Open Access Article
Open Access ArticleAntidiabetic effect of two different Ganoderma species tested in alloxan diabetic rats†
Milena Rašeta *a,
Mira Popovića,
Ivan Čapob,
Nebojša Stilinovićc,
Saša Vukmirovićc,
Biljana Miloševića and
Maja Karamand
*a,
Mira Popovića,
Ivan Čapob,
Nebojša Stilinovićc,
Saša Vukmirovićc,
Biljana Miloševića and
Maja Karamand
aDepartment of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Trg D. Obradovića 3, 21000 Novi Sad, Serbia. E-mail: milena.raseta@dh.uns.ac.rs; Tel: +381214852762
bDepartment of Histology and Embryology, Faculty of Medicine Novi Sad, University of Novi Sad, Hajduk Veljkova 3, 21000 Novi Sad, Serbia
cDepartment of Pharmacology, Toxicology and Clinical Pharmacology, Faculty of Medicine, University of Novi Sad, Hajduk Veljkova 3, 21000 Novi Sad, Serbia
dDepartment of Biology and Ecology, Faculty of Sciences, University of Novi Sad, Trg D. Obradovića 2, 21000 Novi Sad, Serbia
First published on 11th March 2020
Abstract
This study was designed to define total protein, phenol and flavonoid content as well as LC-MS/MS phenolic profile related to antioxidant and antidiabetic activity of ethanolic (EtOH) and water extracts of G. pfeifferi and G. resinaceum. G. resinaceum water extract possessed the highest ability to scavenge DPPH˙ and O2˙−, while the EtOH extract of the same species showed better activity on NO˙ related to other extracts. The highest level of bioactive compounds was determined generally in EtOH extracts. Antidiabetic action was evaluated by the oral glucose tolerance test (OGTT) and histological examination of pancreas and liver in normoglycemic and alloxan-induced diabetic animals. Histological examination of pancreatic tissue demonstrated that G. pfeifferi extracts have protective effects. To conclude, analysed extracts could be considered as a promising candidate for further research with the aim to promote antidiabetic activity, which is for the first time reported for G. pfeifferi.
1. Introduction
An imbalance between formation of reactive oxygen species (ROS) metabolites and the rate at which they are scavenged by enzymatic and non-enzymatic antioxidants is referred to as oxidative stress which can further lead to a range of health disorders in humans.1–3 A series of protective antioxidant mechanisms exist in human organisms, such as catalase (CAT), glutathione-S-transferase (GST) and many others for both prevention of free radical production and reparation of oxidative damage.3–7 Oxidative stress plays an important role in some physiological conditions and in many diseases and disease states, including diabetes mellitus (DM), cancer or neurodegenerative disorders.8 The restriction of the use of cancerogenic synthetic phenolic antioxidants (BHA, BHT, PG and others) in recent years has caused a rapidly increased interest towards naturally occurring antioxidants which could effectively inhibit oxidation in living systems.1,2To date, a large number of bioactive compounds including polysaccharides and their protein complexes, dietary fibres, and other compounds extracted from fungal fruiting bodies, cultured mycelium, or cultured broth in submerged cultivation have been reported to express anti-hyperglycaemic activity.9,10 Natural extracts of G. lucidum have been recognized and used as an alternative therapy for DM since ancient time in China11 in which ganoderan A, B and C have been characterized recently to be useful for treatment of type 1 DM and for type 2 DM.12
Ganoderma species are saprotrophic lignicolous basidiomycete with large, leathery, perennial, woody brackets called “conks”, growing on deciduous trees either with or without a stem. They represent valuable sources of nutrients and different types of bio remedies, which have been used for centuries in Asian countries to promote health and longevity.1,8 Ganoderma is a genus of polypore mushrooms that include about 80 species, many from tropical regions. While the medicinal use of mushrooms has a very long tradition in Asian countries, it has been largely forgotten in Europe; thus, it is not surprising that Ganoderma species that occur only in Europe have been poorly investigated.13 The species G. lucidum, crown fungi, is used for the preparation of infusions and sold as functional supplements worldwide.14 Likewise, there are evidences of potential benefit from using various Ganoderma extracts with respect to antioxidant,1,14–16 antibacterial,13,14 antiviral,9 antidiabetic,8,12 anticancer activity,16–18 cardioprotective and nephroprotective effect.19 They can grow wild or can be cultivated to be used as an important natural source against various diseases caused by oxidative stress.1,2,20 In traditional Chinese ethnomedicine, G. resinaceum Boud. in Pat. 1890 has been also proved to exert many pharmacological effects, for hyperglycaemia, immunoregulation and liver disease including improvement of function of lungs, liver, kidney, spleen and stomach.21 It contains various types of bioactive compounds, namely ganoderic acids A and B,22 β-glucans,10 lanostanoid terpenoides (ganoderesin B, ganoderol B, lucidone A)18 and phenolic compounds.14 A few compounds have been reported for G. resinaceum until nowadays while fifteen nortriterpenoids including six new nortriterpenoids (1–6) and nine known analogs (7–15) have been determined recently supporting results that the side chain of Ganoderma triterpenoids played an important role in α-glucosidase inhibitory activity.22 Structure analysis of a potential bioactive compounds indicated that most powerful compounds have a pentatonic 20(24)-γ-lactone ring, also ganoderlactone D has a 3-OH and 12-OH that correspond to the structure–activity relationships.23 Study reported by Zhao et al.19 suggests that ganoderic acids such as ganoderolactone B, D, E and ganodernoid A may play important roles in the antidiabetic effects of G. lucidum. Therefore, α-glucosidase inhibitors have been proposed as a treatment for type 2 DM by preventing the digestion of carbohydrates.19
Phylogenetic analysis grouped G. pfeifferi together with G. resinaceum, G. subamboinense and three strains of G. lucidum from the United States and Taiwan into one monophyletic group that is characterized by the production of chlamydospores in culture.24 In contrast to G. applanatum and G. lucidum and lately G. resinaceum, from which a great number of biologically and pharmacologically important lanostane triterpenes and polysaccharides have been isolated, G. pfeifferi (Bres 1889) a typical European species is one of the chemically less examined Ganoderma species.9,13,24 G. pfeifferi manifests antimicrobial13,24 activity related to sterols (ergosta-7,22-diene-3-one, ergosta-4,6,8(14),22-tetraene-3-one, 5α,8α-epidioxyergosta-6,22-diene-3β-ol) and triterpenes (lucialdehyde D, ganoderone B and ganoderone C) as well as lanostanoid triterpenes (ganodermadiol, lucidadiol and applanoxidic acid G)9 etc. Among previously described substances, triterpenoids, polysaccharides and glycoproteins could have antidiabetic effects,12 while phenols are significant as antioxidant compounds.14,16 G. pfeifferi contains unique sesquiterpenoids and other small molecular weight compounds among which some exhibits remarkable antimicrobial activities.13 Stimulating effects of G. pfeifferi on the viability of skin cells, the UV protection properties and the antibacterial and anti-aging activities suggest a possible use of this fungal species in the form of cosmetics, perhaps in combination with special minerals. For this purpose, EtOH and water extracts are already registered with the INCI numbers (International Nomenclature of Cosmetic Ingredients).13
To the best of our knowledge, no detailed study has been carried out on the in vivo effect of G. pfeifferi and G. resinaceum extracts on antidiabetic and antioxidant activities, lipid peroxidation and enzyme antioxidants in alloxan-induced DM. Hence, the aim of this study was to determine the in vitro and in vivo antioxidative as well as antidiabetic activities of extracts of both species in rat model of alloxan-induced DM.
