Open Access Article
Open Access ArticleStudy on antibacterial properties and cytocompatibility of EPL coated 3D printed PCL/HA composite scaffolds
Lijiao Tian ab,
Zhenting Zhanga,
Bin Tiana,
Xin Zhangb and
Na Wang*a
ab,
Zhenting Zhanga,
Bin Tiana,
Xin Zhangb and
Na Wang*a
aBeijing Stomatological Hospital, School of Stomatology, Capital Medical University, Beijing 100010, PR China. E-mail: nawangkq@yahoo.com
bLiangxiang Hospital of Beijing Fangshan District, Beijing 100010, PR China
First published on 29th January 2020
Abstract
Biomaterial scaffolds play a critical role in bone tissue engineering. Moreover, 3D printing technology has enormous advantage in the manufacture of bioengineering scaffolds for patient-specific bone defect treatments. In order to provide an aseptic environment for bone regeneration, ε-poly-L-lysine (EPL), an antimicrobic cationic polypeptide, was used for surface modification of 3D printed polycaprolactone/hydroxyapatite (PCL/HA) scaffolds which were fabricated by fused deposition modeling (FDM) technology. The scaffold morphology and micro-structure were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) and transform infrared spectroscopy (FT-IR). The release profile surface roughness, open porosity, and mechanical properties of the scaffolds were evaluated. Cell adhesion, proliferation, differentiation potential and antibacterial properties were also examined. As a result, 3D printed PCL/HA scaffolds with interconnected pores showed a slightly rough surface and improved mechanical properties due to adding hydroxyapatite (HA) particles. After being modified by EPL, favorable biocompatibility and osteoconductivity of ε-poly-L-lysine/polycaprolactone/hydroxyapatite (EPL/PCL/HA) scaffolds were observed. Moreover, antibacterial activity of the EPL/PCL/HA scaffolds was apparent. As a consequence, the EPL/PCL/HA scaffolds had great potential for bone regeneration and prevention of infections. This would yield a patient-specific bioactive and antibacterial composite scaffold for advanced bone tissue engineering applications.
1. Introduction
Bone defect is a common disease. Although autologous bone grafting has been the gold standard of bone replacement for many years,1 its complications, like secondary injury of donor site as well as early and late infection in the post-surgery period, always bring patients more pain and cause the failure of surgery.2–4 Bone tissue engineering provides a new route for the therapy of bone defects. The scaffolds for bone tissue engineering must have pores interconnected in three dimensions, with highly regular pore formation and structure. The porous structure provides space for cell migration, adhesion, and the ingrowth of new bone tissue. Scaffolds for bone tissue engineering should have reasonable strength and bioactivity, without causing any adverse effects.5,6Rapid prototyping (RP) is one of the most promising techniques for designing and producing three-dimensional (3D) porous scaffolds for cell ingrowth.7 Compared with conventional polymer processing techniques, such as salt leaching, gas foaming, solvent casting and phase separation, RP can be easily used to fabricate scaffolds with controllable and reproducible porosity,8,9 offering patients with the exact dimension of individual artificial bone substitute. Fused deposition modeling (FDM), a rapid prototyping technology, is investigated and successfully applied in many research studies to produce novel 3D scaffolds with fully interconnected channel networks, and highly controllable porosity and channel size.10
Polycaprolactone (PCL), bioresorbable aliphatic polyester, is a suitable material for cell attachment and proliferation, its degradation by-products are nontoxic and are usually metabolized and eliminated via natural pathways.11,12 As thermoplastic polymers, PCL can easily be processed into three-dimensional (3D) scaffolds with desired geometry and controlled porosity with interconnectivity using modern computer-based solid free-form fabrication methods.13 However, when used in bone tissue engineering, PCL lacks bioactivity, and the hydrolysis of polyesters exposes carboxylic acid moieties to the local biological environment, known to have caused bone resorption in the past.14,15
Materials for bone defect replacement and repairing process should satisfy characteristics of mechanical capacity for bone-bearing, good biocompatibility, osteoconductivity and osteoinductivity. Hydroxyapatite (HA), the main component of natural bone matrix, has drawn wide attention due to its osteoconductive and osteoinductive properties, bone bonding abilities, and slow degradation in situ.16 However, HA has very low tensile strength and is brittle.17 Therefore, the application of polymeric scaffolds and ceramic particles has shown promising improvements, extending their application as bone patch materials.18 The combination of HA improves stiffness, and osteogenic potential in PCL-based scaffolds for bone tissue engineering applications.19 Moreover, the disadvantages of long absorbable period and acidogenic ability, could be compensated by synchronizing their degradation with that of tri-calcium-phosphate (TCP) and alkaline HA.20
At present, there are few researches about the antibacterial properties of PCL/HA scaffolds for bone tissue engineering. ε-Poly-L-lysine (EPL) is naturally occurring homo-polyamide made of 25–35 L-lysine with antimicrobial activities.21,22 EPL has been used in many novel applications in the fields of food, medicine, environment, agriculture, and electronics because it is biodegradable, water-soluble, thermostable, non-toxic.23–25 It has a wide antimicrobial spectrum.26,27 Microorganisms such as bacteria and fungi do not easily develop resistance to this polypeptide.28
Although, the preparation and properties of PCL/HA scaffolds have been studied in recent years, there is no report about the improvement of biological and antibacterial properties of 3D printed PCL/HA scaffolds modificated by ε-poly-L-lysine (EPL). The goal of the present study was to produce 3D printed PCL/HA composite scaffolds by the FDM technique and to use EPL to modify the surface of PCL/HA scaffolds, as well to characterize and study the properties of the ε-poly-lysine/polycaprolactone/hydroxyapatite (EPL/HA/PCL) composite scaffolds, which have not been reported in previous studies. Furthermore, the biocompatibility and osteoconductivity of the new composite scaffolds were examined with an osteoblast cell line in vitro. Antibacterial annulus was used to observe its antibacterial activity against the Staphylococcus aureus, Escherichia coli and Streptococcus mutans.
