Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diastereoselective synthesis of atropisomeric pyrazolyl pyrrolo[3,4-d]isoxazolidines via pyrazolyl nitrone cycloaddition to facially divergent maleimides: intensive NMR and DFT studies†
Awad I. Said * and
Talaat I. El-Emary
* and
Talaat I. El-Emary
Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt 71516. E-mail: awadsaid@aun.edu.eg; Tel: +20-1012991716
First published on 6th January 2020
Abstract
A pyrazolyl nitrone (2) underwent 1,3-dipolar cycloadditions to afford some N-substituted maleimides (3a–o). An atropisomeric character was introduced into the formed cycloadducts by using maleimides that have a restricted rotation around the C–N bond. Also, facial selectivity of both endo and exo cycloaddition was observed where the major atropisomer was one that is formed by attacking the nitrone from the less hindered face of the dipolarophile. On the other hand, maleimides with free rotation around the C–N bond led to endo and exo cycloadducts without atropisomerism. The presence of atropisomerism in the formed cycloadducts was confirmed by extensive NMR studies and DFT calculations.
Introduction
Chemistry of nitrones has received significant attention since its discovery, more than a century ago.1 Most of the studies have focused on exploring the 1,3-dipole character of nitrones in [3 + 2] dipolar cycloadditions with dipolarophiles to construct functionalized five-membered heterocycles which are important intermediates in organic synthesis.2 The dipolarophiles include alkenes,3 alkynes,4 allenes5 and other cumulated double bonds.6 Isoxazolidines, formed from the [3 + 2] dipolar cycloadditions of nitrones and alkenes, exhibit good biological activities7 and the possibility of their transformation via ring opening into open-chain derivatives8 makes them valuable for the synthesis of natural and biologically important compounds such as amino sugars, amino alcohols, alkaloids, β-lactams, and amino acids.9 Also, pyrazoles have received significant attention because they are widely used as core motifs for a large number of compounds in various applications such as agrochemicals and medicine, due to their broad range of biological activities.10Several computational studies have been carried out to understand the origins of the regio- and stereoselectivities of cycloaddition reactions.11 The stereochemical course of the 1,3-dipolar cycloaddition reaction has been well explained in terms of secondary orbital interactions, steric factors, H-atom bonding and/or dipole–dipole electrostatic interactions.
Atropisomerism12 is a well-known phenomenon resulting from hindered rotation about single bonds where the energy barrier to rotation is high enough to allow the isolation of the conformers. Atropisomeric compounds have several applications including in the design of bioactive molecules, and construction of molecular switches and motors.13 To the best of our knowledge, little work has been reported about the effect of single bond restricted rotation on the route of cycloaddition reactions and the formation of atropisomeric cycloadducts.14 In most of the reported cycloaddition reactions, the used reactants have no restricted rotation near the cycloaddition region of the molecules and the maximum number of cycloadducts was known to be two isomers, namely exo and endo isomers.
In this work, pyrazole-based nitrone 2 underwent 1,3-dipolar cycloaddition with a series of and N-substituted maleimides (3a–o) (as dipolarophiles) with different degrees of rotational restriction around the C–N single bond. The effect of restricted rotation around the C–N single bond on the route of cycloaddition has been studied.
Results and discussion
Nitrone 2 was prepared by reacting 1,3-diphenyl-1H-pyrazole-4-carboxaldehyde (1)15 with N-phenylhydroxylamine16 (Scheme 1). Nitrone 2 was obtained in Z-form as was confirmed by spectral analysis and single-crystal crystallography.17Cycloadditions of 2 to N-substituted maleimides 3a–o (Scheme 2) were performed in toluene under reflux and were monitored by TLC. A high-resolution 1H-NMR spectrum (400 MHz) (ESI†) of the crude reaction products was measured to investigate the formed cycloadducts and estimate their ratio18 (Table 1). Endo and exo isomers were afforded by maleimides 3a–j at a ratio of 3.5–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The majority of endo isomers was interpreted by the presence of a stabilizing secondary orbital interaction in the transition state leading to the endo product. This interaction is not present in the transition state of the exo product.19
1. The majority of endo isomers was interpreted by the presence of a stabilizing secondary orbital interaction in the transition state leading to the endo product. This interaction is not present in the transition state of the exo product.19
| Entry | Conversiona | Endo (%) | Exo (%) | Endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo ratiob exo ratiob |
||
|---|---|---|---|---|---|---|
| Conversiona | Isolatedc | Conversiona | Isolatedd | |||
a Conversions were calculated from 1H-NMR integrations (400 MHz) as the conversion of the nitrone to the products.b Endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo ratio was calculated from 1H-NMR integrations (400 MHz).c Isolated endo isomer as obtained by filtration (first crop).d Isolated exo isomer as obtained using preparative TLC of filtrate. exo ratio was calculated from 1H-NMR integrations (400 MHz).c Isolated endo isomer as obtained by filtration (first crop).d Isolated exo isomer as obtained using preparative TLC of filtrate. |
||||||
| a | 97 | 87 | 80 | 10 | — | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| b | 99 | 92 | 60 | 7 | 0.95 | 13.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| c | 87 | 80 | 65 | 7 | — | 11.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| d | 87 | 83.5 | 60 | 3.5 | — | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| e | 99 | 95 | 55 | 4 | 0.37 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| f | 92 | 88 | 60 | 4 | 0.36 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| g | — | — | 68 | — | — | — |
| h | 99 | 92 | 68 | 7 | 1.8 | 13.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| i | 90 | 78 | 65 | 12 | 1.14 | 6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| j | 75 | 69 | 60 | 6 | — | 11.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| k | 88 | 78 | 58 | 10 | 0.95 | 8.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| l | 93 | 72.5 | 65 | 20.5 | 3.1 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| m | 99 | 85 | 55 | 14 | — | 6.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| n | 97 | 84 | 55 | 13 | — | 6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| o | 97 | 79.5 | 55 | 17.5 | 5.2 | 5.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
On the other hand, maleimides 3k–n gave, strikingly, four cycloadducts as a result of the steric hindrance between the ortho substituent of the phenyl group and the carbonyl group of the maleimide moiety (Fig. 1) that forces phenyl and pyrrolidinedione rings to be tilted away (non-coplanar) to alleviate that steric hindrance.20 So, when the nitrone attacks the dipolarophile, whether the attack is endo or exo, the nitrone will find that the two faces are energetically different (divergent) and hence endo and exo atropisomers can be formed. The major endo or exo atropisomer is the product formed by attacking the nitrone through the dipolarophile face anti to the ortho substituent, where the transition state of the attack has lower energy (Fig. 2).
 | ||
| Fig. 1 (A) The restricted rotation in dipolarophiles 3k–n. (B) The steric hindrance between the ortho substituent and carbonyl group forces the two rings to be not coplanar. | ||
The above mentioned effect of single bond restricted rotation on the route of the cycloaddition was confirmed using 1H-NMR and COSY spectra (ESI†). 1H-NMR spectra of endo isomer 4 formed from cycloaddition using any of dipolarophiles 3a–j showed only three signals in the region 3.8–6.2 ppm, a doublet, a doublet and a singlet corresponding to H3a, H6a and H3, respectively. On the other hand, 1H-NMR spectra of the crude reaction products showed in that region, in addition to the signals corresponding to endo isomer 4, three other signals: a triplet, doublet and doublet corresponding to H3a, H3 and H6a, respectively. The new signals correspond to the exo isomer 5 as was confirmed by 1H-NMR spectra of the separated exo isomers (ESI†). The yield and isomer ratio were determined from 1H-NMR integrations; endo and exo isomers were formed in conversions of 69–95% and 3–20%, respectively (Table 1). The unexpectedly higher δ value of H3 than H6a for endo isomer was ascribed to the presence of H3 in the deshielding zone of the carbonyl group as is shown in the optimized structures of endo and exo isomers (Fig. 3). But H3 in exo isomer is far from this deshielding zone.
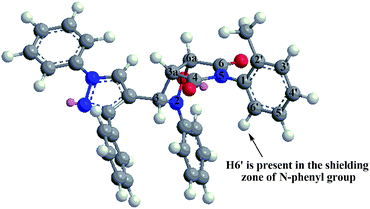
On the other hand, 1H-NMR spectra of endo atropisomers formed from the cycloaddition using any of the dipolarophiles 3k–n showed signals in the region 3.8–6.2 ppm corresponding to seven hydrogens. For example, the 1H-NMR spectrum of endo atropisomers (4k, 4′k) formed using dipolarophile 3k (ESI†) showed two doublets at 4.06 ppm (J 7.32 Hz) and 4.09 ppm (J 7.32 Hz) corresponding to H3a of major and minor endo atropisomers (4k, 4′k), respectively; two doublets at 5.17 ppm (J 7.32 Hz) and 5.22 ppm (J 7.32 Hz) corresponding to H6a of major and minor endo atropisomers (4k, 4′k), respectively; two singlets at 5.71 ppm and 6.06 ppm corresponding to H3 of minor endo and major endo atropisomers (4′k, 4k), respectively; and a doublet at 5.68 ppm (J 8.08 Hz) with integration similar to that of any hydrogen of major endo isomer 4k, the origin of this signal being assigned to H6′. The lower δ value of H6′ for 4k was ascribed to shielding effect induced by the pyrazole ring as deduced from the optimized structure (Fig. 4).
Furthermore, 1H-NMR spectra of the crude reaction product from the cycloaddition using any of dipolarophiles 3k–n were measured (ESI†) and showed signals in the region 3.8–6.2 ppm, in addition to the signals corresponding to seven hydrogens of two endo atropisomers (4, 4′), corresponding to 6 hydrogens of two exo atropisomers (5, 5′) as was confirmed from the 1H-NMR of the separated exo atropisomers. The ratio of endo and exo isomers was determined from the 1H-NMR integrations (Table 2).
| Entry | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4′a 4′a |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5′a 5′a |
||
|---|---|---|---|---|
| Totalb | Isolated | Totalb | Isolated | |
| a 4 and 4′ are major and minor endo atropisomers, respectively, and 5 and 5′ are major and minor exo atropisomers, respectively.b The isomer ratio was determined from 1H-NMR integrations (400 MHz). | ||||
| K | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— |
| L | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| M | 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— |
| N | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— |
Moreover, the effect of single bond restricted rotation on the route of the cycloaddition reaction was confirmed by using dipolarophile 3o, where the ortho substituent (o-fluorine atom) does not cause steric hindrance between the N-maleimide moiety and the carbonyl groups of the pyrrolidine ring. Endo cycloadduct 4o gave only three signals in the region 3.8–6.2 ppm: two doublets and a singlet. At the same time, the 1H-NMR spectrum of the crude reaction product showed, in the same region, only six signals corresponding to two cycloadducts.
Also, GC/mass chromatography (ESI†) confirmed the above mentioned results. The chromatogram obtained for the crude reaction products of the cycloaddition using dipolarophile 3f is simple (fewer peaks) than the chromatogram obtained for the crude reaction products of the cycloaddition using dipolarophile 3m (more peaks), and this confirms the formation of more products in the case of dipolarophile 3m than dipolarophile 3f confirming the occurrence of atropisomerism in the formed cycloadducts obtained by cycloaddition using dipolarophiles with ortho substituent (such as 3m).
Ultimately, non-coplanarity in dipolarophiles 3k–n was confirmed by comparing their UV-visible spectra (Fig. 5). Dipolarophiles with ortho substituents have lower λmax than the corresponding dipolarophiles having the substituent at meta or para position (Table 3).
 | ||
| Fig. 5 Absorption spectra of dipolarophiles with para or ortho substituents: (A) 3b and 3k, (B) 3f and 3m, (C) 3d and 3l, (D) 3h and 3n. | ||
| Dipolarophile | λmax (nm) | Dipolarophile | λmax (nm) |
|---|---|---|---|
| a | 316 | i | 298 |
| b | 320 | k | 298 |
| c | 285 | l | 274 |
| d | 316 | m | 262 |
| e | 314 | n | 288 |
| f | 346 | o | 300 |
| h | 278 |
The formation of atropisomers, as a result of restricted rotation around single bonds, was confirmed by density functional theory (DFT) calculations, which were performed using the Gaussian 09 package. The geometrical optimization and (C6–N5–C1′–C6′) dihedral angle scans of endo and exo cycloadducts, with methyl substitution at either ortho or para position, were performed using DFT at the B3LYP level.21 The solvent effect has been considered based on the polarizable continuum model.22 The solvent used in this calculation was toluene. The 6-311G(d,p) basis sets were employed for all atoms. Fig. 6 reveals the energy barriers for rotation around C–N bond of cycloadducts 4b, 5b, 4k and 5k. The presence of ortho substitution (4k, 5k) increases the energy barrier of rotation regardless of whether the cycloadduct is endo or exo. This confirms that the formation of separable atropisomeric cycloadducts of endo and exo cycloaddition duplicated the formed cycloadducts to be four rather two as in the cases of cycloaddition using dipolarophiles with free single bond rotation.
 | ||
| Fig. 6 DFT calculation of the energy barrier for the C–N rotation in endo and exo isomers with either para or ortho methyl substitution. | ||
Conclusions
We have reported the significant influence of restricted rotation in dipolarophiles on the route of cycloaddition leading to the formation of atropisomeric cycloadducts. Furthermore, this restricted rotation could induce facial selectivity of the addition where the major cycloadduct(s) was the one formed by cycloaddition at the face with less steric hindrance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their gratitude to Assiut University for providing financial support. Also gratefully acknowledged is Prof. R. M. Mahfouz, Professor of Inorganic Chemistry at Assiut University, Egypt for running the 13C-NMR analysis of this paper at King Saud University, Saudi Arabia.Notes and references
- L. I. Smith, Chem. Rev., 1938, 23, 193 CrossRef CAS; J. Hamer and A. Macaluso, Chem. Rev., 1964, 64, 473 CrossRef; O. Wolfgang, Angew. Chem., Int. Ed., 1977, 16, 10 CrossRef; A. Bøgevig, K. V. Gothelf and K. A. Jørgensen, Chem.–Eur. J., 2016, 8(24), 5652 CrossRef.
- R. Huisgen, Angew. Chem., Int. Ed., 1963, 2, 565 CrossRef CAS; S. C. Black, R. F. Crozier and V. C. Davis, Synthesis, 1975, 205 CrossRef; A. Padwa, Angew. Chem., Int. Ed., 1976, 15, 123 CrossRef; W. Opplzer, Angew. Chem., Int. Ed., 1977, 16, 10 CrossRef; C. P. Dell, J. Chem. Soc., Perkin Trans. 1, 1998, 3873 RSC; C. Bhaskar and R. Neelam, J. Heterocycl. Chem., 2018, 55, 1053 CrossRef.
- N. Morita, K. Fukui, J. Irikuchi, H. Sato, Y. Takano, I. Okamoto, H. Ishibashi and O. Tamura, J. Org. Chem., 2008, 73, 7164 CrossRef CAS PubMed; T. B. Nguyen, A. Martel, R. Dhal and G. Dujardin, Org. Lett., 2008, 10, 4493 CrossRef PubMed; P. Surya, Z. Zhe, W. Fang, R. Martin, N. Chuanfa, I. Marc, H. Ralf and A. O. George, Chem.–Eur. J., 2013, 20(3), 831 Search PubMed; R. A. Fernando, A. Amparo, S. R. Maria, E. Marcos, D. P. Carlos and F. Santos, J. Org. Chem., 2017, 82(5), 2505 CrossRef PubMed; M. Tong, Y. Zhang, C. Qin, Y. Fu, Y. Liu, H. Li and W. Wang, Org. Chem. Front., 2018, 5, 2945 RSC; P. La Manna, M. De Rosa, C. Talotta, C. Gaeta, A. Soriente, G. Floresta, A. Rescifin and P. Neri, Org. Chem. Front., 2018, 5, 827 RSC; Y. Yao, W. Yang, Q. Lin, W. Yang, H. Li, L. Wang, F. Gu and D. Yang, Org. Chem. Front., 2019, 6, 3360 RSC.
- C. Lu, A. V. Dubrovskiy and R. C. Larock, J. Org. Chem., 2012, 77, 2279 CrossRef CAS PubMed; S. Bhunia, C. J. Chang and R. S. Liu, Org. Lett., 2012, 14, 5522 CrossRef PubMed; C. S. Mckay, M. Chigrinova, J. A. Blake and J. P. Pezaccki, Org. Biomol. Chem., 2012, 10, 3066 RSC; Z. S. Qi, M. Wang and X. W. Li, Org. Lett., 2013, 15, 5440 CrossRef; R. P. Temming, L. Eggermont, M. B. van Eldijk, J. C. M. van Hest and F. L. van Delft, Org. Biomol. Chem., 2013, 11, 2772 RSC; A. M. Douglas and P. P. John, Can. J. Chem., 2014, 92(4), 337 CrossRef; S. S. Prasad and S. Baskaran, J. Org. Chem., 2018, 83(3), 1558 CrossRef.
- A. Padwa, M. Matzinger, Y. Tomioka and M. K. Venkatramanan, J. Org. Chem., 1988, 53, 955 CrossRef CAS; X. Wu, R. S. Na, H. L. Liu, J. Liu, M. Wang, J. C. Zhong and H. C. Guo, Tetrahedron Lett., 2012, 53, 342 CrossRef; J. Malinina, T. Q. Tran, A. V. Stepakov, V. V. Gurzhiy, G. L. Starova, R. R. Kostikov and A. P. Molchanov, Tetrahedron Lett., 2014, 55, 3663 CrossRef; S. B. Joyann, D. S. Evan, V. P. Hung, C. M. Travis, K. N. Houk and K. G. Neil, J. Am. Chem. Soc., 2016, 138(8), 2512 CrossRef PubMed; M. A. Kroc, A. Prajapati, D. J Wink and L. L. Anderson, J. Org. Chem., 2018, 83(3), 1085 CrossRef PubMed.
- N. Murai, M. Komatsu, Y. Ohshiro and T. Agawa, J. Org. Chem., 1977, 42, 448 CrossRef CAS; K. Namitharan and K. Pitchumani, Org. Lett., 2011, 13, 5728 CrossRef PubMed; S. Joyann, E. D. Barber, H. V. Styduhar, T. C. Pham, N. McMahonK and N. K. Houkand, J. Am. Chem. Soc., 2016, 138(8), 2512 CrossRef PubMed.
- K. R. R. Kumar, H. Mallesha and K. S. Rangappa, Synth. Commun., 2003, 33, 1545 CrossRef CAS; D. G. Piotrowska, J. Balzarini and I. E. Glowacka, Eur. J. Med. Chem., 2012, 47, 501 CrossRef PubMed; A. R. Minter, B. B. Brennan and A. K. Mapp, J. Am. Chem. Soc., 2004, 126, 10504 CrossRef PubMed; T. Yurie, I. Takaoki, O. Hirohisa, I. Tomohiro, C. S. Martin, M. Seiji, T. Tohru, M. Kenji and S. Masaya, Chem.–Eur. J., 2017, 23(35), 8400 CrossRef PubMed.
- B. Alberto, C. Francesca, C. Stefano, M. C. Franca and G. Andrea, Chem.–Eur. J., 2009, 15(32), 7808 CrossRef CAS PubMed; J. Hoogenboom, H. Zuilhof and T. Wennekes, Org. Lett., 2015, 17, 5550 CrossRef PubMed.
- A. Kaïss, M. Moncef and P. Jean-Pierre, Tetrahedron, 2012, 68, 1762 CrossRef CAS; S. S. Bhella, A. P. S. Pannu, M. Elango, A. Kapoor, M. S. Hundal and M. P. S. Ishar, Tetrahedron, 2009, 65, 5928 CrossRef; M. S. Kirillova, F. M. Miloserdov and A. M. Echavarren, Org. Chem. Front., 2018, 5, 273 RSC; J. Zhong, H. He and S. Gao, Org. Chem. Front., 2019, 6, 3781 RSC.
- A. N. Kost and I. I. Grandberg, Adv. Heterocycl. Chem., 1966, 6, 347 CrossRef CAS; M. H. Elnagdi, G. E. H. Elgemeie and F. A. E. Abd-Elall, Heterocycles, 1985, 23, 3121 CrossRef; P. G. Baradi, B. Cacciari, R. Romagnoli and G. Spalluto, Synthesis, 1999, 3, 453 CrossRef; T. I. El-Emary, A. M. Hussein and H. S. El-Kashef, Pharmazie, 2000, 55, 356 Search PubMed; T. I. El-Emary, A. Khalil, G. A. M. El-Hag and A. A. A. El-Adasy, Phosphorus, Sulfur, Silicon Relat. Elem., 2005, 180, 19 CrossRef; F. Xue, H. Lu, L. He, W. Li, D. Zhang, X. Liu and Y. Qin, J. Org. Chem., 2018, 83(2), 754 CrossRef.
- K. N. Houk, J. Am. Chem. Soc., 1984, 106, 3880 CrossRef CAS; J. Liu, S. Niwayama, Y. You and K. N. Houk, J. Org. Chem., 1998, 63, 1064 CrossRef; P. Merino, T. Tejero and A. Díez-Martínez, J. Org. Chem., 2014, 79(5), 2189 CrossRef; P. Merino, M. A. Chiacchio, L. Legnani, I. Delso and T. Tejero, Org. Chem. Front., 2017, 4, 154 RSC; L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, J. Org. Chem., 2018, 83(4), 2182 CrossRef PubMed.
- R. Gandolfi, M. Ratti, L. Toma and C. De Micheli, Heterocycles, 1979, 12, 897 CrossRef CAS; E. L. Eliel, and S. H. Wilen, Stereochemistry of Organic Compounds, Wiley& Sons, New York, 1994, ch. 14 CrossRef; P. C. Dennis, Q. Hongyan, J. G. Steven and C. D. Nicholas, J. Am. Chem. Soc., 1994, 116(7), 3131 CrossRef; S. Muthuramalingam, J. A. Garg, R. Karthick, T. Fox, O. Blacque, K. Venkatesan, S. Ramanathan, S. Kabilan and K. K. Balasubramanian, Org. Chem. Front., 2019, 6, 134 RSC.
- E. Kumarasamy, R. Raghunathan, M. P. Sibi and J. Sivaguru, Chem. Rev., 2015, 115, 11239 CrossRef CAS; B. S. L. Collins, J. C. M. Kistemaker, E. Otten and B. L. Feringa, Nat. Chem., 2016, 8, 860 CrossRef.
- N. J. Beattie, C. L. Francis, A. J. Liepa and G. P. Savage, Aust. J. Chem., 2010, 63, 445 CrossRef CAS; A. M. Said and G. P. Savage, J. Org. Chem., 2011, 76(11), 6946 CrossRef PubMed; S. L. Harding and G. P. Savage, Org. Biomol. Chem., 2012, 10(24), 4759 RSC.
- M. A. Kira, M. O. Abdel-Rehmaan and K. Z. Gadalla, Tetrahedron Lett., 1969, 10, 109 CrossRef.
- A. I. Vogel, in Practical Organic Chemistry, Longmans, London, 3rd edn, 1974, p. 628 Search PubMed.
- S. K. Mohamed, J. T. Mague, M. Akkurt, A. I. Said, F. E. Hawaiz and S. M. I. Elgarhy, IUCrData, 2018, 3, x180208, DOI:10.1107/S2414314618002080.
- D. X. Hu, P. Grice and S. V. Ley, J. Org. Chem., 2012, 77, 5198 CrossRef CAS PubMed.
- T. H. Lowry, and K. S. Richardson, Mechanism and Theory in Organic Chemistry, Harper Collins, New York, 3rd edn, 1987, p. 925 CrossRef; J. March, Advanced Organic Chemistry-Reaction, Mechanisms and Structure, Wiley, New York, 4th edn, 1992, p. 851 CrossRef; A. Ana and F. P. Cossıo, J. Org. Chem., 2001, 66, 6178 CrossRef.
- M. V. Shiva and B. S. Nripendra, Aust. J. Chem., 1976, 29, 295 CrossRef; P. C. Dennis, G. Steven and D. Nicholas, Tetrahedron, 1999, 55, 5681 CrossRef.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev., 1988, 37, 785 Search PubMed; A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327 CrossRef CAS; V. Barone, M. Cossi and J. Tomasi, J. Comput. Chem., 1998, 19, 404 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1821805. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra10039c |
| This journal is © The Royal Society of Chemistry 2020 |