2. Material and methods
2.1. Fungal material and extracts preparation
Fruiting bodies of two Ganoderma fungal species, G. pfeifferi and G. resinaceum were collected in September 2010, at the location of Nature park Begečka Jama and Campus of University of Novi Sad (Serbia), respectively. Determination and identification of the collected material was carried out at Department of Biology and Ecology, University of Novi Sad. Voucher specimens are deposited in the Herbarium of the University of Novi Sad (BUNS), under number (12-00723, 12-00722).All experiments were performed using ethanol (EtOH) and water extracts which were prepared as described previously.16 Both suspensions were prepared in distilled water to reach the final concentration at 100 mg mL−1 dry weight (d.w.) and stored at +4 °C and −20 °C prior to analysis.
2.2. Laboratory animals and experimental procedures
Experiments were carried out on white Wistar rats of both sexes (weighing 210–340 g and ages up to four months) were purchased from Military Medical Academy of Belgrade (Republic of Serbia). Animals were housed in UniProtect airflow cabinet (Ehret GmbH, Emmendingen, Germany) and standard plexiglass cages at a constant 22 ± 1 °C room temperature, 55% ± 1.5% humidity and with standard circadian rhythm (12 h day/night cycle). They were allowed free access to tap water and standard pelleted laboratory rodent feed (Veterinary Institute Subotica, Serbia) during the whole experiment. The experimental procedures were conducted in accordance with the European Directive (2010/63/EU) for animal experiments and they were reviewed and approved by Ethics Committee for Protection and Welfare of Experimental Animals at University of Novi Sad, Serbia.Animals were randomly divided into ten groups, each group consisting of six animals. Five groups were without alloxan pre-treatment, and five groups were with alloxan-induced diabetes (AID). Normoglycemic and diabetic animals were treated in the same manner with an oral aqueous suspension of the EtOH and water extracts of two analysed fungal species at dose of 1 mg mL−1, whereas the control animals received the same volume of physiological solution. The extract suspensions were administered for 5 days and on day 5 (2 h after administration of the last dose of fungal extracts) the rats were narcotized with a 25% solution of urethane in a dose of 5 mL kg−1 intraperitoneal (i.p.). After losing of the righting reflex, the animals were exsanguinated with intracardial puncture in order to take samples of blood and other tissues for further examination. After sacrifice, samples of the rat's pancreas and liver were fixed in 10% buffered formalin for histological examination. One part of liver was quickly frozen with liquid nitrogen and stored in freezer at −80 °C for the antioxidant activity analysis. All animals were weighed before the experiment and after the 5 day treatment period.
2.3. Antioxidant activity
The method of Espin et al.25 was used for evaluation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, where reaction mixture consisted of 10 μL of extract, 60 μL of DPPH work solution (90 μmol L−1) and 180 μL of methanol. Absorbance was measured after 30 min at 540 nm (Multiskan Ex Thermo Fisher Scientific).
Super oxide anion, SOA scavenging capacity was evaluated according to the method described by Nishikimi et al.26 Reaction mixture consisted of 10 μL of extract, 100 μL of NADH (677 μmol L−1), 100 μL of phenazine methosulfate (PMS) (60 μmol L−1), 200 μL nitro blue tetrazolium (144 μmol L−1) and 1.1 mL of phosphate buffer (pH 8.3). After 5 min, 250 μL aliquots were transferred to a plate and their absorbance was measured at 540 nm using a plate reader (Multiskan Ex Thermo Fisher Scientific).
NO radical scavenging activity of the extracts was determined according to the method reported by Green et al.27 The reaction mixture contained 30 μL of extracts, 500 μL of sodium nitroprusside dihydrate (10 mmol L−1) and 500 μL of phosphate buffer (pH 7.4). Test tubes were incubated at room temperature for 90 min, exposed to light. After incubation time, 1 mL of Griess reagent (0.2% solution of naphtylethylenediamine dihydrochloride and 2% solution of sulfanilamide in 4% phorphoric acid in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to test tubes. 250 μL aliquots were transferred to a plate and their absorbance was measured at 546 nm using a plate reader (Multiskan Ex Thermo Fisher Scientific).
1) was added to test tubes. 250 μL aliquots were transferred to a plate and their absorbance was measured at 546 nm using a plate reader (Multiskan Ex Thermo Fisher Scientific).
FRAP assay was performed according to modified procedure by Benzie and Strain.28 The reaction mixture consisted of 10 μL of extracts, 225 μL of FRAP reagent (acetate buffer, pH 3.6, 2,4,6-tris(2-pyridyl)-s-triazine (10 mmol L−1) and FeCl3 (20 mmol L−1 in the ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and 22.5 μL of distilled water. After 6 min of incubation, the absorbance was measured at 593 nm using a plate reader (Multiskan Ex Thermo Fisher Scientific).
1)) and 22.5 μL of distilled water. After 6 min of incubation, the absorbance was measured at 593 nm using a plate reader (Multiskan Ex Thermo Fisher Scientific).
The radical scavenging capacity (RSC) in used assays was expressed as IC50 (μg mL−1) for DPPH, SOA and NO, whereas reducing power (FRAP) of analysed fungal species was expressed as mg of ascorbic acid equivalents per g dry weight (mg AAE per g d.w.). Synthetic antioxidant propyl gallate (PG) was used as a positive control for RSC assays.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sucrose in a ratio 1
sucrose in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 at +4 °C. The obtained homogenates were filtered and the biochemical parameters were determined. The following biochemical parameters for oxidative stress were analysed in liver homogenate: extent of lipid peroxidation (LPx), activity of peroxidase (Px), catalase (CAT), glutathione peroxidase (GSHPx), glutathione reductase (GSHR), xanthine oxidase (XOD) and content of reduced glutathione (GSH) as described in Kaurinovic et al.3
3 at +4 °C. The obtained homogenates were filtered and the biochemical parameters were determined. The following biochemical parameters for oxidative stress were analysed in liver homogenate: extent of lipid peroxidation (LPx), activity of peroxidase (Px), catalase (CAT), glutathione peroxidase (GSHPx), glutathione reductase (GSHR), xanthine oxidase (XOD) and content of reduced glutathione (GSH) as described in Kaurinovic et al.32.4. Chemical constituents in relation to antioxidative activity
Total phenol content was determined according to Singleton et al.30 125 μL of Folin–Ciocalteu reagent (0.1 M) was added to 25 μL of extracts (or standard solution) and after 10 min 100 μL of sodium carbonate (7.5%) was added and reaction mixture was incubated for 2 h. Absorbance was measured after incubation at 760 nm (Multiskan Ex, Thermo Fisher Scientific). Total phenol content was calculated on the basis of a calibration curve of standard solution of gallic acid and expressed as mg of gallic acid equivalents per g of dry weight (mg GAE per g d.w.).