2. Materials and methods
2.1 Fabrication of 3D scaffolds
3D printed polycaprolactone (PCL) and polycaprolactone/hydroxyapatite (PCL/HA) scaffolds (Jiangyin Recongene Biomedical Technologies, Ltd Inc., Jiangyin City, China). The PCL, PCL/HA scaffolds were prepared by the FDM technique. The powders of PCL (Shenzhen Esun Industrial Co., Ltd) and HA (Kunshan Overseas Chinese New Material Co., Ltd) particles with sizes ranging from approximately 1 to 20 μm, were mixed with the mass ratio as 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in high temperature, then processed as raw materials to produce PCL/HA with the fused deposition modeling (FDM) method. The size of grainy raw materials was less than 2.0 × 2.0 × 2.0 mm3 by cutting. Scaffolds were fabricated using the FDM 3D modeler system independently developed by manufacturer, with the nozzle diameter of 300 μm. The melting temperature of upper equipment was set at 75 °C and the melting temperature of nozzle tip was maintained at 110 °C during the fabrication process. Aseptic package and cobalt-60 sterilization processing were necessary after the preparation of scaffolds.
3 in high temperature, then processed as raw materials to produce PCL/HA with the fused deposition modeling (FDM) method. The size of grainy raw materials was less than 2.0 × 2.0 × 2.0 mm3 by cutting. Scaffolds were fabricated using the FDM 3D modeler system independently developed by manufacturer, with the nozzle diameter of 300 μm. The melting temperature of upper equipment was set at 75 °C and the melting temperature of nozzle tip was maintained at 110 °C during the fabrication process. Aseptic package and cobalt-60 sterilization processing were necessary after the preparation of scaffolds.
2.2 Characterization of scaffolds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 at different desorption temperatures.
000 cm−1 at different desorption temperatures.2.3 Biological compatibility and osteoconductivity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio) and then added to each sample. After 4 h incubation, the CCK-8 suspension was extracted and moved to 96-well plates. The optical density (OD) of the extracted suspension was measured at 450 nm using a microplate reader (SpectraMax Paradigm, Austria).
10 ratio) and then added to each sample. After 4 h incubation, the CCK-8 suspension was extracted and moved to 96-well plates. The optical density (OD) of the extracted suspension was measured at 450 nm using a microplate reader (SpectraMax Paradigm, Austria).2.4 Antibacterial activity
Antibacterial activity was determined by the zone of inhibition test using S. aureus (Gram-positive bacteria), E. coli (Gram-negative bacteria) and S. mutans (oral facultative anaerobic bacteria). A microorganism suspension was prepared in 0.9% NaCl in distilled water. Adding microorganism suspension to the prepared agar medium before it solidified. The density of microorganisms in suspension was approximately 106 colony-forming units (CFU) per ml for S. aureus, 105 CFU ml−1 for E. coli, 104 CFU ml−1 for S. mutans. 13 ml of the agar medium mixed with bacteria was poured into each Petri plate uniformly, then the scaffolds were put into the agar plates. The plates were dried and incubated for 24 h at 37 °C. In the end pictures of all plates were taken using a digital camera.The killing rate was examined by the viable cell counting technique against E. coli. The scaffolds with 15 mm in diameter and 2 mm in height (n = 5 for each group) were immersed in sterilized fluid nutrient medium (5 ml) in test tubes, and 10.0 μl bacterial suspension was inoculated, and cultured at 37 °C. After 24 h, the scaffolds were removed and put into new fluid nutrient medium, then applied consecutively by the ultrasonic shaking. The surviving bacteria on scaffolds were counted by the spread plate method. After inoculation, the plates were kept at 37 °C and the colonies were counted after 24 h. Efficacy against E. coli of the scaffolds was calculated by the following equation: R = (A − B)/A×100%, where A is the number of colonies in the PCL/HA group, and B is the number of colonies in the EPL/PCL/HA group (CFU ml−1).
