Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of nano-octahedral MgO via a solvothermal-solid-decomposition method for the removal of methyl orange from aqueous solutions
Xirui Yan a,
Zixin Tiana,
Wencai Peng
a,
Zixin Tiana,
Wencai Peng *ab,
Jianshu Zhangab,
Yanbin Tonga,
Jun Li*c,
Dekui Sund,
Hui Ged and
Jinli Zhang
*ab,
Jianshu Zhangab,
Yanbin Tonga,
Jun Li*c,
Dekui Sund,
Hui Ged and
Jinli Zhang ae
ae
aSchool of Chemistry and Chemical Engineering, Shihezi University, Shihezi 832003, Xinjiang, China. E-mail: pengwencai@shzu.edu.cn; junli@ipe.ac.cn
bKey Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi, Xinjiang, China
cState Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China
dState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, Shanxi, China
eKey Laboratory for Systems Bioengineering MOE, Tianjin University, Collaborative Innovation Centre of Chemical Science and Chemical Engineering (Tianjin), Tianjin 300072, China
First published on 13th March 2020
Abstract
Nano magnesium oxide has wide applications, and MgO with (111) facets has wider potential applications than MgO with (100) facets (e.g., in catalysis and adsorption). However, nano MgO with (111) polar faces has not been studied throughly, so the preparation of nano-octahedral MgO (N-O-MgO) with eight exposed (111) facets remains a great challenge. Herein, we successfully synthesised N-O-MgO via an effective solvothermal-solid-decomposition method and studied its adsorption performance. The obtained N-O-MgO showed excellent performance (229.36 mg g−1) for methyl orange (MO). The adsorption follows the pseudo-second-order kinetic equation and the Langmuir isotherm model. The dimensionless parameter RL (0.042) and Gibbs free energy ΔG (−6.538 kJ mol−1) revealed that the adsorption of MO on N-O-MgO was a spontaneous and feasible process. The adsorption of MO and methyl blue (MB) on N-O-MgO were studied to determine the adsorption sites. Based on these experiments and analysis, it was determined that the adsorption sites were magnesium ions and the adsorption mechanism was proposed to describe the adsorption process.
1. Introduction
Nano metal oxide materials have attracted numerous researchers due to their unique applications in the fields of energy and environment. As an important nano metal oxide, nano magnesium oxide (nano MgO) shows a wide range of applications, for e.g., as a solid-base catalyst and carrier (for Claisen–Schmidt reaction,1 the preparation of Schiff bases,2 and the loading of nickel atoms3 for CO2 reforming of methane), as an adsorbent (for the removal of organic dyes such as methyl orange,4 heavy metal ions such as cadmium ions and lead ions,5 and fluoride ions6), and as a template and additive (for the preparation of graphene-like materials7 and the additive of CO2 adsorbent8). Nano MgO also shows optical properties9 and bactericidal properties.10 There are some methods used for the synthesis of nano MgO, which include precipitation,11,12 solid state reaction,13 microwave radiation method,14 and sol–gel route,.15 However, in these synthetic procedures,9,10,15 researchers have paid less attention to the crystal facet of nano MgO; MgO usually grows into a cubic structure with (100) facets.16In the past few years, numerous studies have focused on MgO with the (111) polar facet due to its higher reactivity in catalysis17,18 and antibacterial19 applications. However, the reports about nano MgO with (111) polar facets and its properties are rare. Although a large MgO crystal can be mechanically cut and polished to obtain micro- or nano-scale materials, it would be difficult to control the facet that is exposed.20 It is an effective strategy to obtain MgO with the polar (111) facet by wet chemistry methods, which is often necessary to use additives in the reaction system to create a polar environment and to inhibit the rapid disappearance of the polar surface during the reaction. Ryan Richard et al.17 synthesised MgO (111) nanoplates by adding 4-methoxybenzyl alcohol in the reaction system, which has a strong interaction with the inorganic intermediate Mg(OH)–(OCH3). Jupille et al.21 obtained (111) facets via etching of cubic MgO smoke crystals using neutral water in wet conditions, which had an average particle size of 170 nm and a specific surface area of 5.5 m2 g−1. Hao et al.19 obtained MgO with (111) facets exposed via the solution combustion process of the mixture of magnesium nitrate and urea. Xie et al.16,20 prepared micro-scale octahedral MgO with 2.4 m2 g−1 specific surface area by decomposing magnesium nitrate in molten lithium nitrate at 400 °C. Further studies by Jeffrey D. Rimer22 discussed the influence of different factors on the morphology of MgO and demonstrated that the molten salt systems contributed to the formation of (111) facets in the decomposition of magnesium salts. For the molten salt systems, the crystal formed by the free movement of ions must grow to the microscale to reduce surface energy and maintain self-stability. Thus, the synthesis of nano-octahedral MgO with eight (111) facets exposed remains a huge challenge.
In this work, we first successfully prepared nano-scale octahedral MgO with eight (111) facets exposed via an effective solvothermal-solid-decomposition method and evaluated its adsorption performance using MO. The adsorption characteristics and mechanism were studied by a series of experiments and calculations, which included the simulation of different kinetic and isotherm model, and the determination of relevant thermodynamic parameters. Based on these analyses, the adsorption sites were determined and an adsorption mechanism for MO adsorption on N-O-MgO was proposed. These studies should be a useful reference for the preparation of materials and the exploration of adsorption mechanisms as well as for the preparation of nano materials with polar facets, the characteristics of nano materials with polar facet, and the exploration of adsorption mechanisms.
2. Experimental
2.1 Preparation of N-O-MgO
Magnesium nitrate (Mg(NO3)2·6H2O, AR); urea (AR) and ethanol (AR); methyl orange (MO) and methyl blue (MB) were purchased from Aladdin Co., Ltd; Tianjin Chemical Reagent Manufacturing Co., Ltd; Adamas Reagent Co., Ltd, respectively. All of them were used without further purification.N-O-MgO was prepared via a solvothermal-solid-decomposition method. In a typical route, 2.56 g of Mg(NO3)2·6H2O was dissolved in 50 mL of ethanol and the homogeneous solution was transferred into a 100 mL Teflon bottle. Then, the mixture was thoroughly stirred after adding 6.00 g of urea into the solution. Subsequently, the Teflon bottle was placed in a stainless-steel vessel that was sealed and put into a temperature-controlled oven for solvothermal treatment at 200 °C for 24 h. After the heat treatment, fresh precipitates were collected, washed, and dried at 80 °C in air. N-O-MgO was produced by calcining the precipitates at 500 °C and continuing for 5 h.
2.2 Characterisation
The crystal structures of the obtained samples were examined by X-ray diffraction (XRD) on a D8 Advance X-ray diffractometer (Germany) using Cu Kα radiation.The microscopic morphology of MgO was analysed by scanning electron microscopy (SEM) on a Hitachi S-4100 FE-SEM instrument (Japan) at 10 kV and transmission electron microscopy (TEM) on a Tecnai G2 F20 field emission transmission electron microscope (USA) at 200 kV.
N2 adsorption/desorption isotherm was recorded on a Micromeritics ASAP 2020 apparatus (USA) at 77 K for calculating the specific surface area and pore size distribution by the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively.
The vibrational characteristics of the obtained samples were recorded by a Fourier transform spectrometer (FT-IR; Agilent Cary 630, Agilent, America) in pressed KBr pellets.
The X-ray photoelectron spectrum (XPS) of N-O-MgO was obtained on a Kratos AMICUS spectrometer (SHIMADZU, JP) using Al Kα radiation. The binding energy of O element was calibrated relative to the carbon impurity with C 1s at 285 eV.
The concentrations of MO and MB in the aqueous solutions were measured using a Shimadzu UV-2550 UV-visible spectrometer (Japan).
2.3 Adsorption experiments
The batch adsorption experiments were carried out by mixing N-O-MgO particles with 20 mL aqueous MO solution under stirring at pH 6.0 and 303 K temperature at the rate of 150 rpm min−1. The three parameters were changed during the experiments: adsorbent amount (0.5–1.75 g L−1), contact time (5–150 min), and MO concentration (20–200 mg L−1). After the experiments, the mixture was separated and the ultimate concentration of MO was determined.The adsorption capacity of MO on N-O-MgO and the removal ratio of MO were calculated using eqn (1) and (2).
| qe = (C0 − Ce)V/m | (1) |
| η = 100%(C0 − Ce)/C0 | (2) |
In which qe (mg g−1) is the adsorption capacity of MO on N-O-MgO, η (%) represents the MO removal efficiency, C0 (mg L−1) is the MO initial concentration, Ce (mg L−1) is the adsorption equilibrium concentration of MO, V (L) is the MO solution volume, and m (g) is the weight of N-O-MgO particles.
The pseudo-first-order and pseudo-second-order (eqn (3) and (4)) kinetic models were simulated to describe the adsorption process of MO on N-O-MgO.
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t qe − K1t
| (3) |
| t/qt = 1/(K2qe2) + t/qe | (4) |
The adsorption process of MO on the N-O-MgO particles was also simulated based on the Langmuir isotherm and Freundlich isotherm (eqn (5) and (6)). The dimensionless separation factor constant (RL, eqn (7)) was calculated to indicate the type of adsorption.
| qe = qmKLCe/(1 + KLCe) | (5) |
| qe = KFCe1/n | (6) |
| RL = 1/(1 + KLC0) | (7) |
3. Results and discussion
3.1 Characterization of N-O-MgO
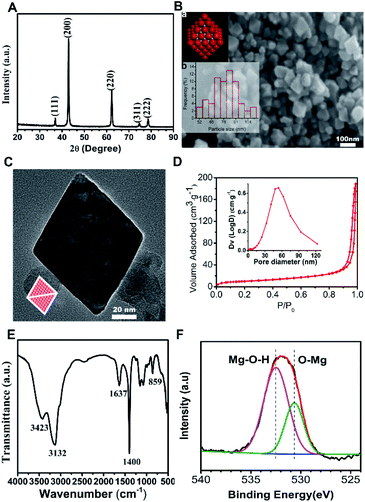
In this ethanol solvent system, urea molecules were a more advantageous ligand than water molecules for magnesium ions, which may inhibit the growth rate of MgO (111) facet and lead to octahedral growth under the decomposition process. Fig. 1(A) shows the XRD pattern of the products, where all the diffraction peaks agreed with the rock-salt structure MgO (JCPDS no. 45-0946), so the products were MgO. As seen in Fig. 1(B), the morphology of the products (MgO) was octahedral with an average particle size of 79.83 nm. This corresponded to the schematic model of rock-salt MgO with eight polar (111) facets exposed (inset a) and the histogram of particle size distribution is shown (inset b). TEM was carried out to characterize the exposed facets of the MgO octahedral. Fig. 1(C) presents the TEM image along the [110] zone axis, in which the octahedron MgO appeared to be a diamond. The diamond model (inset) was drawn by projecting the octahedron along the [110] direction, which was in good agreement with the TEM image. These analyses proved that the as-prepared MgO was nano-octahedral with eight exposed (111) polar facets. The N2 adsorption/desorption isotherm and pore size distribution curve (inset) of N-O-MgO are shown in Fig. 1(D). The N-O-MgO isotherm showed type-IV characteristics with a significant H3 loop. The formation of pores in N-O-MgO was a result of the aggregation of the particles. The BET specific surface area was 36.84 m2 g−1, which was higher than that of micro-scale octahedral MgO (2.4 m2 g−1).16 Fig. 1(E) shows the FT-IR pattern of N-O-MgO. The broad bands located at 3420 cm−1 and 1637 cm−1 correspond to water molecules or hydroxyl groups.23 The peak located at 3132 cm−1 may result from the hydrogen bonds. The peaks at 1400 cm−1 and 859 cm−1 were assigned to magnesium–oxygen (Mg–O) group or magnesium–hydroxyl (Mg–OH) group.24 The FT-IR spectrum revealed abundant hydroxyl groups on N-O-MgO surfaces. The O element of N-O-MgO was investigated by X-ray photoelectron spectroscopy. The O 1s XPS is shown in Fig. 1(F), in which the O 1s spectrum is divided into two peaks. The binding energies of 530 and 532 eV corresponded to the lattice oxygen25 (Mg–O) and magnesium hydroxyl group (Mg–O–H), respectively. The result also indicated abundant hydroxyl groups on N-O-MgO surfaces, which was in accordance with the FT-IR analysis.3.2 Adsorption experiment
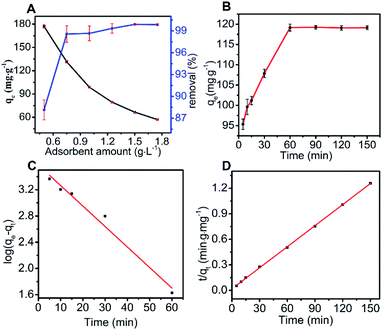
The initial removal percentage was over 81.85% and reached 99.89% when the adsorbent amount was 0.75 g L−1; then, the removal rate increased slowly. This was explained by the increase in adsorption sites for higher N-O-MgO amounts, leading to the increase in removal rate.27
The adsorption kinetic behaviour of MO on N-O-MgO was simulated using pseudo-first-order and pseudo-second-order kinetic models. The fitting results and relevant parameters are shown in Fig. 2(C), (D) and Table 1, respectively. Compared to the simulation by pseudo-first-order model, the calculation results using the pseudo-second-order model were closer to the experimental data and its correlation coefficient R2 was higher than 0.999. This indicated that the adsorption of MO on N-O-MgO can be more adequately described by the pseudo-second-order kinetic model. For this adsorption process, the overall adsorption rate of MO was controlled by the chemical process through ion exchange between MO and N-O-MgO.28 A detailed explanation of the adsorption process is provided in Section 3.3.
| C, mg L−1 | qe,exp, mg g−1 | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|---|
| qe,cal, mg g−1 | K1, g mg−1 min−1 | R2 | qe,cal, mg g−1 | K1, g mg−1 min−1 | R2 | ||
| 100 | 119.42 | 30.62 | 0.0226 | 0.8943 | 121.36 | 0.0037 | 0.9997 |
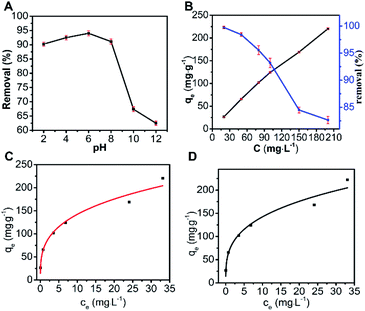
The fitting result and relevant parameters for MO adsorption using the Langmuir and Freundlich isotherm models are presented in Fig. 3(C), (D) and Table 2, respectively. According to the fitting result, the adsorption parameters fitted well with both the Langmuir isotherm (R2 = 0.9520) and Freundlich isotherm (R2 = 0.9594). Here, we used the Langmuir isotherm model to describe the adsorption process for MO. For the Langmuir isotherm model, the adsorption of MO on N-O-MgO was monolayer. The RL value was 0.042 by calculation, implying favourable adsorption of MO on N-O-MgO.
| Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|
| qm, (mg g−1) | KL, (L mg−1) | R2 | RL | KF, (mg g−1) (L mg−1)1/n | n | R2 |
| 229.36 | 0.2280 | 0.9520 | 0.042 | 70.69 | 3.2873 | 0.9594 |
3.3 Adsorption mechanism
① The distance (d, eqn (8)) between two oxygen ions and the area (S2, eqn (9)) of a diamond composed by four adjacent oxygen ions on the surface of N-O-MgO were calculated based on rock-salt contracture.
| 2a2 = (2d)2 | (8) |
| S2 = d2√3/2 | (9) |
② Number of atoms forming a diamond (n, eqn (10)):
| n = 2/6 + 2/3 = 1 | (10) |
③ Calculation of the utilisation ratio (eqn (11)–(13)) of the N-O-MgO adsorption sites.
The amount of outmost layer oxygen ions on the N-O-MgO (111) facet:
| N1 = S1/S2 | (11) |
The number of MO molecules adsorbed on N-O-MgO:
| N2 = (qeAa)/M | (12) |
The utilisation ratio of adsorption sites:
| R = N2/N1 | (13) |
In which a (0.4211 nm) is the cell constant, d (nm) is the distance between two magnesium on the (111) facet, S2 (nm2) is the area of a diamond composed by four adjacent Mg atoms on the (111) facet, S1 (36.84 m2) is the specific surface area of a unit mass of N-O-MgO particles, N1 is the amount of outmost layer O ions on N-O-MgO per unit mass, N2 is the adsorption number of MO molecules on N-O-MgO, R is the utilisation ratio of adsorption sites on N-O-MgO, qe (mg g−1) is the amount of adsorbed MO molecules on the adsorbent surface at equilibrium, Aa (6.022 × 1023 mol−1) is the Avogadro's constant, and M (327.36 g mol−1) is the molar mass of MO.
The calculations indicate that the utilisation ratio of the adsorption sites on N-O-MgO was 91.70%, indicating that MO molecules effectively formed a monolayer on N-O-MgO.
| Dye-SO3Na + N-O-MgO → dye-SO3–Mg–MgO-O-N + NaOH | (14) |
| K = (C0 − Ce)/C0 | (15) |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K
| (16) |
In which C0 (mg L−1), Ce (mg L−1), R (8.314 J mol−1 K−1), and T (303 K) are the initial concentration of MO, equilibrium concentration of MO, gas constant, and absolute temperature, respectively.
ΔG was calculated as −6.538 kJ mol−1, demonstrating that the adsorption process of MO on N-O-MgO is spontaneous and feasible, which is in accordance with the analysis of the Langmuir isotherm model.
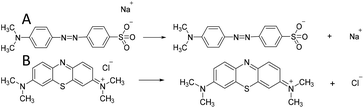
In order to further describe the adsorption process, MO and N-O-MgO after MO adsorption were characterized by FT-IR spectroscopy. Fig. 5(A) shows the FTIR spectra of MO and N-O-MgO after MO adsorption. The FT-IR spectra peaks located at 1605 cm−1, 1120 cm−1, 1692 cm−1 and 1037 cm−1, and 1006 cm−1 were attributed to phenyl groups,30 C–N group,34 and sulfonic acid group (–SO3−),35 and C–H in-plane of benzene rings with bending vibration, respectively. The presence of FT-IR peaks of MO on N-O-MgO after MO adsorption confirmed that the MO molecules were adsorbed on the surface of N-O-MgO. The peaks located at 3699 cm−1 and at 3425 cm−1 were attributed to the stretching of free- and associated-hydroxyl groups, which may be caused by the formation of magnesium hydroxide (Mg(OH)2) and physiosorbed water36 on the surface of N-O-MgO in the adsorption process. Compared with the FT-IR spectra of N-O-MgO shown in Fig. 1(E), the strength of hydroxyl group peaks of N-O-MgO after MO adsorption became weak, indicating that –SO3− groups of MO were substituted with the hydroxyl groups in the MO adsorption process.30,37 The FT-IR spectra of N-O-MgO before and after MO adsorption at 525–950 cm−1 were shown in Fig. 5(B). The peaks of Mg–O–Mg and O–Mg–O (fingerprint area) of N-O-MgO after MO adsorption disappeared or shifted, further confirming that the –SO3− groups of MO were connected with Mg2+ of the N-O-MgO surface.30,38
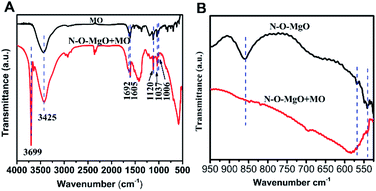 | ||
| Fig. 5 (A) The FT-IR spectra of MO and N-O-MgO after MO adsorption; (B) the FT-IR spectra of N-O-MgO before and after MO adsorption at 525–950 cm−1. | ||
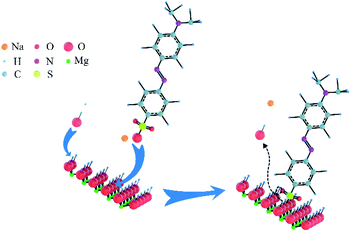
Based on these analyses above, the adsorption mechanism was proposed in Scheme 2. As shown in Scheme 1(A), the exposed –SO3− groups of MO and sodium ions were formed via the ionisation of MO molecules in aqueous solution. For the adsorption process, a coordination hydroxyl group of a magnesium ion broke away from its original position and left a vacancy to form OH− in the aqueous solution; then, a –SO3− group of MO with negative charge exchanged into the vacancy and bonded the magnesium ion by ion exchange and electrostatic interaction. As given in the analysis in Section 3.3.2, the utilisation ratio of adsorption sites on N-O-MgO reached 91.70%, indicating that the exchange of hydroxyls and –SO3− groups of MO were ongoing until the adsorption of MO molecules on N-O-MgO formed a monolayer.
4. Conclusions
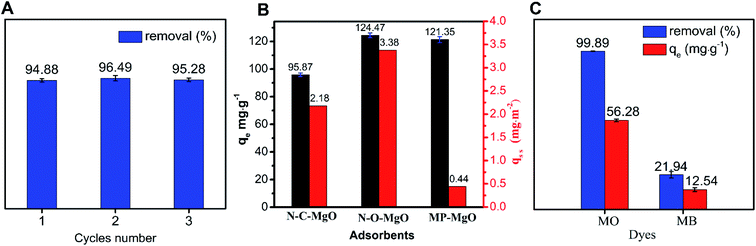
In summary, we have obtained N-O-MgO particles via an effective solvothermal-solid-decomposition method and used it as an adsorbent for MO adsorption to study its adsorption performance. The adsorption process of MO on N-O-MgO in aqueous solution was systematically studied under different experimental conditions (adsorbent amount, contact time, MO concentration, and pH). The results revealed that the pseudo-second-order kinetic model and monolayer adsorption precisely described the spontaneous and feasible adsorption process, which was analysed by the RL and thermodynamic analyses. N-O-MgO had excellent recyclability and higher activity than N-C-MgO and MP-MgO with (100) facets exposed. The adsorption sites were identified as magnesium ions that were effectively utilised on the adsorption process. A possible adsorption mechanism was proposed based on the analysis of experiments, calculation, and characterization. The results of this study may be a reference for the preparation of nanomaterials with polar facets and adsorption mechanism with, for example, different MgO morphologies or even different materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The works were supported by the National Natural Science Foundation of China (NSFC Grant No. 21968029), the Major Science and Technology Project of Xinjiang Bingtuan (2017AA007), the Applied Basic Research Program of Xinjiang Bingtuan (2015AG400) and Science &Technology Research and Achievement Transformation Project of Xinjiang Bingtuan (2015AD027).References
- B. M. Choudary, M. L. Kantam, K. V. Ranganath, K. Mahendar and B. Sreedhar, J. Am. Chem. Soc., 2004, 126, 3396–3397 CrossRef CAS PubMed.
- H. Naeimi, K. Rabiei and Z. Rashid, Curr. Org. Chem., 2016, 20, 316–323 CAS.
- B.-Q. Xu, J.-M. Wei, H.-Y. Wang, K.-Q. Sun and Q.-M. Zhu, Catal. Today, 2001, 68, 217–225 CrossRef CAS.
- M. R. Rezaii Mofrad, G. Mostafaii, R. Nemati, H. Akbari and N. Hakimi, Desalin. Water Treat., 2016, 57, 8330–8335 CrossRef CAS.
- Y. Cai, C. Li, D. Wu, W. Wang, F. Tan, X. Wang, P. K. Wong and X. Qiao, Chem. Eng. J., 2017, 312, 158–166 CrossRef CAS.
- R. R. Devi, I. M. Umlong, P. K. Raul, B. Das, S. Banerjee and L. Singh, J. Exp. Nanosci., 2014, 9, 512–524 CrossRef CAS.
- X. He, H. Zhang, H. Zhang, X. Li, N. Xiao and J. Qiu, J. Mater. Chem. A, 2014, 2, 19633–19640 RSC.
- P. Lan and S. Wu, Chem. Eng. Technol., 2014, 37, 580–586 CrossRef CAS.
- R. Halder and S. Bandyopadhyay, J. Alloys Compd., 2017, 693, 534–542 CrossRef CAS.
- L. Huang, D.-Q. Li, Y.-J. Lin, M. Wei, D. G. Evans and X. Duan, J. Inorg. Biochem., 2005, 99, 986–993 CrossRef CAS PubMed.
- H.-X. Wang, B.-Y. Hu, L.-S. Wang and G.-D. Yang, Chin. J. Inorg. Chem., 2006, 22, 363–366 CAS.
- Y. Jin, X. Pan, R. Dai, Q. Zhang, N. Zhao and J. Zong, Inorg. Chem. Ind., 2014, 46, 33–35 CAS.
- H. Chen, Z. Luo, X. Chen and F. Kang, Micro Nano Lett., 2017, 12, 27–29 CrossRef CAS.
- S. Makhluf, R. Dror, Y. Nitzan, Y. Abramovich, R. Jelinek and A. Gedanken, Adv. Funct. Mater., 2005, 15, 1708–1715 CrossRef CAS.
- A. Kumar and J. Kumar, J. Phys. Chem. Solids, 2008, 69, 2764–2772 CrossRef CAS.
- Z.-Y. Jiang, Q. Kuang, Z.-X. Xie and L.-S. Zheng, Adv. Funct. Mater., 2010, 20, 3634–3645 CrossRef CAS.
- Z. Kake, H. Juncheng, K. Christian and R. Ryan, Angew. Chem., 2006, 118, 7435–7439 CrossRef.
- J. Chen, S. Tian, J. Lu and Y. Xiong, Appl. Catal., A, 2015, 506, 118–125 CrossRef CAS.
- Y.-j. Hao, B. Liu, L.-g. Tian, F.-t. Li, J. Ren, S.-j. Liu, Y. Liu, J. Zhao and X.-j. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 12687–12693 CrossRef CAS PubMed.
- T. Xu, X. Zhou, Z. Jiang, Q. Kuang, Z. Xie and L. Zheng, Cryst. Growth Des., 2009, 9, 192–196 CrossRef CAS.
- R. Hacquart and J. Jupille, Chem. Phys. Lett., 2007, 439, 91–94 CrossRef CAS.
- M. D. Susman, H. N. Pham, A. K. Datye, S. Chinta and J. D. Rimer, Chem. Mater., 2018, 30, 2641–2650 CrossRef CAS.
- Y. Yang, L. Dang, M. J. Shearer, H. Sheng, W. Li, J. Chen, P. Xiao, Y. Zhang, R. J. Hamers and S. Jin, Adv. Energy Mater., 2018, 8, 1703189 CrossRef.
- Y. Zou, X. Wang, F. Wu, S. Yu, Y. Hu, W. Song, Y. Liu, H. Wang, T. Hayat and X. Wang, ACS Sustainable Chem. Eng., 2017, 5, 1173–1185 CrossRef CAS.
- G. Cabailh, R. Lazzari, H. Cruguel, J. Jupille, L. Savio, M. Smerieri, A. Orzelli, L. Vattuone and M. Rocca, J. Phys. Chem. A, 2011, 115, 7161–7168 CrossRef CAS PubMed.
- Y. H. Li, Z. K. Luan, X. Xiao, X. W. Zhou, C. L. Xu, D. H. Wu and B. Q. Wei, Adsorpt. Sci. Technol., 2003, 21, 475–485 CrossRef CAS.
- E. N. E. Qada, S. J. Allen and G. M. Walker, Ind. Eng. Chem. Res., 2006, 45, 6044–6049 CrossRef.
- D. J. Malik, V. Strelko, M. Streat and A. M. Puziy, Water Res., 2002, 36, 1527–1538 CrossRef CAS.
- J. Hu, Z. Song, L. Chen, H. Yang, J. Li and R. Richards, J. Chem. Eng. Data, 2010, 55, 3742–3748 CrossRef CAS.
- W. Yao, S. Yu, J. Wang, Y. Zou, S. Lu, Y. Ai, N. S. Alharbi, A. Alsaedi, T. Hayat and X. Wang, Chem. Eng. J., 2017, 307, 476–486 CrossRef CAS.
- W. Peng, J. Li, B. Chen, W. Ning, G. Luo and W. Fei, Catal. Commun., 2016, 74, 39–42 CrossRef CAS.
- V. K. Lazarov, Z. Cai, K. Yoshida, K. H. Zhang, M. Weinert, K. S. Ziemer and P. J. Hasnip, Phys. Rev. Lett., 2011, 107, 056101 CrossRef PubMed.
- J. Hu, K. Zhu, L. Chen, C. Kübel and R. Richards, J. Phys. Chem. C, 2007, 111, 12038–12044 CrossRef CAS.
- N. Dizge, C. Aydiner, E. Demirbas, M. Kobya and S. Kara, J. Hazard. Mater., 2008, 150, 737–746 CrossRef CAS PubMed.
- Q. Zhou, F. Chen, W. Wu, R. Bu, W. Li and F. Yang, Chem. Eng. J., 2016, 285, 198–206 CrossRef CAS.
- J. Wang, D. Kang, X. Yu, M. Ge and Y. Chen, Chem. Eng. J., 2015, 264, 506–513 CrossRef CAS.
- M. Gao, W. Wang, H. Yang and B.-C. Ye, Chem. Eng. J., 2020, 380, 122459 CrossRef CAS.
- K. El Hassani, B. H. Beakou, D. Kalnina, E. Oukani and A. Anouar, Appl. Clay Sci., 2017, 140, 124–131 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |