Open Access Article
Open Access ArticleSynthesis of the pentasaccharide repeating unit of the O-antigen from Enterobacter cloacae C4115 containing the rare α-D-FucNAc†
Aritra Chaudhury and
Balaram Mukhopadhyay *
*
Sweet Lab, Department of Chemical Sciences, Indian Institute of Science Education and Research Kolkata, Mohanpur, Nadia 741246, India. E-mail: sugarnet73@hotmail.com
First published on 30th January 2020
Abstract
Total synthesis of the pentasaccharide repeating unit associated with the O-antigen of Enterobacter cloacae C4115 is reported. The synthesis of the said oligosaccharide was accomplished through rational protecting group manipulations on commercially available monosaccharides followed by stereoselective glycosylations either by activation of thioglycosides or glycosyl trichloroacetimidates and was found to be productive. Towards the synthesis of the rare sugar unit, α-D-FucNAc in this case, it was established that the methoxymethyl (MOM) group is advantageous over the earlier reported tetrahydro pyran (THP) protection. The effect of MOM-protection was successfully tested for the synthesis of a rare sugar synthon which can serve as a precursor to the rare D-fucosamine residue.
Introduction
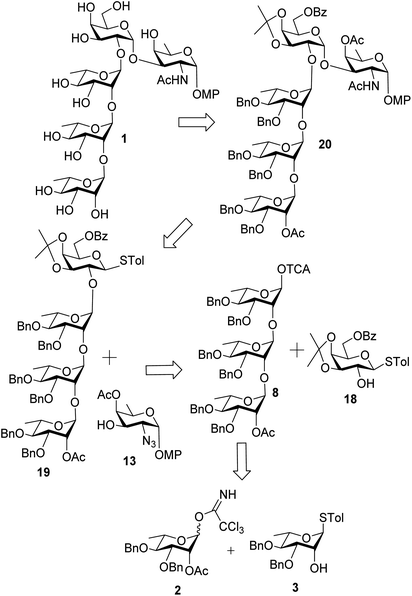
Enterobacter cloacae belong to the genus Enterobacter and are Gram negative anaerobic bacteria of the Enterobacteriaceae family. These bacterial strains have been recognized as nosocomial pathogens affecting immune-compromised patients.1 The cell surface polysaccharide profile of disease causing bacteria is deeply implicated in their pathogenicity.2 The E. cloacae are known for their resistance towards various classes of antibiotics like β-lactam, fluoroquinolones, aminoglycosides and tigecyclin.3 Recently, Knirel et al. have reported the structure of the O-antigen of Enterobacter cloacae C4115 as illustrated below (Fig. 1).4 Considering the growing need for robust and practical strategies to deal with the synthesis of bacterial cell surface oligosaccharides bearing rare amino deoxy sugar residues5 we took up the challenge of developing a concise strategy for the synthesis of this pentasaccharide. Taking a cue from the recent reports directed towards synthesis of rare sugar derivatives,6,7 herein we report the first total synthesis of the aforesaid pentasaccharide in the form of its p-methoxyphenyl glycoside (1, Fig. 1).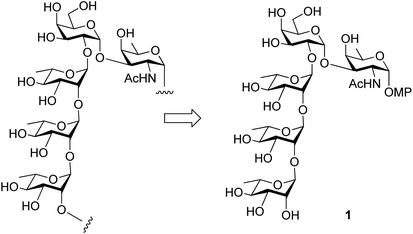 | ||
| Fig. 1 Pentasaccharide repeating unit associated with the O-antigen of Enterobacter cloacae C4115 and the synthetic target (1). | ||
Results and discussion
The challenging aspect associated with the synthesis of this target pentasaccharide is the terminal α-D-fucosamine residue at the reducing end. The α-(1→2) linked L-rhamnose motif is a fairly common oligosaccharide architecture which has been synthesised by our group previously.8,9 This was selected as the first point of retrosynthetic disconnection in contemplation of a (3+1+1) convergent strategy for oligosaccharide assembly.For the non-reducing end α-L-Rhap-(1→2)-α-L-Rhap-(1→2)-α-L-Rhap trisaccharide, iterative glycosylation of the same rhamnosyl trichloroacetimidate donor twice with the rhamnose thioglycoside acceptor was planned. On the other hand, the galactosyl thioglycoside donor bearing non-participating naphthyl protection at O-2 position and remotely participating 6-O-benzoyl protection was envisioned to give the 1,2-cis glycosidic linkage with the D-fucosamine equivalent at its O-3 position. But prior to this, an efficient protocol had to be developed for the synthesis of the D-fucosamine equivalent.
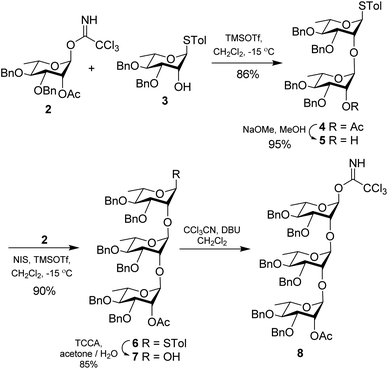
Synthesis of the trisaccharide fragment (Fig. 2) was commenced with the glycosylation of known rhamnosyl donor 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and acceptor 3
10 and acceptor 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 in the presence of TMSOTf to give the disaccharide 4 in 86% yield. De-O-acetylation of 4 under Zemplén conditions12 led to the disaccharide acceptor 5 in 95% yield. Further it was coupled with the donor 2 once again to give the trisaccharide fragment 6 in 90% yield. The α-stereochemistry of the three L-rhamnoside residues were confirmed by their corresponding JC1–H1 coupling constants which were measured at 172.08 Hz, 170.32 Hz and 167.00 Hz respectively.13 Trisaccharide 6 was converted to its corresponding hemi-acetal derivative 7 using trichloroisocyanuric acid (TCCA) in acetone/H2O14 in 85% yield. It was subsequently converted to the corresponding glycosyl trichloroacemidate 8 in 95% yield by treatment with trichloroacetonitrile in the presence of DBU15 (Scheme 1).
11 in the presence of TMSOTf to give the disaccharide 4 in 86% yield. De-O-acetylation of 4 under Zemplén conditions12 led to the disaccharide acceptor 5 in 95% yield. Further it was coupled with the donor 2 once again to give the trisaccharide fragment 6 in 90% yield. The α-stereochemistry of the three L-rhamnoside residues were confirmed by their corresponding JC1–H1 coupling constants which were measured at 172.08 Hz, 170.32 Hz and 167.00 Hz respectively.13 Trisaccharide 6 was converted to its corresponding hemi-acetal derivative 7 using trichloroisocyanuric acid (TCCA) in acetone/H2O14 in 85% yield. It was subsequently converted to the corresponding glycosyl trichloroacemidate 8 in 95% yield by treatment with trichloroacetonitrile in the presence of DBU15 (Scheme 1).
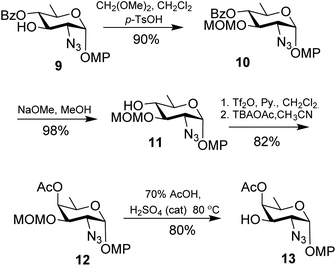
Synthesis of the reducing end residue (Fig. 2) required the development of the D-fucosamine equivalent and we have adopted the method reported by Ghosh et al.16 The synthesis began with di-tert-butylperoxide (DTBP) and tri-isopropylsilanethiol (TIPST) mediated deoxygenation17 leading to the C-6 deoxygenated derivative 9.16 Hereafter, we deviated from the aforesaid protocol as we found the methoxymethyl (MOM) protecting group as a suitable alternative for the tetrahydropyranyl (THP) protection used previously for O-3 protection. Accordingly, compound 9 was converted to 10 in 90% yield using dimethoxymethane in the presence of p-TsOH.18 Subsequent de-benzoylation at O-4 led to intermediate 11 from which epimerisation via Lattrell–Dax inversion19,20 led to the rare sugar derivative 12 in 82% yield over two steps. De-protection of the methoxymethyl group using 70% (aq.) acetic acid in the presence of catalytic conc. H2SO4 at 80 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 gave the desired acceptor 13 in 80% yield (Scheme 2).
21 gave the desired acceptor 13 in 80% yield (Scheme 2).
In their report, Ghosh et al.16 have used the THP group for orthogonal protection of the O-3 position of the glucosamine derivative. However, substantial acid lability of THP protection22 limits its application in glycosylation reaction. Moreover, introduction of THP inevitably leads to the creation of an added asymmetric centre at the C-1 position of the THP group that in turn can make the spectral characterization cumbersome.23
To overcome these issues, we have used MOM protection in place of THP and found that it has little effect on the yield but makes the protocol operationally simpler.
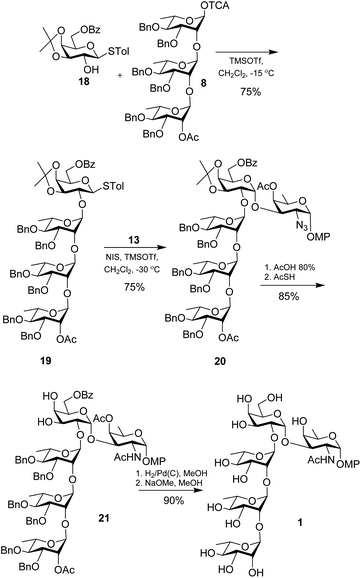
Having the D-fucosamine acceptor 13 in hand we turned our attention towards the galactosyl donor. For the required 1,2-cis glycosylation a non-participating temporary protection at 2-position was desired. However, non-orthogonality with the azido group ruled out the option of a benzyl protection. Therefore, known galactose derivative 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 was subjected to a phase transfer reaction with (2-bromomethyl)naphthalene (NapBr) in the presence of Bu4NBr and 10% aq. NaOH to afford the 2-O-napthylmethyl galactoside 15 which was further acetylated using Ac2O in pyridine to furnish the desired galactosyl donor 16. Unfortunately, glycosylation between galactosyl donor 16 and acceptor 13 through activation of thioglycoside using NIS in the presence TMSOTf failed to provide the disaccharide. Instead, it led to an undesired derivative 17 formed via intra-molecular C-glycosidation25–27 (see Scheme S1 in ESI†).
24 was subjected to a phase transfer reaction with (2-bromomethyl)naphthalene (NapBr) in the presence of Bu4NBr and 10% aq. NaOH to afford the 2-O-napthylmethyl galactoside 15 which was further acetylated using Ac2O in pyridine to furnish the desired galactosyl donor 16. Unfortunately, glycosylation between galactosyl donor 16 and acceptor 13 through activation of thioglycoside using NIS in the presence TMSOTf failed to provide the disaccharide. Instead, it led to an undesired derivative 17 formed via intra-molecular C-glycosidation25–27 (see Scheme S1 in ESI†).
In resort, the known galactoside 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 was glycosylated with trisaccharide trichloroacetimidate donor 8 using TMSOTf giving the tetrasaccharide 19 in 75% yield. Further glycosylation of the tetrasaccharide 19 through activation of thioglycoside using NIS in the presence of TMSOTf furnished the protected pentasaccharide derivative 20 in 75% yield. It is worth noting that in either of the two aforesaid glycosylation reactions we were unable to detect the corresponding β-isomer. Hydrolysis of the isopropylidene group from the pentasaccharide 20 using 80% AcOH at 80 °C
28 was glycosylated with trisaccharide trichloroacetimidate donor 8 using TMSOTf giving the tetrasaccharide 19 in 75% yield. Further glycosylation of the tetrasaccharide 19 through activation of thioglycoside using NIS in the presence of TMSOTf furnished the protected pentasaccharide derivative 20 in 75% yield. It is worth noting that in either of the two aforesaid glycosylation reactions we were unable to detect the corresponding β-isomer. Hydrolysis of the isopropylidene group from the pentasaccharide 20 using 80% AcOH at 80 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 followed by reaction with thioacetic acid to convert the azide group to desired acetamido30 led to compound 21 in 85% yield over 2 steps. Finally, catalytic hydrogenolysis using H2 in the presence of 10% Pd–C31 followed by de-O-acylation under Zemplén conditions12 furnished the target pentasaccharide 1 in 90% yield (Scheme 3).
29 followed by reaction with thioacetic acid to convert the azide group to desired acetamido30 led to compound 21 in 85% yield over 2 steps. Finally, catalytic hydrogenolysis using H2 in the presence of 10% Pd–C31 followed by de-O-acylation under Zemplén conditions12 furnished the target pentasaccharide 1 in 90% yield (Scheme 3).
Conclusions
In conclusion, total synthesis of the pentasaccharide repeating unit of the O-antigen from E. cloacae C4115 is accomplished through a bidirectional glycosylation strategy. Synthetic equivalent of the challenging rare sugar D-FucNAc was derived through improved strategy. The MOM protecting group was shown to be a practical alternative to the previously reported THP protection which was crucial for the efficient synthesis the derivative 13 has been utilized successfully in this particular total synthesis.Experimental section
General
All solvents and reagents were dried prior to use according to literature methods.32 The commercially purchased reagents were used without any further purification unless mentioned otherwise. Dichloromethane was dried and distilled over P2O5 to make it anhydrous and moisture-free. All reactions were monitored by Thin Layer Chromatography (TLC) on silica-gel 60-F254 with detection via fluorescence and by charring after immersion in 10% ethanolic solution of sulphuric acid. Flash chromatography was performed with silica gel 230–400 mesh. Optical rotations were measured on sodium D-line at ambient temperature. 1H and 13C NMR were recorded on Bruker Avance 500 MHz spectrometer at 500 MHz and 125 MHz respectively.p-Tolyl 2-O-acetyl-3′,4′-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-1-thio-α-L-rhamnopyranoside [4]
Trichloroacetimidate donor 2 (430 mg, 0.81 mmol) and acceptor 3 (280 mg, 0.81 mmol) were dissolved in CH2Cl2 (15 mL) and stirred with 4 Å MS (1.5 g) for 15 minutes under N2 atmosphere. Thereafter the temperature was lowered to −15 °C and TMSOTf (0.03 mL, 0.16 mmol) was added and the reaction was allowed to continue for 30 minutes. TLC (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.6) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (100 mL) and brine (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (6
1 n-hexane/EtOAc, Rf = 0.6) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (100 mL) and brine (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give the disaccharide product 4 (570 mg, 86%) as colourless foam.
1 n-hexane/EtOAc) to give the disaccharide product 4 (570 mg, 86%) as colourless foam.
[α]25D +28 (c 1.0, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 7.37–7.08 (m, 24H, ArH), 5.51 (s, 1H, H-2′), 5.34 (s, 1H, H-1), 4.94 (s, 1H, H-1′), 4.89 (t, 2H, J = 10.5 Hz, Ph-CH2), 4.74–4.53 (m, 6H, Ph-CH2), 4.16 (s, 1H, H-2), 4.12–4.08 (m, 1H, H-5), 3.93 (dd, 1H, J = 2.5 Hz 9.5 Hz, H-3′), 3.85 (dd, 1H, J = 2.0 Hz 9.0 Hz, H-3), 3.80–3.77 (m, 1H, H-5′) 3.47 (t, 1H, J = 9.0 Hz, H-4), 3.40 (t, 1H, J = 9.5 Hz, H-4′), 2.31 (s, 3H, Ar-CH3), 2.13 (s, 3H, COCH3), 1.28 (d, 3H, J = 6.0 Hz, H-6), 1.22 (d, 3H, J = 6.0 Hz, H-6′).
13C NMR (CDCl3, 125 MHz) δ: 170.1 (CO), 138.4, 138.1, 138.0, 137.5, 131.9, 129.8, 128.5, 128.4, 128.3 (Ar-C), 99.5 (C-1′), 87.6 (C-1), 80.2 (C-4), 80.0 (C-4′), 79.9 (C-3), 77.6 (C-3′), 76.6 (C-2), 75.4 (Ph-CH2) 72.2 (Ph-CH2), 71.8 (Ph-CH2), 69.3 (C-5), 68.9 (C-2′), 68.4 (C-5′), 21.1 (Ar-CH3), 21.0 (COCH3), 17.9 (C-6), 17.8 (C-6′).
HRMS calculated for C49H54O9SNa (M + Na)+: 841.3386, found: 841.3378.
p-Tolyl 3′,4′-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-1-thio-α-L-rhamnopyranoside [5]
Disaccharide 4 (570 mg, 0.7 mmol) was stirred in NaOMe/MeOH (20 mL, 0.05 M) at room temperature for 2 hours. TLC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.35) at this point showed the reaction to be complete and the reaction mixture was quenched with DOWEX 50W resin. The resin was filtered out and the filtrate was evaporated to dryness under reduced pressure to give the crude residue which was purified by column chromatography (5
1 n-hexane/EtOAc, Rf = 0.35) at this point showed the reaction to be complete and the reaction mixture was quenched with DOWEX 50W resin. The resin was filtered out and the filtrate was evaporated to dryness under reduced pressure to give the crude residue which was purified by column chromatography (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give the disaccharide acceptor 5 (540 mg) in 95% yield.
1 n-hexane/EtOAc) to give the disaccharide acceptor 5 (540 mg) in 95% yield.
[α]25D +57 (c 0.9, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 7.41–7.30 (m, 22H, Ar-H), 7.12 (d, 1H, J = 7.5 Hz, Ar-H), 5.41 (d, 1H, J = 1.5 Hz, H-1′), 5.07 (d, 1H, J = 1.5 Hz, H-1), 4.94–4.64 (m, 8H, PhCH2), 4.2–4.23 (m, 1H, H-2′), 4.17–4.14 (m, 2H, H-2, H-5), 3.89 (dd, 1H, J = 3.0 Hz, 10.0 Hz, H-3′, H-3), 3.82 (m, 1H, H-5′), 3.49 (m, 2H, H-4, H-4′), 2.34 (s, 3H, ArCH3), 1.34 (d, 3H, J = 6.0 Hz, H-6), 1.25 (d, 3H, J = 6.5 Hz, H-6′).
13C NMR (CDCl3, 125 MHz) δ: 138.4, 138.3, 137.9, 137.9, 137.4, 131.8, 130.6, 129.8, 128.4, 128.3, 128.1, 128.0, 127.9, 127.9, 127.8, 127.6, 127.6 (ArC), 100.9 (C-1′), 87.7 (C-1), 80.4 (C-4), 79.9 (C-4′), 79.9 (C-3), 79.5 (C-3′), 76.6 (C-2′), 75.3 (PhCH2), 75.2 (PhCH2), 72.3 (PhCH2), 72.1 (PhCH2), 69.2 (C-2), 68.7 (C-5), 68.0 (C-5′), 21.0 (ArCH3), 17.9 (C-6), 17.7 (C-6′).
HRMS calculated for C47H52O8SNa (M + Na)+: 799.3281, found: 799.3274.
p-Tolyl 2′′-O-acetyl-3′′,4′′-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3′,4′-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-1-thio-α-L-rhamnopyranoside [6]
Trichloroacetimidate donor 2 (600 mg, 0.94 mmol) and disaccharide acceptor 5 (540 mg, 0.69 mmol) were dissolved in CH2Cl2 (20 mL) and stirred with 4 Å MS (2 g) for 15 minutes under N2 atmosphere. Thereafter the temperature was lowered to −15 °C and TMSOTf (0.04 mL, 0.18 mmol) was added and the reaction was allowed to continue for 1 hour. TLC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.5) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (100 mL) and brine (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (6
1 n-hexane/EtOAc, Rf = 0.5) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (100 mL) and brine (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give the trisaccharide product 6 (800 mg, 90%) as white foam.
1 n-hexane/EtOAc) to give the trisaccharide product 6 (800 mg, 90%) as white foam.
[α]25D +44 (c 0.8, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 7.40–7.26 (m, 32H, ArH), 7.13–7.11 (m, 2H, ArH), 5.58 (s, 1H, H-2′′), 5.36 (s, 1H, H-1), 5.07 (s, 1H, H-1′), 5.05 (s, 1H, H-1′′), 4.95–4.89 (m, 3H, PhCH2), 4.79–4.56 (m, 9H, PhCH2), 4.17–4.10 (m, 3H, H-2, H-5, H-2′), 4.16 (dd, 1H, J = 9.0 Hz, 3.0 Hz, H-3′′), 3.91–3.85 (m, 3H, H-3, H-3′, H-5′), 3.77–3.74 (m, 1H, H-5′′), 3.49–3.43 (m, 3H, H-4, H-4′, H-4′′), 2.34 (s, 3H, ArCH3), 2.17 (s, 3H, COCH3), 1.31 (d, 3H, J = 6.0 Hz, H-6), 1.29 (d, 3H, J = 6.0 Hz, H-6′), 1.22 (d, 3H, J = 6.0 Hz, H-6′′).
13C NMR (CDCl3, 125 MHz) δ: 170.0 (CO), 138.5–127.5 (Ar-C), 100.6 (C-1′), 99.0 (C-1′′), 87.7 (C-1), 80.4 (C-4), 80.0 (C-4′, C-4′′), 79.6 (C-3), 79.0 (C-3′), 77.7 (C-3′′), 76.4 (C-2), 75.4 (PhCH2), 75.3 (PhCH2), 75.2 (PhCH2), 74.5 (C-5), 72.2 (PhCH2), 72.1 (PhCH2), 71.8 (PhCH2), 69.3 (C-2′), 68.9 (C-2′′), 68.6 (C-5′′), 68.3 (C-5′), 21.1 (Ar-CH3), 21.0 (COCH3), 17.9 (C-6 × 2), 17.8 (C-6).
HRMS calculated for C69H76O13SNa (M + Na)+: 1167.4904, found: 1167.4899.
p-Methoxyphenyl 2-azido-4-O-benzoyl-2,6-dideoxy-3-O-methoxymethyl-α-D-glucopyranoside [10]
To a solution of the substrate 9 (400 mg, 1.04 mmol) dissolved in CH2Cl2 (20 mL), dimethoxymethane (0.16 mL, 2.1 mmol) and p-TSA (40 mg, 0.2 mmol) were added and the reaction mixture was refluxed for 12 hours. The reaction mixture was quenched with Et3N (0.1 mL) and the solvent was concentrated under reduced pressure. The crude product was purified by column chromatography (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.4) to give the pure product 10 (412 mg) in 90% yield as a light yellow oil.
1 n-hexane/EtOAc, Rf = 0.4) to give the pure product 10 (412 mg) in 90% yield as a light yellow oil.
[α]25D +104 (c 1.1, CHCl3).
1H NMR (CDCl3, 500 MHz) δ 8.09–6.85 (m, 9H, ArH), 5.47 (d, 1H, J = 3.5 Hz, H-1), 5.15 (t, 1H, J = 9.5 Hz, H-4), 4.79 (d, 1H, J = 7.0 Hz, MOM-CH2), 4.75 (d, 1H, J = 7.0 Hz, MOM-CH2), 4.38 (t, 1H, J = 9.5 Hz, H-3), 4.18–4.13 (m, 1H, H-5), 3.78 (s, 3H, Ar-OCH3), 3.44 (dd, 1H, J = 10.5 Hz, 3.5 Hz, H-2), 3.26 (s, 3H, MOM-OCH3), 1.22 (d, 3H, J = 6.5 Hz, H-6).
13C NMR (CDCl3, 125 MHz) δ: 165.4 (CO), 155.3, 150.4, 133.3, 129.7, 129.4, 128.4, 117.8, 114.6 (ArC), 97.8 (C-1), 97.6 (MOM-CH2), 75.5 (C-3), 75.4 (C-4), 66.5 (C-5), 62.7 (C-2), 55.8 (Ar-OCH3), 55.6 (MOM-OCH3), 17.3 (C-6).
HRMS calculated for C22H25N3O7Na (M + Na)+: 466.1590, found: 466.1584.
p-Methoxyphenyl 2-azido-2,6-dideoxy-3-O-methoxymethyl-α-D-glucopyranoside [11]
The substrate 10 (412 mg, 1.0 mmol) was treated with NaOMe (0.05 M) in methanol (20 mL) and the reaction mixture was stirred at room temperature for 8 hours. The reaction was complete by this time as shown by TLC (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.4) and it was then quenched with DOWEX 50W H+ resin. The reaction mixture was filtered and the methanol was evaporated to dryness under vacuum. The crude residue was purified by column chromatography to give the product as a white foam 11 (305 mg) which was purified by column chromatography (3
1 n-hexane/EtOAc, Rf = 0.4) and it was then quenched with DOWEX 50W H+ resin. The reaction mixture was filtered and the methanol was evaporated to dryness under vacuum. The crude residue was purified by column chromatography to give the product as a white foam 11 (305 mg) which was purified by column chromatography (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) and obtained in 98% yield.
1 n-hexane/EtOAc) and obtained in 98% yield.
[α]25D +97 (c 1.0, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 7.03 (d, 2H, J = 9.0 Hz, ArH), 6.83 (d, 2H, J = 9.0 Hz, ArH), 5.35 (d, 1H, J = 3.5 Hz, H-1), 4.90 (d, 1H, J = 7.0 Hz, MOM-CH2), 4.86 (d, 1H, J = 7.0 Hz, MOM-CH2), 4.42 (s, 1H, 4-OH), 3.93–3.87 (m, 2H, H-3, H-5), 3.77 (s, 3H, OMP-OCH3), 3.52 (s, 3H, MOM-OCH3), 3.42 (dd, 1H, J = 3.5 Hz, 10.5 Hz, H-2), 3.25 (d, 1H, J = 9.0 Hz, H-4), 1.30 (d, 3H, J = 6.0 Hz, H-6).
13C NMR (CDCl3, 125 MHz) δ: 155.3, 150.5, 118.1, 114.6, 98.3 (MOM-CH2), 97.4 (C-1), 83.2 (C-3), 74.9 (C-4), 68.3 (C-5), 61.8 (C-2), 56.1 (OMP-OCH3), 55.6 (MOM-OCH3), 17.7 (C-6).
HRMS calculated for C15H21N3O6Na (M + Na)+: 362.1328, found: 362.1327.
p-Methoxyphenyl 4-O-acetyl-2-azido-2,6-dideoxy-3-O-methoxymethyl-α-D-glucopyranoside [12]
To a solution of the substrate 11 (305 mg, 0.94 mmol) dissolved in CH2Cl2 (15 mL) and pyridine (7.5 mmol) was added. The temperature was lowered to 0 °C. Triflic anhydride (0.5 mL, 3.0 mmol) was added and then the temperature was raised. The reaction mixture was stirred at room temperature for 2 hours. The reaction mixture was diluted with CH2Cl2 (30 mL) and the combined organic layer was washed with ice-cold HCl (5% aq., 100 mL). The organic layer was collected and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude triflate which was carried forward to the next step without further purification.The crude triflate was dissolved in CH3CN (15 mL) and TBAOAc (427 mg, 1.4 mmol) was added and the reaction mixture was stirred at room temperature for 2.5 hours when TLC (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.7) showed complete conversion. Thereafter the solvent was concentrated under reduced pressure and the crude residue was dissolved in CH2Cl2 (30 mL) and washed with water (100 mL). The organic layer was collected and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the product 12 (285 mg) and was purified by column chromatography (3
1 n-hexane/EtOAc, Rf = 0.7) showed complete conversion. Thereafter the solvent was concentrated under reduced pressure and the crude residue was dissolved in CH2Cl2 (30 mL) and washed with water (100 mL). The organic layer was collected and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the product 12 (285 mg) and was purified by column chromatography (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) and obtained in 82% yield over 2 steps as a white foam.
1 n-hexane/EtOAc) and obtained in 82% yield over 2 steps as a white foam.
[α]25D +112 (c 0.9, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 7.03 (d, 2H, J = 8.5 Hz, ArH), 6.83 (d, 2H, J = 8.5 Hz, ArH), 5.47 (d, 1H, J = 3.5 Hz, H-1), 5.36 (d, 1H, J = 2.5 Hz, H-4), 4.85 (d, 1H, J = 7.0 Hz, MOM-CH2), 4.65 (d, 1H, J = 7.0 Hz, MOM-CH2), 4.39 (dd, 1H, J = 3.0 Hz, 11.5 Hz, H-3), 4.22 (m, 1H, H-5), 3.77 (s, 3H, OMP-OCH3), 3.66 (dd, 1H, J = 3.5 Hz 11.0 Hz, H-2), 3.48 (s, 3H, MOM-OCH3), 2.18 (s, 3H, CH3), 1.13 (d, 3H, J = 6.5 Hz, H-6).
13C NMR (CDCl3, 125 MHz) δ: 170.5 (CO), 155.3, 150.5, 117.8, 114.6, 97.9 (C-1), 94.9 (MOM-CH2), 70.7 (C-3), 70.3 (C-4), 65.7 (C-5), 58.8 (C-2), 56.1 (MOM-OCH3), 55.6 (OMP-OCH3), 20.7 (CH3), 16.1 (C-6).
HRMS calculated for C17H23N3O7Na (M + Na)+: 404.1434, found: 404.1429.
p-Methoxyphenyl 4-O-acetyl-2-azido-2,6-dideoxy-α-D-glucopyranoside [13]
Compound 12 (230 mg, 0.60 mmol) was suspended in 70% aq. AcOH (20 mL) and H2SO4 (conc.) was added in catalytic amount. The reaction mixture was heated at 80 °C for 8 hours when TLC (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.5) showed complete conversion. The acetic acid was removed under reduced pressure and the crude residue was purified by column chromatography (2.5
1 n-hexane/EtOAc, Rf = 0.5) showed complete conversion. The acetic acid was removed under reduced pressure and the crude residue was purified by column chromatography (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to obtain compound 13 (168 mg, 80%) as a white foam.
1 n-hexane/EtOAc) to obtain compound 13 (168 mg, 80%) as a white foam.
[α]25D +76 (c 1.0, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 7.03 (d, 2H, J = 8.5 Hz, ArH), 6.84 (d, 2H, J = 8.5 Hz, ArH), 5.45 (d, 1H, J = 3.5 Hz, H-1), 5.27 (m, 1H, H-4), 4.48 (dd, 1H, J = 3.0 Hz, 11.5 Hz, H-3), 4.28–4.24 (m, 1H, H-5), 3.77 (s, 3H, CH3), 3.66 (dd, 1H, J = 3.5 Hz 10.5 Hz, H-2), 2.21 (s, 3H, CH3), 1.13 (d, 3H, J = 6.5 Hz, H-6).
13C NMR (CDCl3, 125 MHz) δ: 171.5 (CO), 155.4, 150.7, 117.9, 114.7, 98.0 (C-1), 73.1 (C-4), 67.3 (C-3), 65.7 (C-5), 60.0 (C-2), 55.6 (OMP-OCH3), 20.8 (COCH3), 16.1 (C-6).
HRMS calculated for C15H19N3O6Na (M + Na)+: 360.1172, found: 360.1166.
p-Tolyl 2-O-acetyl-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-6-O-benzoyl-3,4-di-O-isopropylidene-1-thio-α-D-galactopyranoside [19]
Trisaccharide 6 (800 mg, 0.68 mmol) was dissolved in acetone/H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 35 mL) and trichloroisocyanuric acid (175 mg, 0.68 mmol) was added and the reaction mixture was stirred at room temperature for 2 hours. TLC (5
1, 35 mL) and trichloroisocyanuric acid (175 mg, 0.68 mmol) was added and the reaction mixture was stirred at room temperature for 2 hours. TLC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.15) at this point showed the reaction to be complete. The solvent was removed under reduced pressure and the residue was dissolved in CH2Cl2 (40 mL). The solution was washed with NaHCO3 aq. (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (3
1 n-hexane/EtOAc, Rf = 0.15) at this point showed the reaction to be complete. The solvent was removed under reduced pressure and the residue was dissolved in CH2Cl2 (40 mL). The solution was washed with NaHCO3 aq. (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 n-hexane/EtOAc) to give the trisaccharide hemi-acetal 7 (615 mg) in 85% yield. The hemi-acetal derivative was taken forward to the next step without further characterisation.
2 n-hexane/EtOAc) to give the trisaccharide hemi-acetal 7 (615 mg) in 85% yield. The hemi-acetal derivative was taken forward to the next step without further characterisation.
A part of the hemi-acetal derivative 7 (250 mg, 0.24 mmol) was dissolved in freshly dried CH2Cl2 (15 mL) and then it was treated with DBU (0.1 mL, 1.2 mmol) followed by trichloroacetonitrile (0.14 mL, 1.2 mmol). The reaction mixture was stirred at room temperature for 4 hours. TLC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) at this point showed the reaction to be complete. The solvent was removed under reduced pressure and the residue was purified by column chromatography (2.5
1 n-hexane/EtOAc) at this point showed the reaction to be complete. The solvent was removed under reduced pressure and the residue was purified by column chromatography (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give the trichloroacetimidate 8 (290 mg) in 95% yield. The trichloroacetimidate derivative was taken forward to the next step without further characterisation.
1 n-hexane/EtOAc) to give the trichloroacetimidate 8 (290 mg) in 95% yield. The trichloroacetimidate derivative was taken forward to the next step without further characterisation.
The trichloroacetimidate donor 8 (290 mg, 0.24 mmol) and galactosyl acceptor 18 (110 mg, 0.26 mmol) were dissolved in CH2Cl2 (15 mL) and stirred with 4 Å MS (1.5 g) for 15 minutes under N2 atmosphere. Thereafter the temperature was lowered to −15 °C and TMSOTf (0.01 mL, 0.06 mmol) was added and the reaction was allowed to continue for 45 minutes. TLC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf = 0.5) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (100 mL) and brine (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (3
1 n-hexane/EtOAc, Rf = 0.5) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (100 mL) and brine (100 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give the tetrasaccharide 19 (280 mg) in 75% yield.
1 n-hexane/EtOAc) to give the tetrasaccharide 19 (280 mg) in 75% yield.
[α]25D +109 (c 0.8, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 8.07–6.87 (m, 39H, ArH), 5.60 (s, 1H, H-2′′′), 5.29 (s, 1H, H-1′), 5.12 (s, 1H, H-1′′), 5.06 (s, 1H, H-1′′′), 4.96–4.57 (m, 14H, PhCH2, H6a, H6b), 4.50 (d, 1H, J = 10.0 Hz, H-1), 4.24–4.17 (m, 3H, H-2′′, H-3, H-4′), 4.09–4.01 (m, 4H, H-2′, H-3′′′, H-5, H-5′), 4.24–4.17 (dd, 1H, J = 3.0 Hz, 9.0 Hz, H-3′′), 3.92–3.81 (m, 4H, H-2, H-3′, H-5′′, H-5′′′), 3.51 (t, 1H, J = 9.5 Hz), 3.47 (t, 1H, J = 9.5 Hz), 3.46 (t, 2H, J = 9.5 Hz, H-4′′, H-4′′′), 2.25 (s, 3H, ArCH3), 2.17 (s, 3H, COCH3), 1.52 (s, 3H, CH3), 1.35–1.31 (m, 6H, H-6′, H-6′′′), 1.26 (d, 3H, J = 6.5 Hz, H-6′′).
13C NMR (CDCl3, 125 MHz) δ: 170.1 (CO), 166.4 (CO), 155.3, 150.7, 138.7, 138.6, 138.5, 138.0, 132.9, 129.6, 128.15, 128.3, 128.1, 127.9, 127.8, 127.6, 127.5, 127.4, 127.3 (Ar-C), 110.7 (O2CMe2), 100.4 (C-1′′), 99.1 (C-1′′′), 98.2 (C-1′), 86.2 (C-1), 80.2 (C-4′), 80.1 (C-4′′, C-4′′′), 79.9 (C-3), 79.2 (C-3′′), 79.0 (C-3′), 77.8 (C-3′′′), 75.4 (PhCH2), 75.3 (PhCH2), 75.0 (C-2), 74.9 (PhCH2), 74.8 (PhCH2), 74.6 (C-5), 74.3 (C-5′), 73.7 (C-2′′), 72.2 (PhCH2), 71.8 (PhCH2), 69.0 (C-2′′′), 68.9 (C-2′), 68.5 (C-5′′), 68.3 (C-5′′′), 64.2 (C-6), 27.8, 26.3 (2 × CH3), 21.1 (Ar-CH3), 21.0 (COCH3), 17.9 (C-6′′), 17.9 (C-6′′′), 17.8 (C-6′′).
HRMS calculated for C85H94O19S (M + Na)+: 1473.6008, found: 1473.6001.
p-Methoxyphenyl 2-O-acetyl-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-6-O-benzoyl-3,4-di-O-isopropylidene-α-D-galactopyranosyl-(1→3)-4-O-acetyl-2-azido-2,6-dideoxy-α-D-glucopyranoside [20]
Tetrasaccharide donor 19 (280 mg, 0.19 mmol) and acceptor 13 (70 mg, 0.21 mmol) were dissolved in CH2Cl2 (15 mL) and stirred with 4 Å MS (1.5 g) and NIS (56 mg, 0.23 mmol) for 15 minutes under N2 atmosphere. Thereafter the temperature was lowered to −30 °C and TMSOTf (7 μL, 0.03 mmol) was added and the reaction was allowed to continue for 1 hour. TLC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc, Rf=0.45) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (50 mL), Na2S2O3 (50 mL) and brine (50 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (2.5
1 n-hexane/EtOAc, Rf=0.45) at this point showed the reaction to be complete. The MS was filtered out and the filtrate was washed successively with NaHCO3 aq. (50 mL), Na2S2O3 (50 mL) and brine (50 mL). The organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude residue which was purified by column chromatography (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-hexane/EtOAc) to give the fully protected pentasaccharide 20 (240 mg, 75%) as colourless syrup.
1 n-hexane/EtOAc) to give the fully protected pentasaccharide 20 (240 mg, 75%) as colourless syrup.
[α]25D +57 (c 0.8, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 8.09 (d, 2H, J = 8.0 Hz, ArH), 7.48–7.22 (m, 33H, ArH), 6.90 (d, 2H, J = 9.0 Hz, ArH), 6.78 (d, 2H, J = 9.0 Hz, ArH), 5.55 (d, 1H, J = 1.5 Hz, H-2′′′′), 5.39 (d, 1H, J = 8.5 Hz, H-1), 5.36 (d, 1H, J = 3.0 Hz, H-4), 5.21 (d, 1H, J = 1.0 Hz, H-1′′), 5.10 (d, 1H, J = 2.5 Hz, H-1′), 5.07 (s, 1H, H-1′′′), 5.00 (s, 1H, H-1′′′′), 4.92–4.86 (m, 3H, PhCH2), 4.76–4.54 (m, 11H, PhCH2, H-6a, H-6b), 4.41–4.38 (m, 2H, H-3′, H-3), 4.31 (dd, 1H, J = 3.0 Hz 5.5 Hz, H-3′′′), 4.10 (m, 3H, H-2′′, H-2′′′, H-5), 3.99 (dd, 1H, J = 3.0 Hz 9.0 Hz, H-3′′′′), 3.93–3.89 (m, 2H, H-2′, H-5′), 3.86–3.78 (m, 8H, H-5′′, H-5′′′, H-3′′, H-2, H-4′, CH3), 3.74–3.71 (m, 1H, H-5′′′′), 3.47 (t, 1H, J = 9.5 Hz), 3.42 (t, 1H, J = 9.5 Hz), 3.37 (t, 1H, J = 9.5 Hz, H-4′′, H-4′′′, H-4′′′′), 2.15 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 1.52 (s, 3H, CH3), 1.32–1.27 (m, 12H, CH3, H-6 × 3), 1.22 (d, 3H, J = 6.5 Hz, H-6).
13C NMR (CDCl3, 125 MHz) δ: 170.1 (CO), 170.0 (CO), 166.4 (CO), 155.3, 150.7, 138.7, 138.6, 138.5, 138.4, 138.0, 118.3, 114.6 (CO), 109.8 (O2CMe2), 100.4 (C-1′′′), 99.1 (C-1′′′′), 98.9 (C-1′′), 98.0 (C-1), 94.8 (C-1′), 80.2, 80.1, 80.1 (H-4′′, H-4′′′, H-4′′′′), 79.2 (C-5), 78.9 (C-5′), 77.7 (C-3′′′′), 75.7 (C-3′), 75.3 (PhCH2), 75.2 (PhCH2), 74.8, 74.5 (C-2′′, C-2′′′), 73.5 (C-3′′′), 72.8 (C-2′), 72.1 (PhCH2), 71.7 (PhCH2), 71.7 (C-6), 71.2 (C-3), 68.9 (C-2′′′′), 68.6 (C-4), 68.4, 68.2 (C-5′′, C-2, C-3′′), 68.1 (C-5′′′), 67.2 (PhCH2), 65.9 (C-5′′′′), 64.3 (PhCH2), 58.9 (C-4′), 55.6 (CH3), 27.9, 26.2 (2 × CH3), 21.1 (COCH3), 20.6 (COCH3), 18.0 (C-6), 17.9 (C-6), 16.1 (C-6).
HRMS calculated for C93H105N3O25Na (M + Na)+: 1686.6935, found: 1686.6929.
p-Methoxyphenyl 2-O-acetyl-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-3,4-di-O-benzyl-α-L-rhamnopyranosyl-(1→2)-6-O-benzoyl-α-D-galactopyranosyl-(1→3)-4-O-acetyl-2-N-acetimido-2,6-dideoxy-α-D-glucopyranoside [21]
The protected pentasaccharide 20 (140 mg, 0.08 mmol) was suspended in 80% acetic acid (10 mL) at 80 °C for 4 hours. The acetic acid was removed under reduced and the residue was treated with thioacetic acid (10 mL). The reaction mixture was kept standing at room temperature for 7 days. Thereafter the thioacetic acid was removed under reduced pressure and the crude residue which was purified by column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 n-hexane/EtOAc) to give the partially deprotected pentasaccharide derivative 21 (120 mg) in 85% yield.
3 n-hexane/EtOAc) to give the partially deprotected pentasaccharide derivative 21 (120 mg) in 85% yield.
[α]25D +84 (c 0.7, CHCl3).
1H NMR (CDCl3, 500 MHz) δ: 8.03–8.01 (m, 2H, ArH), 7.56–7.16 (m, 33H, ArH), 6.87 (d, 2H, J = 9.0 Hz, ArH), 6.78 (d, 2H, J = 9.0 Hz, ArH), 6.22 (d, 1H, J = 10.0 Hz, NHAc), 5.52 (m, 1H, H-2′), 5.36 (s, 1H, H-4), 5.30 (d, 1H, J = 3.5 Hz, H-1), 5.11 (d, 1H, J = 2.5 Hz, H-1′′′), 5.06 (d, 1H, J = 2.5 Hz, H-1′), 5.00 (s, 1H, H-1′′), 4.96 (s, 1H, H-1′′′′), 4.88 (dd, 2H, J = 5.0 Hz 11.0 Hz, PhCH2), 4.74–4.53 (m, 13H, H-2, H-6a, H-6b, PhCH2), 4.30 (t, 1H, J = 7.0 Hz, H-5′), 4.15–4.07 (m, 3H, H-3, H-2′′′, H-5′′), 4.02–3.84 (m, 7H, H-2′′, H-2′, H-5′′′, H-5′′′′, H-3′′, H-3′′′′, H-3′), 3.80–3.75 (m, 6H, H-4′, CH3, H-5), 3.45–3.37 (m, 2H), 3.32 (t, 1H, J = 9.0 Hz, H-4′′, H-4′′′, H-4′′′′), 2.13 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 1.86 (s, 3H, COCH3), 1.30–1.20 (m, 9H, H-6 × 3), 1.02 (d, 3H, J = 6.5 Hz, H-6).
13C NMR (CDCl3, 125 MHz) δ: 170.6 (CO), 170.0 (CO), 169.9 (CO), 167.6 (CO), 155.2, 150.5, 138.6, 138.4, 138.4, 138.3, 138.2, 138.0, 133.5, 129.7, 129.2, 128.5, 128.3, 128.1, 127.9, 127.8, 127.7, 127.6, 127.5, 127.5, 127.4, 127.3, 118.0, 114.6 (Ar-C), 100.7 (C-1′′′), 99.2 (C-1′′), 99.0 (C-1′′′′), 98.0 (C-1), 95.5 (C-1′), 80.4, 80.2, 80.0 (H-4′′, H-4′′′, H-4′′′′), 79.0 (C-3′′′′), 78.3 (C-5′′′′), 77.6 (C-5′′, C-5′′′), 75.4 (PhCH2), 75.3 (C-6′), 74.3 (C-2′′), 74.2 (PhCH2), 73.7 (C-2′′′), 73.4 (C-2′), 72.0 (PhCH2), 71.9 (PhCH2), 71.7 (PhCH2), 70.5 (C-3), 69.5 (C-4′), 69.2 (C-3′′), 69.0 (C-2′′′′), 68.3 (C-3′′′), 68.2 (C-3′), 68.0 (C-4), 67.0 (C-5′), 66.0 (C-5), 63.3 (PhCH2), 55.6 (CH3), 47.9 (C-2), 23.1 (COCH3), 21.0 (COCH3), 20.5 (COCH3), 18.3 (C-6 × 2), 17.8 (C-6), 16.2 (C-6).
HRMS calculated for C92H105O26NNa (M + Na)+: 1662.6823 found: 1662.6819.
p-Methoxyphenyl α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-α-D-galactopyranosyl-(1→3)-2 N-acetamido-2,6-dideoxy-α-D-glucopyranoside [1]
The pentasaccharide derivative 26 (105 mg, 0.06 mmol) was dissolved in methanol (10 mL) and 10% Pd–C (100 mg) was added. The reaction mixture was kept stirring under a hydrogen atmosphere at room temperature and pressure for 36 hours. Thereafter the reaction mixture was filtered out and the filtrate was evaporated to dryness under reduced pressure. The crude residue was dissolved in freshly dried methanol (10 mL) and NaOMe (20 mg) was added. The reaction mixture was stirred at room temperature for 24 hours. Thereafter the reaction mixture was quenched with DOWEX 50W resin. The reaction mixture was filtered and the methanol was evaporated to dryness under vacuum to give the deprotected target 1 (55 mg, 90%) as off white amorphous mass.[α]25D +41 (c 0.6, MeOH).
1H NMR (CD3OD, 500 MHz) δ: 7.00 (d, 2H, J = 9.0 Hz, ArH), 6.83 (d, 2H, J = 9.0 Hz, ArH), 5.32 (s, 1H, H-1), 5.30 (d, 1H, J = 3.0 Hz, H-1′′′), 5.11 (s, 1H, H-1′), 5.06 (d, 1H, J = 3.5 Hz, H-1′′) 4.92 (s, 1H, H-1′′′′), 4.53 (dd, 1H, J = 3.0 Hz, 10.5 Hz), 4.17–4.11 (m, 2H), 4.03–4.00 (m, 2H), 3.96–3.93 (m, 4H), 3.87–3.84 (m, 2H), 3.82–3.76 (m, 2H), 3.73 (s, 3H, OCH3), 3.71–3.60 (m, 4H), 3.39–3.30 (m, 5H), 2.05 (s, 3H, NHCOCH3), 1.28–1.24 (m, 12H, 4 × H-6).
13C NMR (CD3OD, 125 MHz) δ: 155.3, 150.9, 117.9, 114.2 (ArC), 102.4 (C-1′′′′), 100.9 (C-1′), 100.1 (C-1), 97.5 (C-1′′′), 96.5 (C-1′′), 78.4, 77.7, 74.6, 72.9, 72.8, 72.6, 72.5, 71.7, 70.8, 70.6, 70.5, 70.4, 69.8, 69.0, 68.9, 68.8, 68.1, 66.4, 61.6, 54.6, 21.2 (NHCOCH3), 16.8, 16.6, 16.4, 15.3 (C-6 × 4).
HRMS calculated for C39H61O23NNa (M + Na)+: 934.3532, found: 934.3532.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
AC is thankful to Science and Engineering Research Board (SERB), New Delhi for National Postdoctoral Fellowship (PDF/2017/000919). The work is supported by the research grant EMR/2017/003043 from SERB, New Delhi to BM.Notes and references
- W. E. Sanders Jr and C. C. Sanders, Clin. Microbiol. Rev., 1999, 10, 221–241 Search PubMed.
- A. Varki, Essentials of Glycobiology, ed. A. Varki, R. D. Cummings, J. D. Esko, P. Stanley, G. W. Hart, M. Aebi, A. G. Darvill, T. Kinoshita, N. H. Packer, J. H. Prestegard, R. L. Schnaar and P. H. Seeberger, Cold Spring Harbor Laboratory Press, 3rd edn, 2015 Search PubMed.
- M. L. Mezzatesta, F. Gona and S. Stefani, Future Microbiol., 2012, 7, 887–902 CrossRef CAS PubMed.
- A. V. Perepelov, X. Guangnan, A. V. Filatov, X. Zhang, A. S. Shashkov, M. Wang and Y. A. Knirel, Carbohydr. Res., 2017, 448, 110–114 CrossRef CAS PubMed.
- M. Emmadi and S. S. Kulkarni, Nat. Prod. Rep., 2014, 31, 870–879 RSC.
- E. Glibstrup and C. M. Pedersen, Org. Lett., 2016, 18, 4424–4427 CrossRef CAS PubMed.
- S. S. Kulkarni and M. Emmadi, Nat. Protoc., 2013, 8, 1870–1889 CrossRef PubMed.
- D. Buddhadev and B. Mukhopadhyay, RSC Adv., 2015, 5, 98033–98040 RSC.
- V. Sarkar and B. Mukhopadhyay, RSC Adv., 2016, 6, 40147–40154 RSC.
- J. Zhang, J. Mao and H. Chen, Tetrahedron: Asymmetry, 1994, 5, 2283–2290 CrossRef CAS.
- R. Das and B. Mukhopadhyay, Carbohydr. Res., 2016, 376, 1–6 CrossRef PubMed.
- G. Zemplén, Ber. Dtsch. Chem. Ges., 1926, 59, 1254–1266 CrossRef.
- K. Bock and C. Pedersen, J. Chem. Soc., Perkin Trans. 2, 1974, 293–297 RSC.
- N. Basu, S. K. Maity, A. Chaudhury and R. Ghosh, Carbohydr. Res., 2006, 369, 10–13 CrossRef PubMed.
- R. R. Schmidt and J. Michel, Angew. Chem., Int. Ed. Engl., 1980, 19, 731–732 CrossRef.
- A. Chaudhury and R. Ghosh, Org. Biomol. Chem., 2017, 15, 1444–1452 RSC.
- H.-S. Dang, B. P. Roberts, J. Sekhon and T. M. Smit, Org. Biomol. Chem., 2003, 1, 1330–1341 RSC.
- J.-L. Gras, Y.-Y. K. W. Chang and A. Guerin, Synthesis, 1985, 74–75 CrossRef CAS.
- R. Lattrell and G. Lohaus, Justus Liebigs Ann. Chem., 1974, 901–920 CrossRef CAS.
- R. Albert, K. Dax, R. W. Link and A. E. Stutz, Carbohydr. Res., 1983, 118, C5–C6 CrossRef CAS.
- F. B. LaForge, J. Am. Chem. Soc., 1933, 55, 3040–3048 CrossRef CAS.
- R. Beier and B. P. Mundy, Synth. Commun., 1979, 9, 271–273 CrossRef CAS.
- B. Kumar, M. A. Aga, A. Rouf, B. A. Shah and S. C. Taneja, RSC Adv., 2014, 4, 21121–21131 RSC.
- G. Catelani, Carbohydr. Res., 1988, 182, 297–300 CrossRef CAS.
- G. Vessella, A. Casillo, A. Fabozzi, S. Traboni, A. Iadonisi, M. M. Corsaro and E. Bedini, Org. Biomol. Chem., 2019, 17, 3129–3140 RSC.
- D. Crich and A. A. Bowers, J. Org. Chem., 2006, 71, 3452–3463 CrossRef CAS PubMed.
- S. S. Kulkarni, Y.-H. Liu and S.-C. Hung, J. Org. Chem., 2005, 70, 2808–2811 CrossRef CAS PubMed.
- K. B. Pal, V. Sarkar and B. Mukhopadhyay, CrystEngComm, 2016, 18, 1156–1164 RSC.
- G. Medgyes, G. Jerkovich and J. Kuszmann, Carbohydr. Res., 1989, 186, 225–239 CrossRef CAS PubMed.
- Y. Nakahara, Y. Nakahara and T. Ogawa, Carbohydr. Res., 1996, 292, 71–81 CrossRef CAS.
- W. M. Perlman, Tetrahedron Lett., 1967, 8, 1663–1664 CrossRef.
- D. D. Perrin, W. L. Amarego and D. R. Perrin, Purification of Laboratory Chemicals, Pergamon, London, 1996 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of the 1H and 13C NMR spectra of all new compounds. See DOI: 10.1039/c9ra09807k |
| This journal is © The Royal Society of Chemistry 2020 |