2.5 Statistical analysis
The experiment data have been expressed as the mean ± standard deviation (S.D.). Statistical significance was figured out by means of ANOVA and Student's t test analyses using software spss22.0. The value of P < 0.05 was considered statistically significant.3. Results and discussion
3.1. Characterization of scaffolds
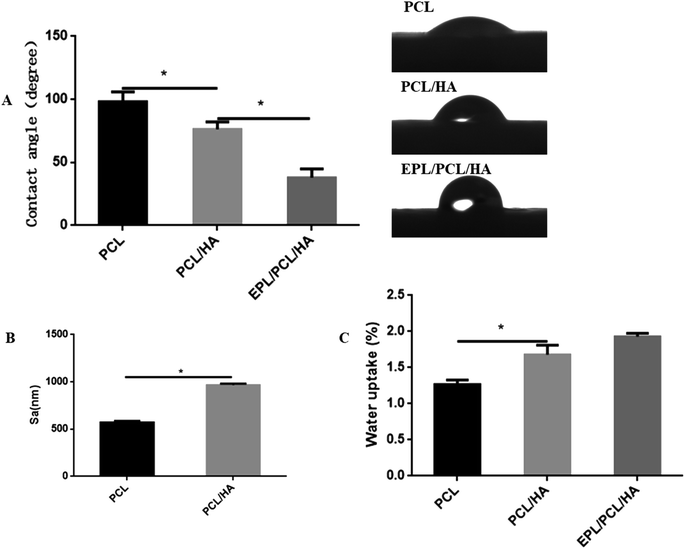 | ||
| Fig. 3 Contact angle (A), surface roughness (B) and water uptake (C) of PCL, PCL/HA and EPL/PCL/HA scaffolds. Data were presented as mean ± standard deviation. (*p < 0.05). | ||
The static contact angles of PCL, PCL/HA and EPL/PCL/HA scaffolds were showed in Fig. 3A. The static contact angle of the PCL and PCL/HA scaffolds were 98.08 ± 3.580° and 76.26 ± 2.635° respectively. When PCL/HA scaffolds surface coated by EPL, the contact angle decreased to 37.90 ± 3.087°. Compared to PCL scaffolds, the contact angle of the PCL/HA scaffolds decreased significantly due to the adding of HA particles, while the contact angle of the EPL/PCL/HA scaffolds surface markedly decreased due to EPL coating. A smaller contact angle means a more hydrophilic surface of a material.45 The results indicated that the hydrophilicity of EPL/PCL/HA scaffolds improved obviously. Fig. 3C showed the differences in water-uptake ability among PCL, PCL/HA and EPL/PCL/HA scaffolds. By adding the HA into PCL scaffolds, the water-uptake ability slightly increased. Furthermore, after EPL was immobilized on the surface of the PCL/HA scaffolds, the water uptake ability of the scaffolds did not significantly increase compared to the PCL/HA scaffolds.
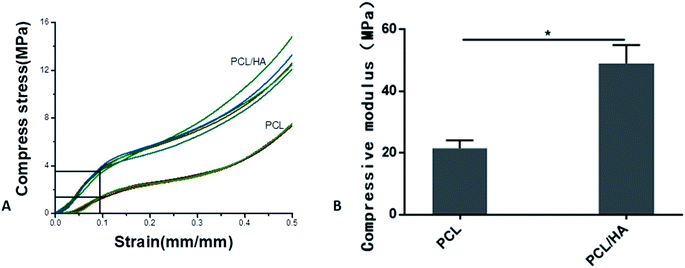
The open porosity of the scaffolds was estimated using liquid displacement method, porosities of the PCL and PCL/HA scaffolds were about 60.25% and 49.05%, respectively, which in the range of the porosity of natural cancellous bone (50–90%).46
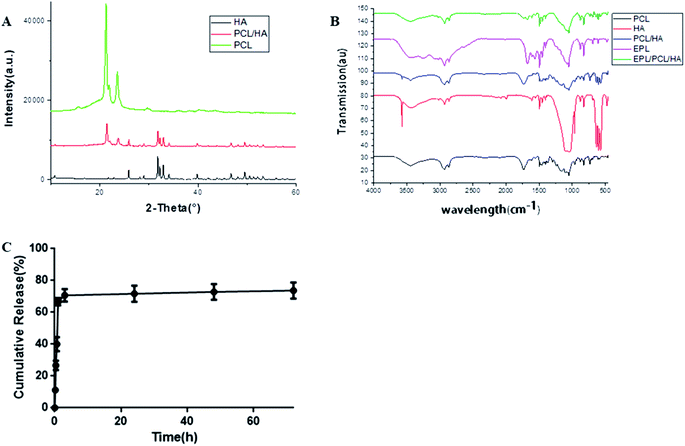 | ||
| Fig. 4 (A) XRD spectra of HA, PCL and PCL/HA; (B) FT-IR analysis. (C) Release kinetics of EPL from EPL/PCL/HA scaffolds. | ||
The Fourier transform infrared spectroscopy (FTIR) spectra of the EPL/PCL/HA scaffolds clearly showed chemical bands related to pure PCL, HA and EPL (Fig. 4B). There were no significant changes in the band structures when PCL and HA were mixed, suggesting that there was no chemical reaction between PCL and HA in the PCL/HA scaffolds. EPL peaks can be observed in EPL/PCL/HA peaks, which indicated that the EPL existed on the coated surface.
