 992 Search PubMed.
- Q. Jing, X. Dong, X. Chen, Z. Yang, S. Pan and C. Lei, The lone-pairs enhanced birefringence and SHG response: A DFT investigation on M2B5O9Cl (M = Sr, Ba, and Pb), Chem. Phys., 2015, 453–454, 42–46 CrossRef CAS.
- J. Perdew and M. Levy, Physical Content of the Exact Kohn–Sham Orbital Energies: Band Gap and Derivative Discontinuities, Phys. Rev. Lett., 1983, 51, 1884–1887 CrossRef CAS.
- A. Walsh, D. J. Payne, R. G. Egdell and G. W. Watson, Stereochemistry of post-transition metal oxides: revision of the classical lone pair model, Chem. Soc. Rev., 2011, 40, 4455–4463 RSC.
- A. Walsh and G. Watson, The origin of the stereochemically active Pb(II) lone pair : DFT calculations on PbO and PbS, J. Solid State Chem., 2005, 178, 1422–1428 CrossRef CAS.
- A. Walsh and G. W. Watson, Influence of the anion on lone pair formation in Sn(II) monochalcogenides: a DFT study, J. Phys. Chem. B, 2005, 109, 18868–18875 CrossRef CAS PubMed.
- J. Raulot, G. Baldinozzi, R. Seshadri and P. Cortona, An ab-initio Study of the Role of Ione Pairs in the Structure and Insulator-Metal Transition in SnO and PbO, Solid State Sci., 2002, 4, 467–474 CrossRef CAS.
- D. J. Payne, R. G. Egdell, A. Walsh, G. W. Watson, J. Guo, P. A. Glans, T. Learmonth and K. E. Smith, Electronic Origins of Structural Distortions in Post-Transition Metal Oxides: Experimental and Theoretical Evidence for a Revision of the Lone Pair Model, Phys. Rev. Lett., 2006, 96, 157403 CrossRef CAS PubMed.
- P. Hohenberg and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, B864 CrossRef.
- A. Filippetti and N. A. Spaldin, Strong-correlation effects in Born effective charges, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 045111 CrossRef.
- A. Roy, R. Prasad, S. Auluck and A. Garg, First-Principles Calculation of Born Effective Charges and Spontaneous Polarization of Ferroelectric Bismuth Titanate, J. Phys.: Condens. Matter, 2010, 22, 165902 CrossRef PubMed.
- R. Resta and D. Vanderbilt, Theory of Polarization: A Modern Approach, Top. Appl. Phys., 2007, 105, 31–68 CrossRef CAS.
- R. Resta, Modern theory of polarization in ferroelectrics, Ferroelectrics, 1994, 151, 49–58 CrossRef CAS.
- Q. Zhang, Q. Jing, H. Duan and H. Cao, The relationship between covalent bonds and the optical response in a nonpolar family ATeMoO6 (A=Mg, Zn, Cd): A Berry-Phase investigation, J. Solid State Chem., 2018, 264, 22–28 CrossRef CAS.
- N. A. Spaldin, A beginner's guide to the modern theory of polarization, J. Solid State Chem., 2012, 195, 2–10 CrossRef CAS.
- Q. Jing, G. Yang, J. Hou, M. Sun and H. Cao, Positive and negative contribution to birefringence in a family of carbonates: A Born effective charges analysis, J. Solid State Chem., 2016, 244, 69–74 CrossRef CAS.
- A. V. Krukau, O. A. Vydrov, A. F. Izmaylov and G. E. Scuseria, Influence of the Exchange Screening Parameter on the Performance of Screened Hybrid Functionals, J. Chem. Phys., 2006, 125, 224106 CrossRef PubMed.
- W. Jia, J. Fu and Z. Cao, et al., Fast plane wave density functional theory molecular dynamics calculations on multi-GPU machines, J. Comput. Phys., 2013, 251, 102–115 CrossRef.
- W. Jia, Z. Cao and L. Wang, et al., The analysis of a plane wave pseudopotential density functional theory code on a GPU machine, Comput. Phys. Commun., 2013, 184, 9–18 CrossRef CAS.
- W. H. J. A. C. Baur, The geometry of polyhedral distortions. Predictive relationships for the phosphate group, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30(5), 1195–1215 CrossRef CAS.
- K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10653g |
| This journal is © The Royal Society of Chemistry 2020 |