Total flavonoid content was determined according to Chang et al.31 30 μL of extracts (or standard solution) was diluted with 90 μL of methanol and 6 μL of aluminium trichloride (0.75 mol L−1), 6 μL of sodium acetate (1 mol L−1) and 170 μL of distilled water was added. Absorbance was measured after 30 min at 415 nm (Multiskan Ex, Thermo Fisher Scientific). From the calibration curve of quercetin solution was calculated total flavonoid content which is expressed as mg of quercetin equivalents per g of dry weight (mg QE per g d.w.).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, were used to dilute analyzed extracts to the final concentration of 2 mg mL−1. Gradient elution was performed using the following solvent gradient: starting with 70% A/30% B, reaching 30% A/70% B in 6.00 min, then 100% B at 9.00 min, holding until 12.00 min, with re-equilibration time of 3 min. The flow rate was 1 mL min−1 and the injection volume was 5 μL. ESI-MS was used to detect phenolic compounds by using the ion source parameters as follows: nebulization gas (N2) pressure 276 kPa, drying gas (N2) flow 9 L min−1 and temperature 350 °C, capillary voltage 4 kV, and negative polarity. The data were acquired in dynamic MRM mode by using the optimized compound specific parameters given before.32 The identification of the peaks was done by using standards and the total of identified phenolics was also calculated.
1 ratio, were used to dilute analyzed extracts to the final concentration of 2 mg mL−1. Gradient elution was performed using the following solvent gradient: starting with 70% A/30% B, reaching 30% A/70% B in 6.00 min, then 100% B at 9.00 min, holding until 12.00 min, with re-equilibration time of 3 min. The flow rate was 1 mL min−1 and the injection volume was 5 μL. ESI-MS was used to detect phenolic compounds by using the ion source parameters as follows: nebulization gas (N2) pressure 276 kPa, drying gas (N2) flow 9 L min−1 and temperature 350 °C, capillary voltage 4 kV, and negative polarity. The data were acquired in dynamic MRM mode by using the optimized compound specific parameters given before.32 The identification of the peaks was done by using standards and the total of identified phenolics was also calculated.2.5. Antidiabetic activity
Alloxan (alloxan monohydrate, Sigma Chemicals Co, St Louis, MO, USA) was used for diabetes induction in the laboratory animals. Alloxan was dissolved in physiological solution and administered in a dose of 100 mg kg−1 i.p. Hyperglycaemia was detected 48 h after the application of alloxan. Animals with a glycemia higher than 15 mmol L−1 were included in further experiment. Normoglycemic animals underwent oral glucose tolerance test. In this test for the induction of hyperglycaemia we used saturated solution of glucose at dose of 3 g kg−1. Blood glucose level was measured from rat's tail vein using commercial Accu Check Active kits for human use (Roche, Switzerland). Blood glucose measurements were at the beginning of experiment, 48 h after the administration of alloxan and 2 h after the administration of the last dose of mushroom extract. For the oral glucose tolerance test blood glucose measurements were just before and 30 min after the administration of glucose solution.2.6. Statistical analysis
Statistical analysis was performed using IBM SPSS statistical software, version 19.0 (IBM Corp., Armonk, NY, USA). Data were reported as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) or Kruskal–Wallis test was employed for the comparisons between experimental groups. Post-hoc testing for ANOVA was performed using Tukey's test. Mann–Whitney U test was used for post-hoc testing in Kruskal–Wallis analysis. Furthermore, paired-samples t-test was used for the evaluation of antidiabetic activity, by comparing pre-test and post-test glycaemia values. IC50 values (mg mL−1) were determined by the linear regression analysis of RSC (OriginLab 8), while statistical data correlation was obtained by Pearson correlation coefficient to estimate the relationship between the antioxidant potential of analysed fungal extracts and total protein, total phenol and total flavonoid contents. A difference between groups was considered statistically significant for a P value less than 0.05 (P < 0.05).3. Results and discussion
3.1. Antioxidant activity
| Assay | G. pfeifferi | G. resinaceum | PG | ||
|---|---|---|---|---|---|
| EtOH | H2O | EtOH | H2O | ||
| a TPR, total protein content; TP, total phenol content; TF, total flavonoid content; EtOH, ethanolic extract; H2O, water extract; n.a., non active. Data are reported as mean ± standard deviation of triplicates. a,b,c,d,e – different letters in the same row indicate significant difference between extracts (p < 0.01). R2* – all values are statistically significant (p < 0.05). | |||||
| DPPH (μg mL−1) | 18.9 ± 0.4 c | 36.1 ± 0.9 e | 22 ± 1 d | 14.0 ± 0.6 b | 0.39 ± 0.01 a |
| SOA (μg mL−1) | 91.9 ± 0.2 e | 65.7 ± 0.1 d | 62.7 ± 0.4 c | 30.0 ± 0.2 b | 10.0 ± 0.5 a |
| NO (μg mL−1) | 370 ± 1 d | 273.8 ± 0.4 c | 215.2 ± 0.8 b | 830.8 ± 0.3 e | 6.1 ± 0.8 a |
| FRAP (mg AAE per g d.w.) | 151.5 ± 0.2 b | 65 ± 1 d | 80.2 ± 0.5 c | 297 ± 1 a | n.a. |
| TPR (mg BSAE per mL) | 11.6 ± 0.3 a | 2.2 ± 0.3 d | 7.2 ± 0.7 b | 3.4 ± 0.2 c | — |
| TP (mg GAE per g d.w.) | 43.7 ± 0.6 a | 23.1 ± 0.9 b | 44.0 ± 0.2 a | 11.4 ± 0.4 c | — |
| TF (mg QE per g d.w.) | 26.3 ± 0.7 a | 10.1 ± 0.8 c | 22.9 ± 0.2 b | 4.4 ± 0.9 d | — |
| Correlation coefficient – R2* | |||||
|---|---|---|---|---|---|
| DPPH | TPR | 0.99* | 0.47 | 0.97* | 0.99* |
| TP | 0.72* | 0.03 | 0.93* | 0.40 | |
| TF | 0.83* | 0.01 | 0.96* | 0.04 | |
| SOA | TPR | 0.72* | 0.04 | 0.99* | 0.99* |
| TP | 0.99* | 0.51* | 0.98* | 0.54* | |
| TF | 0.40 | 0.60* | 0.99* | 0.11 | |
| NO | TPR | 0.96* | 0.51* | 0.97* | 0.99* |
| TP | 0.44 | 0.97* | 0.94* | 0.54* | |
| TF | 0.98* | 0.99* | 0.97* | 0.11 | |
| FRAP | TPR | 0.99* | 0.86* | 0.99* | 0.99* |
| TP | 0.59* | 0.96* | 0.99* | 0.38 | |
| TF | 0.92* | 0.91* | 0.99* | 0.03 | |
For the FRAP assay, the most effective activity was obtained for water extract of G. resinaceum (297 ± 1 mg AAE per g d.w.), followed by EtOH extracts of G. pfeifferi and G. resinaceum, while the lowest activity was obtained for water extract of G. pfeifferi (65 ± 1 mg AAE per g d.w.) (Table 1). Our results are in accordance with previously published data14 in which the activity of water G. resinaceum extract was more powerful compared to EtOH extract. At the same time analysed extracts of G. resinaceum species from Serbia showed better potential compared to extracts of G. resinaceum species from Turkey.14 Also, high correlation between total phenol content and FRAP activity in EtOH extract of G. resinaceum was detected (R2 = 0.99) probably due to its richness of active ingredients which possess an antioxidant effect such as α tocopherol, linoleic α-acid, phenolic acids etc.1
In this study, G. pfeifferi (compared to G. resinaceum) proved to be a better source of phenols (with the exception of EtOH extract of G. resinaceum) which are regarded as one of the most powerful natural antioxidants.1,2,12 Among phenols, preliminary the dominant content of flavonoids was detected in EtOH extracts as well as the content of proteins which is, best to our knowledge, the first scientific report.
As shown in Table 2, in comparison with control samples intensity of lipid peroxidation (LPx) was significantly reduced in animals treated with analysed extracts, except in animals treated with G. pfeifferi EtOH extract where the intensity of LPx was slightly increased (11.0 ± 0.5 nmol MDA per mg P). In groups treated with G. resinaceum extracts both with and without alloxan pre-treatment, similar activity in comparison to control samples was detected, which points the effects of this less examined fungal species. Teng et al.11 indicate the importance of proteoglycans as potent hypoglycaemic agents in G. lucidum extracts.
| Parameter | Control | G. pfeifferi EtOH | G. pfeifferi H2O | Alloxan + saline | Alloxan + saline + G. pfeifferi EtOH | Alloxan + saline + G. pfeifferi H2O |
|---|---|---|---|---|---|---|
| LPx | 10.8 ± 0.7 b | 11.0 ± 0.5 b | 8.7 ± 0.2 a | 9.69 ± 0.01 a | 7.4 ± 0.1 a | 6.3 ± 0.6 a |
| GSH | 124 ± 10 b | 285 ± 38 a | 231 ± 7 a | 99.3 ± 0.4 c | 104 ± 7 c | 133 ± 18 b |
| GSHR | 6.21 ± 0.01 b | 3.3 ± 0.2 d | 4.2 ± 0.2 c | 6.7 ± 0.1 a | 6.25 ± 0.03 b | 4.2 ± 0.4 c |
| GSHPx | 21 ± 1 a | 16 ± 2 b | 14.8 ± 0.6 b | 14.9 ± 0.6 b | 17 ± 1 b | 14 ± 3 b |
| Px | 11.7 ± 0.1 c | 15 ± 1 a | 13.0 ± 0.1 b | 8.1 ± 0.8 d | 10 ± 1 c | 6.6 ± 0.1 e |
| CAT | 61 ± 10 d | 108 ± 12 b | 133 ± 4 a | 97 ± 2 c | 89 ± 7 c | 109 ± 3 b |
| XOD | 55 ± 2 c | 47 ± 1 d | 45.2 ± 0.4 d | 66 ± 2 a | 69 ± 1 a | 58.7 ± 0.4 b |
The amount of GSH, secondary metabolite important in detoxification pathways of organisms via conjugation with toxic metabolites,3 was statistically significant increased mostly in animals treated with G. pfeifferi EtOH and water extracts (285 ± 38 and 231 ± 7 nmol GSH per mg P, respectively), as well as in animals treated with G. resinaceum EtOH extract (alloxan-pre-treated) (213 ± 10 nmol GSH per mg P). Related to GSH content, increased GSHR and GSHPx activities were confirmed only in animals treated with G. pfeifferi EtOH extract (alloxan-pretreated) (6.25 ± 0.03 and 6.21 ± 0.01 nmol per mg per min P, respectively) (Table 2). Chemical compounds with –SH groups could express antioxidative activity in lower concentrations, while in higher concentrations predominant activity could be prooxidant.1,2 Reduction of alloxan to dialuric acid takes over the thiol, most often GSH which is oxidized, and final product is alloxan–GSH.33 Administration of three extracts in animals pre-treated with alloxan (EtOH and water of G. pfeifferi and EtOH of G. resinaceum) caused statistically significant changes of LPx and GSH. This result certainly indicates that there exist some protective effects of these fungal species of the genus Ganoderma and supports the above-mentioned mechanism (Tables 2 and 3).
| Parameter | Control | G. resinaceum EtOH | G. resinaceum H2O | Alloxan + saline | Alloxan + saline + G. resinaceum EtOH | Alloxan + saline + G. resinaceum H2O |
|---|---|---|---|---|---|---|
| a EtOH, ethanolic extracts; H2O, water extracts. GSH content is expressed as nmol GSH per mg of proteins. GSHR, GSHPx, Px, CAT and XOD activity are expressed in nmol per mg per min of proteins. Intensity of lipid peroxidation is expressed in nmol malondialdehyde per mg of proteins. Results are expressed as means ± SD. a,b,c,d,e different letters in the same column indicate significant difference between analyzed samples (Tukey's test, p < 0.01). | ||||||
| LPx | 10.8 ± 0.7 b | 9.9 ± 0.2 b | 10.1 ± 0.3 b | 9.69 ± 0.01 a | 10.5 ± 0.4 b | 9.6 ± 0.3 a |
| GSH | 124 ± 10 c | 166 ± 13 b | 153 ± 19 b | 99.3 ± 0.4 d | 213 ± 10 a | 171 ± 1 b |
| GSHR | 6.21 ± 0.01 a | 2.3 ± 0.3 c | 1.2 ± 0.1 d | 6.7 ± 0.1 a | 5.3 ± 0.6 b | 5.04 ± 0.04 b |
| GSHPx | 21 ± 1 a | 14 ± 1 b | 15 ± 1 b | 14.9 ± 0.6 b | 13.2 ± 0.8 c | 15.2 ± 0.8 b |
| Px | 11.7 ± 0.1 a | 8.3 ± 0.3 b | 8 ± 1 b | 8.1 ± 0.8 b | 8 ± 2 b | 7.9 ± 0.9 b |
| CAT | 61 ± 10 d | 58 ± 4 e | 65 ± 2 d | 97 ± 2 b | 110 ± 8 a | 86 ± 3 c |
| XOD | 54.6 ± 2 c | 77 ± 1 a | 65 ± 5 b | 66 ± 2 b | 63 ± 2 b | 73 ± 2 a |
Treatment with all G. pfeifferi extracts (except water with alloxan-pre-treatment) produced increase in Px activity, which was not statistically significant (Table 2). Based on data showed in Table 3, treatment with G. resinaceum extracts in animals pre-treated with alloxan resulted in reduced Px activity, which may be result of the fact that phenolic compounds as hydrogen donors in Px-catalysed reaction were not present in a sufficient concentration or do not contribute to increase in Px activity.34 This is in accordance with determined in vitro antioxidant potential in this study. G. resinaceum extracts expressed better in vitro antioxidant potential with good correlation between this potential and total phenol and total protein content, indicating a synergistic effect on antioxidant activity which is probably not based only on the presence of phenolic compounds but also on the presence of proteins.
Administration of both G. pfeifferi extracts caused a decrease in the values of XOD activity in experimental animals (47 ± 1 for EtOH and 45.2 ± 0.4 nmol per mg per min P for water, respectively) compared to control sample (55 ± 2 nmol per mg per min P). Based on published data, flavonoids are considered as significant inhibitors or activators of certain enzymes from the group of oxidoreductase, which is in accordance with results of our study, since higher total flavonoid content was noticed in G. pfeifferi extracts (Table 1). Treatment with alloxan and analyzed extracts (Tables 2 and 3) increased the activity of XOD compared to control group, which is characteristic of liver damage in diabetes and points to the existence of oxidative stress.35
Values of CAT showed an insignificant decrease of activity in animals treated only with G. resinaceum EtOH extracts (58 ± 4 nmol per mg per min P) compared to control group (61 ± 10 nmol per mg per min P), while in other groups treated with G. resinaceum EtOH extracts, the significant increase of activity was detected (Table 3). Treatment with G. pfeifferi extracts resulted in statistically significant increase of CAT activity (Table 2). Increase in CAT activity in groups treated with extracts of both species, may be the result of the stronger activity of Px enzyme.20
3.2. Chemical composition of analysed fungal species
Total protein content in analysed extracts ranged from 2.2 to 11.6 mg BSAE per mL (Table 1) with EtOH extracts of both species being the richest. To the best of our knowledge these results on total protein content of the analysed fungal species are preliminary, while total protein content for the same extracts of G. applanatum and G. lucidum (39 and 27 mg BSAE per mL, respectively)15 was higher compared to obtained results (Table 1). Still, high correlation between total protein content and the antioxidant potential (Table 1) suggests sufficient antioxidant potential of protein–polysaccharide complexes in examined extracts, previously detected.1Similar to total protein, higher total phenol and total flavonoid contents were also detected in EtOH extracts for both species (Table 1) which is in accordance with previous results for other Ganoderma species.16 Compared to our study, total phenol content in MeOH and water extracts of G. resinaceum from Turkey14 was 37.32 and 36.39 mg GAE per g d.w., is higher only regarding water extract of the same species (11.4 ± 0.4 mg GAE per g d.w.). High correlations between total phenol and total flavonoid content and antioxidative activity (Table 1) for both G. resinaceum extracts might suggest that phenolic compounds play important role in the expressed antioxidative potential.14,16 Although similar in total phenol and total flavonoid contents, antioxidative activity of G. pfeifferi was weaker than for G. resinaceum (Table 1). Overall, total phenol and total flavonoid contents were higher in EtOH extracts most probably due to the polarity of the extraction solvent which strongly affects the level of phenolic compounds.16
High correlation between both total protein and total phenol contents and antioxidative potentials points out the role of these bioactive compounds in analysed extracts. Best to our knowledge, antioxidative potential of G. pfeifferi extracts was detected for the first time in this research, and these results indicate the importance of this poorly researched species.
Considering the antioxidant activity as a function of the phenolic constituents, beside total phenol content, we have also quantified eight phenolic compounds using LC-MS/MS technique, while concentration of other detected compounds were under the limits of quantification (LOQ) of used technique (Table 4). Only, G. pfeifferi EtOH contained all detected phenolic compounds and the highest content (Table 4). p-Hydroxybenzoic and gallic acids represented the major compounds in EtOH extracts of both examined fungal species. Among the examined extracts, the highest level of p-hydroxybenzoic and gallic acid was determined in G. pfeifferi EtOH (23.00 and 30.50 μg per g d.w., respectively). In examined extracts, p-coumaric and quinic acid were determined in all examined extracts in a similar concentration range (0.25–1.5 μg per g d.w.). With respect to G. resinaceum extracts, the highest concentration was noticed for gallic acid (15.85 μg per g d.w.) which is preliminary determined in this species as well as quinic and p-coumaric acid.14 Other quantified compounds in G. resinaceum were noticed at lower concentration in relation to literature data.14
| Phenolic compound | Fungal species and extract type | |||
|---|---|---|---|---|
| Amount of compound detected (μg per g d.w.) | ||||
| G. pfeifferi EtOH | G. pfeifferi H2O | G. resinaceum EtOH | G. resinaceum H2O | |
| a EtOH, ethanolic extract; H2O, water extract. Bold number: amount of qualified phenolic compounds in examined extracts.b Number: detected compound – peak observed, concentration is lower than the LoQ (limit of quantification), but higher than LoD (limit of detection). | ||||
| p-Hydroxybenzoic acid | 23.00 | 5.10 | 12.20 | <0.30b |
| Protocatechuic acid | 6.50 | 6.20 | 4.01 | 2.65 |
| p-Coumaric acid | 1.50 | 1.00 | 0.80 | 0.60 |
| Vanillic acid | 6.50 | 4.50 | <4.00b | <4.00b |
| Gallic acid | 30.50 | 1.50 | 15.85 | 1.20 |
| Caffeic acid | 0.80 | 0.60 | 0.40 | 0.25 |
| Quinic acid | 8.51 | 6.35 | 6.90 | 3.00 |
| Chlorogenic acid | 1.26 | 0.80 | <0.30b | <0.30b |
Determined phenolic acids in this study, with the highest concentration of p-hydroxybenzoic and gallic acid agrees with the fact that the most common fungal benzoic acid derivates with antioxidant potential are p-hydroxybenzoic, protocatechuic, gallic, vanillic, syringic acid etc.2
Finally, this study reported LC-MS/MS phenolic profile of G. pfeifferi and EtOH extract of G. resinaceum for the first time.
3.3. Pharmacodynamic activity
| Experimental group | Glycaemia before alloxan administration (mmol L−1) | Glycaemia 48 h after alloxan administration (mmol L−1) | Glycaemia on the last day of treatment (mmol L−1) |
|---|---|---|---|
| a EtOH, ethanolic extract; H2O, water extract.b p < 0.05 statistically significant results vs. control group (alloxan + saline). | |||
| Alloxan + saline | 6.8 ± 0.6 | 33.1 ± 0.2 | 32.8 ± 0.4 |
| Alloxan + G. pfeifferi, EtOH | 7.1 ± 0.5 | 33 ± 2 | 31 ± 2 |
| Alloxan + G. pfeifferi, H2O | 7.2 ± 0.5 | 32 ± 3 | 31 ± 2 |
| Alloxan + G. resinaceum, EtOH | 7.1 ± 0.4 | 32 ± 2 | 28 ± 2 |
| Alloxan + G. resinaceum, H2O | 7.0 ± 0.4 | 31 ± 2 | 27.3 ± 0.3b |
| Experimental group | Glycaemia before treatment (mmol L−1) | Glycaemia before glucose administration (mmol L−1) | Glycaemia 30 min after glucoseadministration (mmol L−1) |
|---|---|---|---|
| a EtOH, ethanolic extract; H2O, water extract.b p < 0.05 statistically significant results vs. control group (saline). | |||
| Control | 6.7 ± 0.4 | 6.6 ± 0.6 | 9.6 ± 0.7 |
| G. pfeifferi, EtOH | 7.1 ± 0.6 | 7.1 ± 0.5 | 10.3 ± 0.7 |
| G. pfeifferi, H2O | 7.1 ± 0.5 | 7.0 ± 0.6 | 10.9 ± 0.9b |
| G. resinaceum, EtOH | 6.9 ± 0.4 | 7.1 ± 0.4 | 8.7 ± 0.3 |
| G. resinaceum, H2O | 6.8 ± 0.5 | 7.3 ± 0.5 | 9.1 ± 0.6 |
A possible explanation of antihyperglycemic effect of examined extracts could be the activity of polysaccharides (glucans – ganoderan A and B),10 that have been recently proven to express the antidiabetic effect in animals treated with G. lucidum22 which has resemblance to examined G. resinaceum. This effect may suggest that polysaccharides from water extract cannot stimulate the insulin synthesis but could stimulate the insulin release from the preserved pancreatic islets directly.10 De Silva et al.17 explain hypoglycaemic effects of G. lucidum polysaccharides through the release of Ca2+ ions into β-pancreatic cells, which leads to the release of insulin.10 Other studies suggest that triterpenes (ganoderic acid B) could be acting as antidiabetic agents via inhibition of α-glucosidase.12,22
Based on literature data,36 phenolic compounds may affect carbohydrate metabolism at various levels: improving postprandial and fasting blood glucose levels, insulin secretion, insulin sensitivity, helping on DM prevention and limiting the rate of glucose absorption from the intestines into the bloodstream. Among quantified phenolics of analysed extracts, gallic acid which has not yet been determined in these fungal species, is outspread by content in both EtOH extracts (Table 4). Therapeutic efficacy of this phenolic may lead to an improvement in insulin-dependent glucose transport in tissue through the translocation under signalling pathways.36 The results presented in OGTT (Table 6) suggest that, beside antioxidant activity, gallic acid may be one of the compounds responsible for glycaemia reduction in 5 day treatment with fungal extracts.
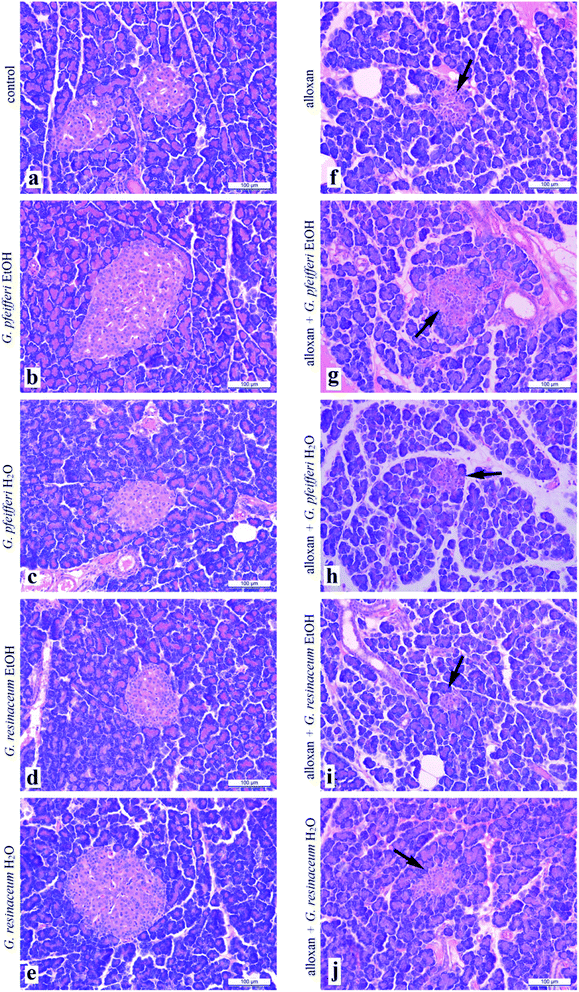 | ||
| Fig. 1 Histological cross section of pancreas treated with EtOH and H2O extracts of G. pfeifferi and G. resinaceum (arrow points to Langerhans islet). | ||
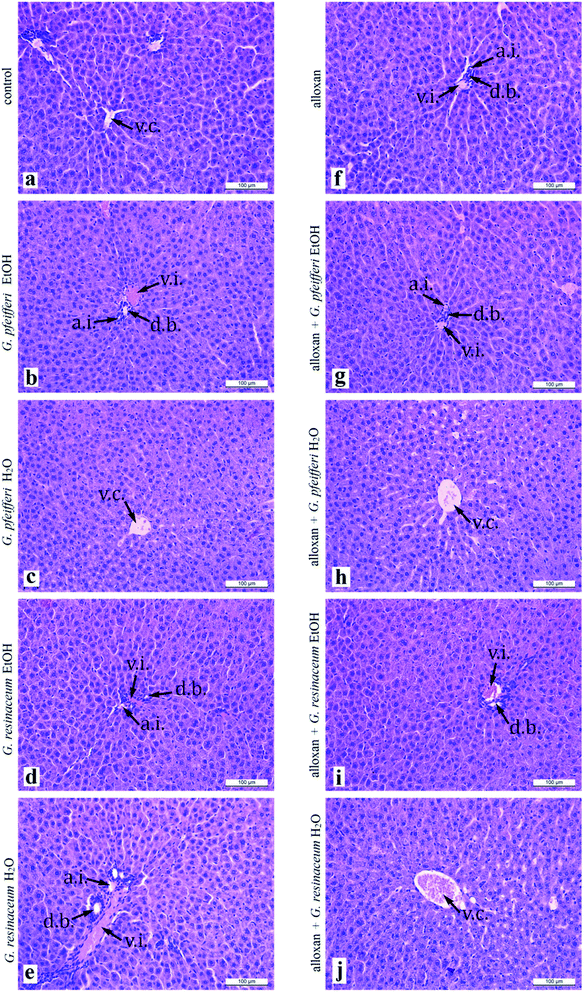
Analysis of liver histology sections (Fig. 2a–j) in both experimental groups, control and alloxan-pre-treated did not show any pathohistological changes in liver tissue, even though the applied doses of alloxan were sufficient for diabetes induction. The hepatocytes characterised usual shape, with centrally placed nucleus, and the light, highly granulated acidophilic cytoplasm. They were arranged in linear cords of cells which radiate from the central vein to the periphery of the classic liver lobule. In each of the lobes corners, we easily identified branches of the hepatic artery proper, hepatic portal vein and bile ducts, which represent components of portal triad. These results suggest that 5 day treatment with G. pfeifferi and G. resinaceum extracts after alloxan administration resulted in certain hepatoprotective effects. Related to hypoglycaemic activity, hepatoprotective ability of these samples to protect β-cells from the diabetogenic action of alloxan, possibly by the presence of antioxidant substances such as gallic acid.40
4. Conclusion
This study revealed for the first-time antidiabetic activity via alloxan-induced diabetes of examined Ganoderma species. Neither antidiabetic activity of G. pfeifferi and G. resinaceum nor the antioxidant potential of G. pfeifferi have been reported before. Water extracts of G. resinaceum showed the highest effect as free radical scavengers of DPPH˙ and O2˙− as well as on FRAP assay, except EtOH extract which showed the most powerful activity in NO assay. Five days treatment with both G. pfeifferi extracts had protective effects on liver biochemical parameters (GSH, LPx, GSHPx, GSHR, CAT and XOD) while G. resinaceum extracts had the most powerful reduction of the lipid peroxidation intensity suggesting protective role in oxidative stress. Based on correlation and antioxidant potential it was clearly indicated that the presence of phenolic compounds such as gallic acid (quantified in the highest concentration in G. pfeifferi EtOH extract), which is characterised as effective antioxidants due to their existence of hydroxyl groups.In animals treated with G. pfeifferi EtOH extract, after alloxan pre-treatment, pancreas cross-sections revealed increase in a number of slightly larger Langerhans islets with a lower density of β-cells, which was not observed in the cross-sections from control animals. Further studies on different animal and human models are essential to verify the beneficial effects of fungal species including more studies for the isolation and characterization of the triterpenes, polysaccharides or individual phenolic acids as active principles responsible for these activities.
Conflicts of interest
Authors declared that there are is no conflict of interest.Acknowledgements
This study was supported by Ministry of Education, Science and Technological Development of the Republic of Serbia (No. 172058). The authors are thankful to Dr Filip Šibul for LC-MS/MS analysis of extracts.References
- I. C. Ferreira, L. Barros and R. M. Abreu, Antioxidants in wild mushrooms, Curr. Med. Chem., 2009, 16, 1543–1560 CrossRef CAS PubMed.
- M. Kozarski, A. Klaus, D. Jakovljević, N. Todorović, J. Vunduk, P. Petrović, M. Nikšić, M. M. Vrvić and L. Van Griensven, Antioxidants of edible mushrooms, Molecules, 2015, 20, 19489–19525 CrossRef CAS PubMed.
- B. Kaurinovic, M. Popovic, S. Vlaisavljevic and M. Raseta, Antioxidant activities of Melittis melissophyllum L. (Lamiaceae), Molecules, 2011, 16, 3152–3167 CrossRef CAS PubMed.
- I. Gülçin, Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid), Toxicology, 2006, 217, 213–220 CrossRef PubMed.
- I. Gülçin, Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa, Amino Acids, 2007, 32, 431–438 CrossRef PubMed.
- T. Ak and I. Gülçin, Antioxidant and radical scavenging properties of curcumin, Chem.-Biol. Interact., 2008, 174, 27–37 CrossRef CAS PubMed.
- I. Gülçin, Antioxidant activity of food constituents: an overview, Arch. Toxicol., 2012, 86, 345–391 CrossRef PubMed.
- F. Li, Y. Zhang and Z. Zhong, Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice, Int. J. Mol. Sci., 2011, 12, 6135–6145 CrossRef CAS PubMed.
- T. H. J. Niedermeyer, U. Lindequist, R. Mentel, D. Gordes, E. Schmidt, K. Thurow and M. Lalk, Antiviral terpenoid constituents of Ganoderma pfeifferi, J. Nat. Prod., 2005, 68, 1728–1731 CrossRef CAS PubMed.
- H. N. Zhang and Z. B. Lin, Hypoglycemic effect of Ganoderma lucidum polysaccharides, Acta Pharmacol. Sin., 2004, 25, 191–195 CAS.
- B. S. Teng, C. D. Wang, D. Zhang, J. S. Wu, D. Pan, L. F. Pan, H. J. Yang and P. Zhou, Hypoglycemic effect and mechanism of a proteoglycan from Ganoderma lucidum on streptozotocin-induced type 2 diabetic rats, Eur. Rev. Med. Pharmacol. Sci., 2012, 16, 166–175 CAS.
- H. T. Ma, J. F. Hsieh and S. T. Chen, Anti-diabetic effects of Ganoderma lucidum, Phytochemistry, 2015, 114, 109–113 CrossRef CAS PubMed.
- U. Lindequist, W. D. Jülich and S. Witt, Ganoderma pfeifferi – A European relative of Ganoderma lucidum, Phytochemistry, 2015, 114, 102–108 CrossRef CAS PubMed.
- G. Zengin, C. Sarikurkcu, E. Gunes, A. Uysal, R. Ceylan, S. Uysal, H. Gungor and A. Aktumsek, Two Ganoderma species: profiling of phenolic compounds by HPLC-DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer's diseases and skin disorders, Food Funct., 2015, 6, 2794–2802 RSC.
- M. Kozarski, A. Klaus, M. Nikšić, M. M. Vrvić, N. Todorović, D. Jakovljević and L. J. L. D Van Griensven, Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor, J. Food Compos. Anal., 2012, 26, 144–153 CrossRef CAS.
- M. Rašeta, M. Karaman, M. Jakšić, F. Šibul F, M. Kebert, A. Novaković and M. Popović, Mineral composition, antioxidant and cytotoxic biopotentials of wild-growing Ganoderma species (Serbia): G. lucidum (Curtis) P. Karst vs. G. applanatum (Pers.) Pat, International Journal of Food Science and Technology, 2016, 51, 2583–2590 CrossRef.
- D. De Silva, S. Rapior, F. Fons, A. Bahkali and K. Hyde, Medicinal mushrooms in supportive cancer therapies: an approach to anti-cancer effects and putative mechanisms of action, Fungal Divers., 2012, 55, 1–35 CrossRef.
- X. R. Peng, J. Q. Liu, Z. H. Han, X. X. Yuan, H. R. Luo and M. H. Qiu, Protective effects of triterpenoids from Ganoderma resinaceum on H2O2-induced toxicity in HepG2 cells, Food Chem., 2013, 141, 920–926 CrossRef CAS.
- X. R. Zhao, X. K. Huo, P. P. Dong, C. Wang, S. S. Huang, B. J. Zhang, H. L. Zhang, S. Deng, K. X. Liu and X. C. Ma, Inhibitory effects of highly oxygenated lanostane derivatives from the fungus Ganoderma lucidum on P-glycoprotein and α-glucosidase, J. Nat. Prod., 2015, 78, 1868–1876 CrossRef CAS.
- M. Popović, S. Vukmirović, N. Stilinović, I. Čapo and V. Jakovljević, Anti-oxidative activity of an aqueous suspension of commercial preparation of the mushroom C. comatus, Molecules, 2010, 15, 4564–4571 CrossRef.
- B. Chen, B. Ke, L. Ye, S. Jin, F. Jie, L. Zhao and X. Wu, Isolation and varietal characterization of Ganoderma resinaceum from areas of Ganoderma lucidum production in China, Sci. Hortic., 2017, 224, 109–114 CrossRef CAS.
- X. Q. Chen, L. X. Chen, J. Zhao, Y. P. Tang and S. P. Li, Nortriterpenoids from the fruiting bodies of the mushroom Ganoderma resinaceum, Molecules, 2017, 22, e1073, DOI:10.3390/molecules22071073.
- V. T. Nguyen, N. T. Tung, T. D. Cuong, T. M. Hung, J. A. Kim and M. H. Woo, et al., Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum, Phytochem. Lett., 2015, 12, 69–74 CrossRef CAS.
- S. G. Hong and H. S. Jung, Phylogenetic analysis of Ganoderma based on nearly complete mitochondrial small-subunit ribosomal DNA sequences, Mycologia, 2004, 96, 742–755 CrossRef PubMed.
- C. J. Espin, G. Soler-Rivas and J. H. Wichers, Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical, J. Agric. Food Chem., 2000, 48, 648–656 CrossRef PubMed.
- M. Nishikimi, N. Rao and K. Yagi, The occurrence of super oxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen, Biochem. Biophys. Res. Commun., 1972, 46, 849–853 CrossRef CAS.
- C. E. Green, D. A. Wagner, J. Glogowski, P. L. Skiper, J. S. Wishnok and S. R. Tannenabaum, Analysis of nitrate, nitrite and [15N] nitrate in biological fluids, Anal. Biochem., 1982, 243, 709–714 Search PubMed.
- I. F. F. Benzie and J. J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power“: the FRAP assay, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- M. Bradford, A rapid and sensitive method for the quantitation of quantities microgram of protein utilizing the principle of dye binding protein, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventos, Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent, Methods Enzymol., 1999, 299, 152–178 CAS.
- C. C. Chang, M. H. Yang, H. M. Wen and J. C. Chern, Estimation of total flavonoid content in propolis by two complementary colorimetric methods, J. Food Drug Anal., 2002, 10, 178–182 CAS.
- D. Orčić, M. Francišković, K. Bekvalac, E. Svirčev, I. Beara, M. Lesjak and N. Mimica-Dukić, Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection, Food Chem., 2014, 143, 48–53 CrossRef PubMed.
- S. Lenzen, The mechanisms of alloxan- and streptozotocin-induced diabetes, Diabetologia, 2008, 51, 216–226 CrossRef CAS PubMed.
- N. C. Veitch, Structural determinants of plant peroxidase function, Phytochem. Rev., 2004, 3, 3–17 CrossRef CAS.
- M. E. Inkester, M. A. Cotter and N. E. Cameron, Treatment with the xanthine oxidase inhibitor, allopurinol, improves nerve and vascular function in diabetic rats, Eur. J. Pharmacol., 2007, 561, 63–71 CrossRef PubMed.
- T. F. De Miranda Pedroso, T. R. Bonamigo, J. da Silva, P. Vasconcelosa, J. P. Félix, C. A. Lima Cardoso, R. I. Carvalho Souza, A. C. dos Santos, C. R. Ferreira Volobuff, A. S. Nazari Formagio and V. D. Kappel Trichez, Chemical constituents of Cochlospermum regium (Schrank) Pilg. root and its antioxidant, antidiabetic, antiglycation, and anticholinesterase effects in Wistar rats, Biomed. Pharmacother., 2019, 111, 1383–1392 CrossRef PubMed.
- A. Sabo, N. Stilinovic, S. Vukmirovic, Z. Bukumiric, I. Capo and V. Jakovljevic, Pharmacodynamic action of a commercial preparation of the mushroom Coprinus comatus in rats, Phytother. Res., 2010, 24, 1532–1537 CrossRef CAS PubMed.
- S. Yazdankhah, M. Hojjati and M. H. Azizi, The Antidiabetic potential of black mulberry extract-enriched pasta through inhibition of enzymes and glycemic index, Plant Foods Hum. Nutr., 2019, 74, 149–155 CrossRef PubMed.
- X. Liu, J. P. Yuan, C. K. Chung and X. J. Chen, Antitumor activity of the sporoderm-broken germinating spores of Ganoderma lucidum, Cancer Lett., 2002, 182, 155–161 CrossRef CAS.
- A. M. Zanoelo, C. Melazzo-Mazzanti, J. Kerpel Gindri, A. Filappi, D. Prestes and M. Cecim, Efeito Protetor do Syzygium cumini contra Diabetes mellitus induzidos por aloxano em ratos, Acta Farm. Bonaerense, 2002, 21, 3–36 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10158f |
| This journal is © The Royal Society of Chemistry 2020 |