3.2 Biological compatibility and osteoconductivity
The proliferation of MC3T3-E1 cells cultured on PCL, PCL/HA and EPL/PCL/HA scaffolds were assessed using the CCK-8 assay (Fig. 6). The optical density (OD) value of the CCK-8 solution was measured at 1, 3, and 5 days after the cells were seeded. The OD values of the EPL/PCL/HA scaffolds were significantly higher than that of the PCL and PCL/HA scaffolds, and the OD values of PCL/HA scaffolds were much higher than that of pure PCL scaffolds, which indicated that cell proliferation on the EPL/PCL/HA scaffolds was superior to that on the PCL and PCL/HA scaffolds.
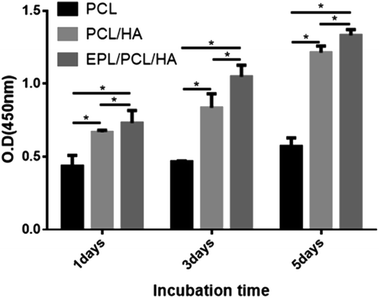 | ||
| Fig. 6 CCK-8 assay of MC3T3-E1 cells on the scaffolds. Asterisks denote significant differences (*p < 0.05). | ||
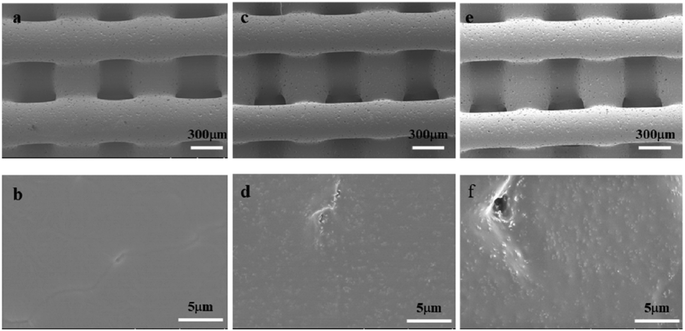
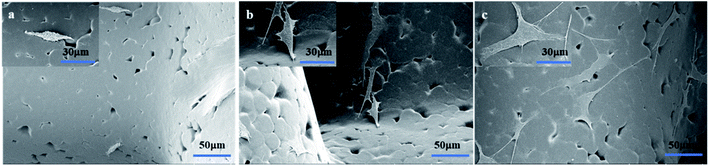
It has been demonstrated that moderately rough surfaces frequently favor the attachment and proliferation of several different cell types compared to smooth surface.43 Fiber surface roughness can increase the membrane surface area and improve membrane hydrophilicity.47 In our study, high magnification SEM images (Fig. 1b, d and f) showed that the fibers of PCL/HA and EPL/PCL/HA scaffolds had a moderately rough surface, while the fibers of PCL scaffolds had a smooth surface. Furthermore, the fiber surface roughness of scaffolds was detected by using a 3D surface profiler based on scanning white light interferometry (Fig. 3B), demonstrating the incorporation of HA particles into the polymer resulted in scaffolds with a more heterogeneous morphology and structure. After seeded on scaffolds, MC3T3-E1 cells with longer pseudopodia and processus spread better on PCL/HA and EPL/PCL/HA scaffolds than that on PCL scaffolds (Fig. 5), which in turn demonstrated that moderately rough surfaces of PCL/HA and EPL/PCL/HA scaffolds promoted more cells attachment and proliferation.
Many researchers stated that more hydrophilic than hydrophobic surfaces were better for cell attachment, cell spreading, and cell proliferation.48 In addition, the hydrophilicity of the material aids in the absorption of fibronectin, which is essential for osteoblast adhesion in vitro.49 Compared to PCL and PCL/HA scaffolds, the static contact angle of the EPL/PCL/HA scaffolds decreased significantly (Fig. 3A). HA can improve the hydrophilicity of polymer material by offering the free –OH group.50 EPL is a hydrophilic polypeptide, and the amidogens of EPL bound with proteoglycans on the cells' surface facilitate the adhesion effect for cells.51,52 Therefore, the hydrophilicity of EPL/PCL/HA scaffolds was significantly improved by HA and EPL, which positively affected the behavior of MC3T3-E1 cells. Hence, in our study, results showed EPL/PCL/HA scaffolds were most favourable for cell adhesion and proliferation in three groups.
Lysine residues of EPL coated on the surface of the material with a positive charge could promote the cells adhesion with a negative charge.53 As a result, after modified by EPL the scaffolds were much more suitable for cells adhesion and proliferation. As shown in photographs (Fig. 5), it was obvious that cytoplasm of MC3T3-E1 cells extended more pseudopodia of filament or sheet shape, and there were more cell–cell interactions inside the pores on EPL/PCL/HA scaffolds than those on PCL/HA scaffolds. What's more, the results of CCK-8 test showed that there was most cells proliferation on EPL/PCL/HA scaffolds.
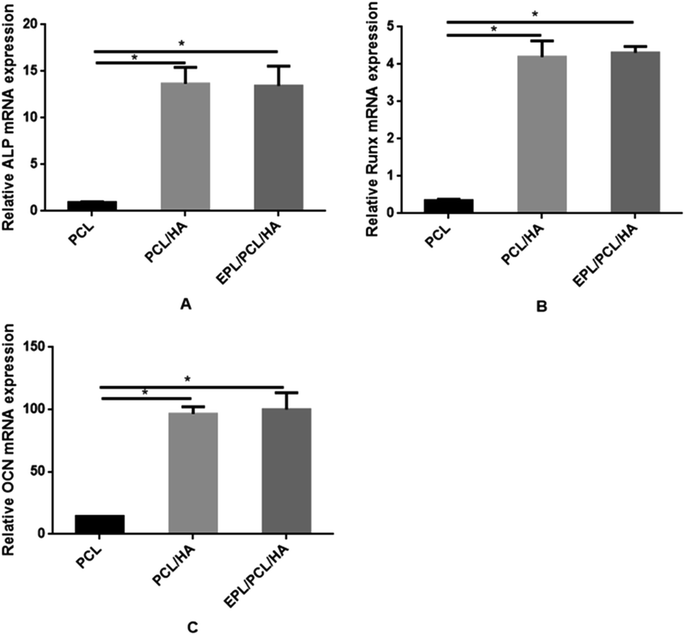 | ||
| Fig. 9 Quantitative analyses of osteogenesis-related gene expressions (i.e., ALP (A), Runx2 (B) and OCN (C)). The data were represented as mean ± standard deviation (n = 3; *p < 0.05). | ||
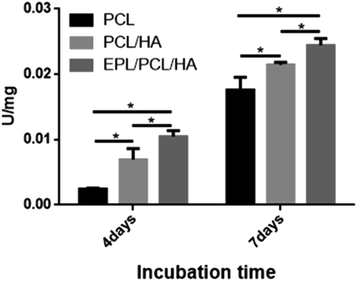
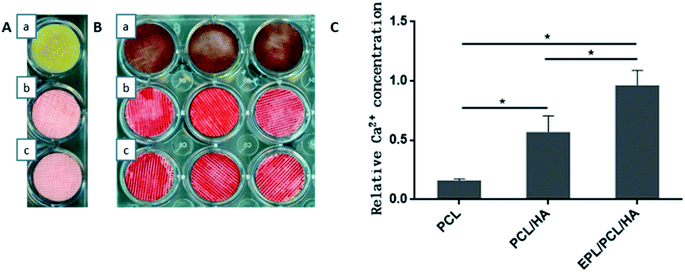
No bone formation was observed in Runx2 null mice,56 suggesting that Runx2 is a key transcription factor for osteoblast differentiation.57 ALP is considered to play an important role in the process of mineral formation in tissues like bone, cartilage tooth root cementum and dentin.58 Osteocalcin (OCN) is expressed during the postproliferative period and reaches its maximum expression during mineralization and accumulates in the mineralized bone.59 The conclusion can be drawn that the addition of the HA particles in this study induced the high expression of ALP, Runx2 and OCN in MC3T3-E1 cells cultured on EPL/PCL/HA and PCL/HA scaffolds, and in turn promoted the process of osteoblast differentiation and bone formation. The ability of HA in composite materials to stimulate osteogenesis had been reported in several studies.60,61 Research suggests that HA powder particle size can contribute to modulation of osteoblast gene expression.62 Besides, the moderately rough surface of scaffolds may promote the osteogenic differentiation of MC3T3-E1. Studies show that moderately rough surface of materials have the potential to promote expression of the osteogenic and osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells (hMSCs).63 The results had proved that EPL/PCL/HA and PCL/HA scaffolds had moderately rough surface which also could cause the high osteogenic differentiation and more mineralization. On the other hand, our results showed that EPL/PCL/HA scaffolds had higher ALP activity (Fig. 7) and more mineralization in Alizarin Red staining and quantitative analysis (Fig. 8) than PCL/HA scaffolds, while there was no significant difference between the expressions of the osteogenesis-related genes of the EPL/PCL/HA scaffolds and that of the PCL/HA scaffolds. This may result from more MC3T3-E1 cells can adhere and proliferate on EPL/PCL/HA scaffolds due to EPL modification.
3.3 Antibacterial activity
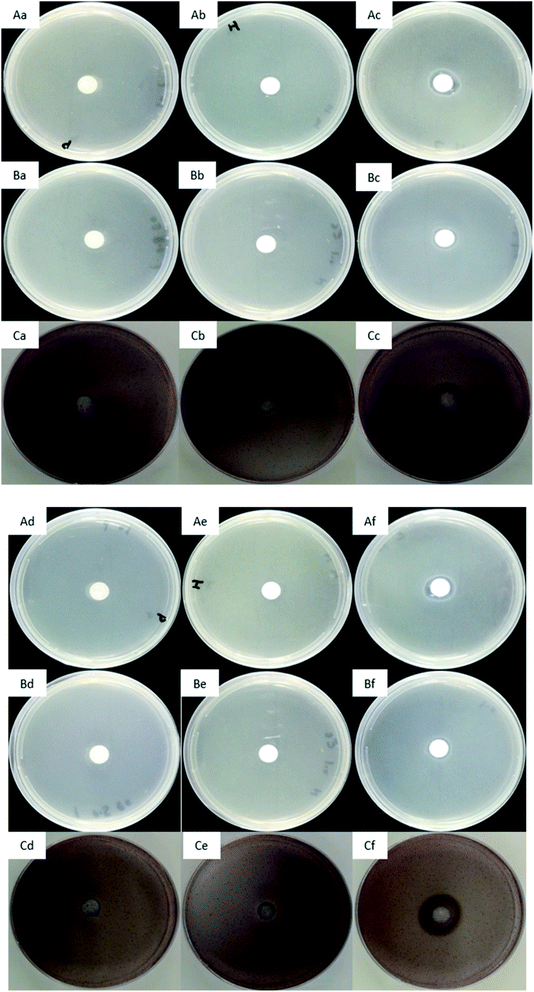
Images of agar plates antimicrobial assay results were showed in Fig. 10. After 24 hours incubation, a transparent zone was observed around the EPL/PCL/HA scaffolds (Fig. 10(Ac, Bc and Cc)), in contrast to the opaque parts over the zone where bacteria were alive. While there was no transparent inhibition zone around the PCL and PCL/HA scaffolds (Fig. 10(Aa, Ab, Ba, Bb, Ca and Cb)). The antibacterial effect did not obviously change after subsequent culture for 3 days (Fig. 10(Af, Bf and Cf)). It turned out to be that the EPL/PCL/HA scaffolds composite exhibited an excellent broad-spectrum antibacterial activities against S. aureus, E. coli and S. mutans in vitro, and the antimicrobial activity of EPL/PCL/HA scaffolds was retained for an extended time period. When PCL and PCL/HA scaffolds were taken as control samples, antibacterial rate of EPL/PCL/HA scaffolds against E. coli was 92.42%. The antimicrobial nature of any implanted graft material aids in the persistence of the implant by preventing microbial attacks associated with surgeries. In our case, we used EPL to modify the surface of PCL/HA scaffold, to obtain a bone tissue engineering scaffold with antibacterial property. ε-Poly-L-lysine (EPL) is a linear L-lysine homopolymer biosynthesized by actinomyces, and it has a unique structure linking ε-amino and α-carboxylic acid functional groups,64 known to have a broad-spectrum antibacterial activity against Gram-positive and Gram-negative bacteria.65 The mechanism of inhibited microbial growth was studied and concluded that the electrostatic adsorption of EPL to the cell surface followed by the stripping of the outer membrane and abnormal distribution of the cytoplasm ultimately led to physiological damage of the EPL treated microbes.26 Marian Fürsatz et al., evaluated the biopolymer of EPL functionalized bacterial cellulose (BC) in vitro and had demonstrated that polymer exhibited efficient contact inhibition of S. epidermidis and did not affect the cytocompatibility to cultured human fibroblasts.66 In our study, FITR exam proved the existence of EPL in composite scaffolds and no chemical reaction produced among EPL, HA and PCL, illustrating that chemical property of EPL did not change. In summary, EPL is a promising, green and versatile modified material to improve the performance of 3D-printed PCL/HA scaffolds for the earlier period of bone formation.4. Conclusions
In this study, 3D printed PCL/HA scaffold has been modified using ε-poly-L-lysine (EPL) to build a new EPL/HA/PCL composite scaffold for infection prevention and better bone formation in patient-specific bone defect treatments. The created scaffolds were found to be cytocompatible as well as capable of osteogenic differentiation and antimicrobial activity in vitro, which is beneficial not only to bone regeneration, but to reduce or prevent the incidence of the infective complications in reparative bone formation. Further investigations are needed to determine if the present scaffolds can support functional tissue regeneration in vivo.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (81171004 and 81300916).References
- C. Myeroff and M. Archdeacon, J. Bone Jt. Surg., Am. Vol., 2011, 93, 2227–2236 CrossRef PubMed.
- C. Lakhiani, M. V. DeFazio, K. Han, R. Falola and K. Evans, J. Reconstr. Microsurg., 2016, 32, 342–357 CrossRef PubMed.
- P. Li, Q. Fang, J. Qi, R. Luo and C. Sun, J. Oral Maxillofac. Surg., 2015, 73, 1637–1640 CrossRef PubMed.
- J. M. Toto, E. I. Chang, R. Agag, K. Devarajan, S. A. Patel and N. S. Topham, Head & Neck, 2015, 37, 1660–1664 CrossRef PubMed.
- S. J. Heo, S. E. Kim, J. Wei, Y. T. Hyun, H. S. Yun, D. H. Kim, J. W. Shin and J. W. Shin, J. Biomed. Mater. Res., Part A, 2009, 89, 108–116 Search PubMed.
- X. Y. Zhang, G. Fang and J. Zhou, Materials, 2017, 10, 50 CrossRef PubMed.
- J. M. Sobral, S. G. Caridade, R. A. Sousa, J. F. Mano and R. L. Reis, Acta Biomater., 2011, 7, 1009–1018 CrossRef CAS PubMed.
- J. Y. Lee, B. Choi, B. Wu and M. Lee, Biofabrication, 2013, 5, 045003 CrossRef PubMed.
- S. A. Park, S. H. Lee and W. D. Kim, Bioprocess Biosyst. Eng., 2011, 34, 505–513 CrossRef CAS PubMed.
- M. Nikzad, S. H. Masood and I. Sbarski, Mater. Des., 2011, 32, 3448–3456 CrossRef CAS.
- C. X. Lam, D. W. Hutmacher, J. T. Schantz, M. A. Woodruff and S. H. Teoh, J. Biomed. Mater. Res., Part A, 2009, 90, 906–919 CrossRef PubMed.
- M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS.
- S. Eshraghi and S. Das, Acta Biomater., 2010, 6, 2467–2476 CrossRef CAS PubMed.
- G. L. Siparsky, K. J. Voorhees and F. D. Miao, J. Environ. Polym. Degrad., 1998, 6, 31–41 CrossRef CAS.
- O. M. Bostman, J. Bone Jt. Surg. Br. Vol., 1991, 73, 679–682 CrossRef CAS.
- H. Zhou and J. Lee, Acta Biomater., 2011, 7, 2769–2781 CrossRef CAS PubMed.
- A. Azari, S. Nikzad, A. Yazdani, F. Atri and A. F. Anvari-Yazdi, J. Mater. Sci.: Mater. Med., 2017, 28, 111 CrossRef PubMed.
- M. Okamoto and B. John, Prog. Polym. Sci., 2013, 38, 1487–1503 CrossRef CAS.
- N. Koupaei and A. Karkhaneh, J. Tissue Eng. Regener. Med., 2016, 13, 251–260 CrossRef CAS PubMed.
- V. Uskokovic and D. P. Uskokovic, J. Biomed. Mater. Res., Part B, 2011, 96, 152–191 CrossRef PubMed.
- S. Shima and H. Sakai, Agric. Biol. Chem., 2014, 45, 2497–2502 Search PubMed.
- S. Shima and H. Sakai, Agric. Biol. Chem., 2014, 45, 2503–2508 Search PubMed.
- A. H. Chheda and M. R. Vernekar, Int. Food Res. J., 2015, 22, 23–30 CAS.
- M. Laura, M. Núria, F. Vicente, d. l. T. Cristina, A. Alessandro, M.-M. e. Ramón, S. Félix, A. Pedro, P.-P. Enrique and O. Mar, Chemistry, 2014, 20, 5271–5281 CrossRef PubMed.
- H. Mandal, S. S. Katiyar, R. Swami, V. Kushwah, P. B. Katare, A. M. Kumar, S. K. Banerjee, A. Popat and S. Jain, Int. J. Pharm., 2018, 542, 142–152 CrossRef CAS PubMed.
- S. Shima, J. Antibiot., 1984, 37, 1449–1455 CrossRef CAS PubMed.
- A. K. Pandey and A. Kumar, Process Biochem., 2014, 49, 496–505 CrossRef CAS.
- H. Gao and S.-Z. Luo, RSC Adv., 2016, 6, 58521–58528 RSC.
- M. E. Hoque, D. W. Hutmacher, W. Feng, S. Li, M. H. Huang, M. Vert and Y. S. Wong, J. Biomater. Sci., Polym. Ed., 2005, 16, 1595–1610 CrossRef CAS PubMed.
- D. W. Hutmacher, T. Schantz, I. Zein, K. W. Ng, S. H. Teoh and K. C. Tan, J. Biomed. Mater. Res., 2001, 55, 203–216 CrossRef CAS.
- J. Venkatesan, Z.-J. Qian, B. Ryu, N. Ashok Kumar and S.-K. Kim, Carbohydr. Polym., 2011, 83, 569–577 CrossRef CAS.
- D. D. Liu, J. C. Zhang, C. Q. Yi and M. S. Yang, Sci. Bull., 2010, 55, 1013–1019 CrossRef.
- I. H. Pereira, E. Ayres, L. Averous, G. Schlatter, A. Hebraud, A. C. de Paula, P. H. Viana, A. M. Goes and R. L. Oréfice, J. Mater. Sci.: Mater. Med., 2014, 25, 1137–1148 CrossRef CAS PubMed.
- Q. Yao, B. Wei, Y. Guo, C. Jin, X. Du, C. Yan, J. Yan, W. Hu, Y. Xu, Z. Zhou, Y. Wang and L. Wang, J. Mater. Sci.: Mater. Med., 2015, 26, 5360 Search PubMed.
- J. Lee, M. J. Cuddihy and N. A. Kotov, Tissue Eng., Part B, 2008, 14, 61 CrossRef CAS PubMed.
- H. Seitz, W. Rieder, S. Irsen, B. Leukers and C. Tille, J. Biomed. Mater. Res., Part B, 2005, 74, 782–788 CrossRef PubMed.
- C. M. Murphy, M. G. Haugh and F. J. O'Brien, Biomaterials, 2010, 31, 461–466 CrossRef CAS PubMed.
- I. Martin, R. F. Padera, G. Vunjaknovakovic and L. E. Freed, J. Orthop. Res., 1998, 16, 181 CrossRef CAS PubMed.
- S. Nguyen and R. H. Marchessault, J. Biomed. Mater. Res., Part B, 2006, 77, 5 CrossRef PubMed.
- G. V. Salmoria, E. A. Fancello, C. R. M. Roesler and F. Dabbas, Int. J. Adv. Manuf. Technol., 2013, 65, 1529–1534 CrossRef.
- O. Y. Alothman, F. N. Almajhdi and H. Fouad, Biomed. Eng. Online, 2013, 12, 95 CrossRef PubMed.
- T. Almela, I. M. Brook, K. Khoshroo, M. Rasoulianboroujeni, F. Fahimipour, M. Tahriri, E. Dashtimoghadam, A. El-Awa, L. Tayebi and K. Moharamzadeh, Bioprinting, 2017, 4, 1 CrossRef.
- J. H. Shim, J. B. Huh, Y. P. Ju, Y. C. Jeon, S. S. Kang, J. Y. Kim, J. W. Rhie and D. W. Cho, Tissue Eng., Part A, 2013, 19, 317–328 CrossRef CAS PubMed.
- A. Itälä, H. O. Ylänen, J. Yrjans, T. Heino, T. Hentunen, M. Hupa and H. T. Aro, J. Biomed. Mater. Res., 2002, 62, 404–411 CrossRef PubMed.
- Y. Yang, X. Ding, T. Zou, G. Peng, H. Liu and Y. Fan, RSC Adv., 2017, 7, 7954–7963 RSC.
- L. A. Di, A. Longoni, G. Criscenti, C. Mota, B. C. Van and L. Moroni, Biofabrication, 2016, 8, 045007 CrossRef PubMed.
- Q. Liu, Y. F. Song, B. Grottkau, M. X. Ma, C. Ye and B. Guo, BioMed Res. Int., 2017, 2017, 1–12 Search PubMed.
- J. Wei, M. Yoshinari, S. Takemoto, M. Hattori, E. Kawada, B. Liu and Y. Oda, J. Biomed. Mater. Res., Part B, 2007, 81, 66–75 CrossRef PubMed.
- M. Yang, S. Zhu, Y. Chen, Z. Chang, G. Chen, Y. Gong, N. Zhao and X. Zhang, Biomaterials, 2004, 25, 1365–1373 CrossRef CAS PubMed.
- S. Dhivya, S. Saravanan, T. P. Sastry and N. Selvamurugan, J. Nanobiotechnol., 2015, 13, 40 CrossRef CAS PubMed.
- L. Qian and W. M. Saltzman, Biomaterials, 2004, 25, 1331–1337 CrossRef CAS PubMed.
- Y. E. Qing, K. Chen, W. U. Huang, H. E. Yunsong, M. Nong, L. I. Chunxiang and T. Liang, Exp. Ther. Med., 2015, 9, 25–32 CrossRef PubMed.
- C. R. Wittmer, J. A. Phelps, W. M. Saltzman and P. R. Van Tassel, Biomaterials, 2007, 28, 851–860 CrossRef CAS PubMed.
- Y. F. Chou, J. C. Dunn and B. M. Wu, J. Biomed. Mater. Res., Part B, 2005, 75, 81–90 CrossRef PubMed.
- S. Kwak, A. Haider, K. C. Gupta, S. Kim and I. K. Kang, Nanoscale Res. Lett., 2016, 11, 323 CrossRef PubMed.
- T. Komori, H. Yagi, S. Nomura, A. Yamaguchi, K. Sasaki, K. Deguchi, Y. Shimizu, R. T. Bronson, Y. H. Gao and M. Inada, Cell, 1997, 89, 755–764 CrossRef CAS.
- Z. Maruyama, C. A. Yoshida, T. Furuichi, N. Amizuka, M. Ito, R. Fukuyama, T. Miyazaki, H. Kitaura, K. Nakamura and T. Fujita, Dev. Dyn., 2010, 236, 1876–1890 CrossRef.
- G. Harrison, I. M. Shapiro and E. E. Golub, J. Bone Miner. Res., 2010, 10, 568–573 CrossRef PubMed.
- D. Liu, J. Zhang, Y. Li, S. Wang and M. Yang, Biol. Trace Elem. Res., 2012, 149, 291–297 CrossRef CAS.
- M. E. Frohbergh, A. Katsman, M. J. Mondrinos, C. T. Stabler, K. D. Hankenson, J. T. Oristaglio and P. I. Lelkes, Tissue Eng., Part A, 2015, 21, 970 CrossRef CAS PubMed.
- X. Liu, L. A. Smith, J. Hu and P. X. Ma, Biomaterials, 2009, 30, 2252–2258 CrossRef CAS PubMed.
- J. Xie, M. J. Baumann and L. R. McCabe, J. Biomed. Mater. Res., Part A, 2004, 71, 108–117 CrossRef PubMed.
- I. Wall, N. Donos, K. Carlqvist, F. Jones and P. Brett, Bone, 2009, 45, 17–26 CrossRef CAS PubMed.
- T. Bo, P. P. Han, Q. Z. Su, P. Fu, F. Z. Guo, Z. X. Zheng, Z. L. Tan, C. Zhong and S. R. Jia, Food Control, 2015, 7135 Search PubMed.
- R. Ye, H. Xu, C. Wan, S. Peng, L. Wang, H. Xu, Z. P. Aguilar, Y. Xiong, Z. Zeng and H. Wei, Biochem. Biophys. Res. Commun., 2013, 439, 148–153 CrossRef CAS PubMed.
- M. Fürsatz, M. Skog, P. Sivler, E. Palm, C. Aronsson, A. Skallberg, G. Greczynski, H. Khalaf, T. Bengtsson and D. Aili, Biomed. Mater., 2018, 13, 025014 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |