Open Access Article
Open Access ArticleElucidating the effect of precursor decomposition time on the structural and optical properties of copper(I) nitride nanocubes†
Rudo
Kadzutu-Sithole
a,
Lerato F. E.
Machogo-Phao
a,
Tshwarela
Kolokoto
a,
Memory
Zimuwandeyi
a,
Siziwe S.
Gqoba
a,
Kalenga P.
Mubiayi
a,
Makwena J.
Moloto
 b,
Juanita
Van Wyk
*a and
Nosipho
Moloto
b,
Juanita
Van Wyk
*a and
Nosipho
Moloto
 *a
*a
aMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Private Bag 3, Wits, 2050, South Africa. E-mail: Juanita.VanWyk@wits.ac.za; Nosipho.Moloto@wits.ac.za; Fax: +27117176749; Tel: +27117176732 Tel: +27117176774
bDepartment of Chemistry, Faculty of Applied and Computer Science, Vaal University of Technology, Private Bag X021, Vanderbijlpark, South Africa
First published on 15th September 2020
Abstract
To study the effect of time on the colloidal synthesis of Cu3N nanoparticles, copper(II) nitrate was thermally decomposed at 260 °C for up to 60 min in octadecylamine as a stabilizing ligand. Thermolysis of the nitrate followed four steps which included; nucleation, growth, ripening and decomposition. At 5 min, partially developed nanocubes were found in a dense population of Cu3N nuclei. Well-defined Cu3N nanocubes were obtained at 15 min with no presence of the nuclei. TEM images showed disintegration of the cubes at 20 min and as time progressed, all the Cu3N decomposed to Cu by 60 min. The formation of the Cu3N nanocubes was confirmed by XRD and XPS. FTIR suggested the formation of a nitrile (RCN) as a result of the thermal decomposition in octadecylamine (ODA) and this was confirmed using NMR and hence, a reaction mechanism was then proposed. The optical properties of the as-synthesized Cu3N were studied using UV-vis and photoluminescence spectroscopies. The absorption spectra for particles synthesized from 5 min to 15 min showed a singular exciton peak while from 20 min to 60 min two peaks were observed. The two peaks may both be associated with the two direct transitions observed in Cu3N or the more red-shifted peak could be a result of localized surface plasmon resonance due to the Cu nanoparticles. Nevertheless, similar to other studies, it is clear that the optical properties of Cu3N are complex.
1. Introduction
The industrial application of semiconducting nanomaterials cannot be overemphasized, more especially in photonics and medicine. Colloidal synthesis of Cu3N continues to receive tremendous research focus marking a variation from the popular solid-state methods which involve the use of RF magnetron sputtering. In literature, there are many discrepancies in the data that have been reported for Cu3N nanoparticles (NPs). For example, the band gap and thermal stability and this has been attributed to the unstable nature of Cu3N acquired during the growth stage.1 Researchers are slowly turning their focus to solution-based synthesis of Cu3N NPs due to the ease with which the desired morphology and properties of the NPs can be engineered. Few reports have demonstrated that Cu3N NPs can be synthesized by employing ammonolysis or use of N sources such as azide, hexamethylenetetramine and urea but the challenge with these methods is that the resultant NPs are ill-shaped.2–8 Special attention is recently being given to the synthesis of Cu3N NPs using direct thermal decomposition of Cu(NO3)2·3H2O in octadecylamine (ODA) to obtain well defined nanocubes.9–13 Thermal decomposition of other metal nitrates for example, Mn(NO3)2, Ni(NO3)2·6H2O, Zn(NO3)2·6H2O, Ce(NO3)3·6H2O, Co(NO3)2·6H2O in ODA resulted in the formation of corresponding metal oxides and not metal nitrides.14 Wang et al.11 demonstrated that it is very crucial to control the concentration of Cu(NO3)2 in ODA in order to get Cu3N and not Cu or its oxides. Xi et al.13 reported on the formation of Cu3N upon decomposing Cu(NO3)2 in a mixture of two reducing agents, ODA and oleylamine (OLA) at 240 °C for up to 10 min. The report revealed that very few cubes were observed amongst small nuclei of Cu3N after 2 min but only cubes were present after 10 min. However, the authors did not report on the effect of time beyond 10 min. Herein, we probe the effect of precursor decomposition time on the formation of Cu3N nanocubes through thermolysis of Cu(NO3)2 at 260 °C for up to 60 min in ODA as the capping and reducing agent. Progression in the formation of the Cu3N nanocubes could be summarized in four stages namely; nucleation, growth, ripening and decomposition. In order to verify our findings, the same synthetic procedure was followed using hexadecylamine (HDA) as the capping and reducing agent.2. Experimental section
Materials
Copper(II) nitrate trihydrate (puriss. p. a, 99–104%), 97% octadecylamine (ODA), octadecene (≥95.0% GC), 98% hexadecylamine (HDA), 99.5% chloroform and ethanol absolute (≥99.8% GC) were purchased from Sigma-Aldrich. Chloroform was refluxed in calcium hydride and distilled in nitrogen before use and ethanol was stored under 3A molecular sieves. All other chemicals were used as received.Synthesis of Cu3N nanoparticles
The controlled synthesis of Cu3N nanoparticles was achieved by thermal decomposition of copper(II) nitrate in ODA. Cu(NO3)2·3H2O (1 mmol) was dissolved in octadecene (1 mL), mixed with ODA (10 mL) and degassed under a continuous flow of N2 gas (©Afrox, 99.997%) at 110 °C. After an hour, the temperature was slowly raised to 260 °C. Aliquots were then extracted at 5, 10, 15, 20, 30 and 60 min. Dry absolute ethanol was then added to the aliquots to flocculate the particles. The particles were then collected by centrifugation at 5000 rpm. The samples were then dissolved in dry chloroform, flocculated using dry absolute ethanol and centrifuged 5 times. The final powder samples were dried in a vacuum oven and kept under inert conditions before characterization.Characterization
A Varian Cary Eclipse (Cary 50) UV-vis spectrophotometer was used to carry out the optical properties of the NPs. The photoluminescence spectra of the NPs were recorded on a Varian Cary Eclipse EL04103870 spectrofluorometer with a medium PMT voltage at an excitation wavelength of 200 nm. The absorption and emission spectra were obtained in chloroform and placed in quartz cuvettes (1 cm path length). Powdered XRD patterns of the samples were measured on a Bruker MeasSrv D2-205530 diffractometer using secondary graphite monochromated Cu Kα radiation (λ 1.5406 Å) at 30 kV/30 mA. Measurements were taken using a glancing angle of incidence detector at an angle of 2°, for 2θ values over 10–90° in steps of 0.026° with a step time of 37 s and at a temperature of 25 °C. X-ray photoelectron spectroscopy measurements were performed with a PHI 5000 Versaprobe – Scanning ESCA Microprobe operating with a 100 μm 25 W 15 kV Al monochromatic X-ray beam. The transmission electron microscopy (TEM) was carried out on a FEI Technai T12 operated at an acceleration voltage of 200 kV with a beam spot size of 20–100 nm in TEM mode. The vibrational modes were measured on a Bruker Tensor 27 FT-IR and the nuclear magnetic resonance (NMR) data was obtained using a 500 MHz Bruker AVANCE III, the samples were run using CDCl3 as a solvent at 300 K.3. Results and discussion
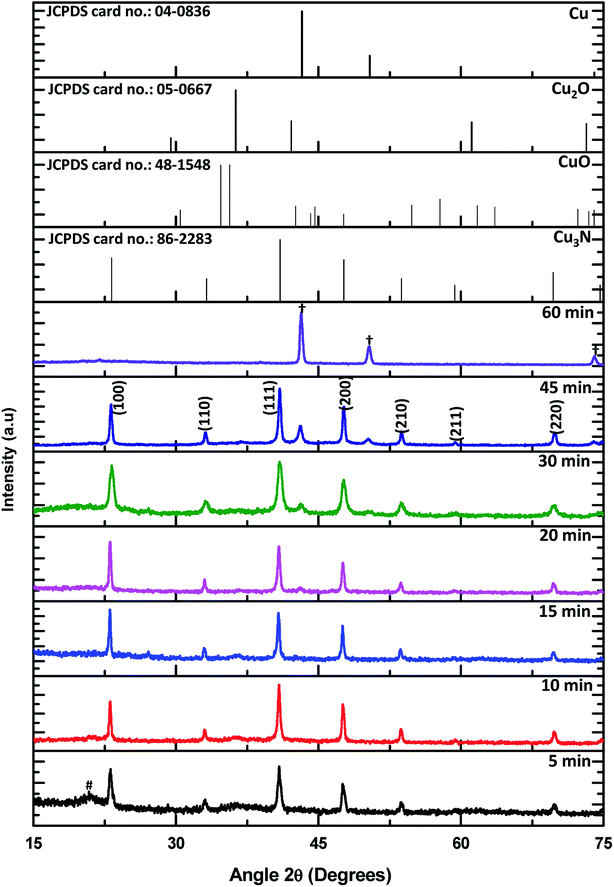
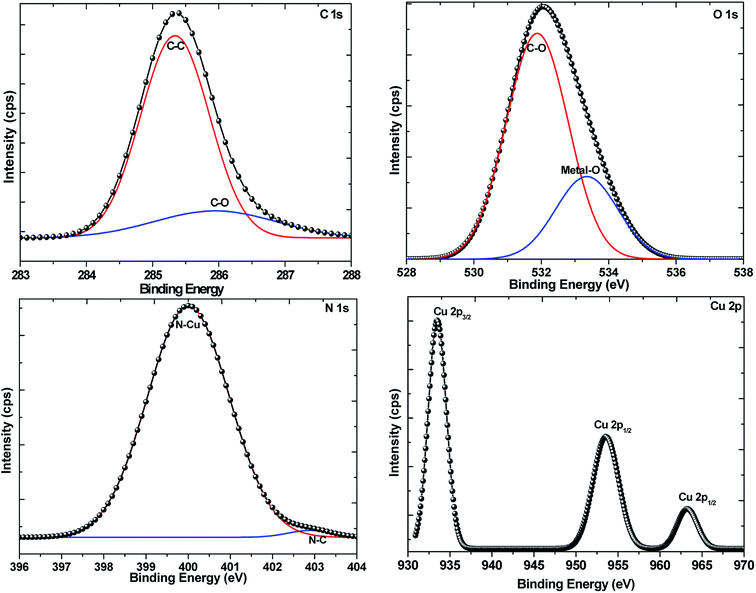
Powder X-ray diffraction was used to determine the crystal phases of the resultant NPs and for the detection of any impurities. Shown in Fig. 1 are the XRD patterns of the NPs synthesized from 5 min to 60 min in ODA as well as the reference patterns of Cu3N and possible impurities. The series of Bragg reflections in the experimental patterns show peaks that correspond to planes (100), (110), (111), (200), (210), (211) and (220) of the cubic phase of Cu3N (card number JCPDS card no.: 86-2283). An additional peak from the 20 min sample is observed at 2θ = 42.98° showing the initial stage of Cu formation. More copper peaks emerge till all the Cu3N is converted to Cu (JCPDS card no.: 04-0836) at 60 min. These sets of data show that thermal decomposition of Cu(NO3)2 in ODA at 260 °C result in the formation of Cu3N after about 5 min. Cu3N continues to be the only product present until about 20 min, then a mixture of Cu3N and Cu NPs begin to form until only Cu is detected at 60 min. Understanding of the metallization of Cu3N can come in handy in making microscopic Cu metallic links during the fabrication of optical recording media15 and optical data storage.16 The peak assigned as # is associated with the degradation products of ODA and the corresponding XRD patterns are shown in Fig. 1S.† In addition, no oxide impurities were observed from the XRD.The composition of the formed Cu3N NPs and oxidation states of Cu and N was further confirmed by employing X-ray photoelectron spectroscopy (XPS) and the results are shown in Fig. 2 and 3. From the survey spectrum, it can be deduced that the as-synthesized Cu3N NPs contain C, O, N and Cu. Majumdar et al.17 discovered that Cu3N films that were synthesized by RF magnetron sputtering consisted of 20.4% C and 35.5% O after being exposed to air. As such, exposure of the as-synthesized Cu3N NPs to air and the presence of the organic stabilizer could explain the observed pivotal amount of C and O in the sample as seen in Fig. 2. The copper auger peak (Cu LMM) is indicative of the presence of Cu2+, this suggests the presence of CuO which is a readily observed impurity in Cu3N due to the oxidation. The XRD in Fig. 1 however showed no evidence of CuO, suggesting only surface oxidation. Two peaks centralized at 285.35 and 285.95 eV from the C 1s high-resolution are assigned to C–C and C–O respectively. The C–C is due to the long chain alkylamine capping agent. The presence of C–O is attributed to the oxidation of the capping agent as later shown in the FTIR results (Fig. 6). The O 1s spectrum has a broad peak that is centered at 532.04 eV and deconvolution of the O 1s spectrum gives rise to two peaks at 531.87 and 533.36 eV that are ascribed to the presence of C–O and metal-O specie respectively.12 The C–O further confirms the oxidation of the capping agent while the metal–oxygen bonded specie is indicative of the formation of CuO.
The NPs were synthesized in a nitrogen-rich atmosphere and the N 1s high-resolution XPS depicts the main peak centered at 399.70 eV showing the main bonding of nitrogen to copper (N–Cu) in the Cu3N nanocubes. This value is higher than the 397.7 eV that has been reported.18–22 The peak at 403.01 eV is attributed to N–C bonding in stearonitrile. Interestingly, Navio et al. and others were of the idea that the 397.7 eV peak is attributed to the presence of nitrogen that is adsorbed to the surface of the Cu3N(100) and the second peak that they obtained at 398.7 eV was attributed to the presence of N that is in the bulk of the Cu3N.20,23
In the Cu 2p spectrum, the expected Cu+ peaks were obtained at 933.48 eV and 953.52 eV of the Cu 2p3/2 and Cu 2p1/2 states respectively, consistent with previous reports.24 Corresponding shakeup peaks at around 934.4 and 954.63 eV (ref. 24) were not obtained hence suggesting the lack of Cu2+ in the NPs. However, a peak of relatively lower intensity was obtained around 963.20 eV and reports25–27 have associated this peak as a shakeup peak to Cu 2p1/2 suggesting the presence of CuO. In the XRD pattern, there were no evident peaks that could be ascribed to CuO hence, the detected oxide in the Cu3N nanocubes could be a result of mild surface oxidation due to exposure to atmospheric oxygen.
To study the morphology of the as-synthesized NPs, the powders were dispersed in chloroform and drop-casted onto Cu and Ni grids. After drying, they were then analyzed and imaged under the transmission electron microscope and the images are shown in Fig. 4. The images obtained show that the sample that is collected after 5 min at 260 °C consists of mixed morphology. Relatively few nanocubes are found in between a cloud of very small dot-like NPs. A closer look reveals that some of the nanocubes are incompletely formed and in close contact with a number of the dot-like NPs. In another study, Xi et al. obtained a similar mixture of nuclei and cubes after 5 min of thermal decomposition of Cu(NO3)2 in a mixture of ODA and oleylamine at 240 °C.13
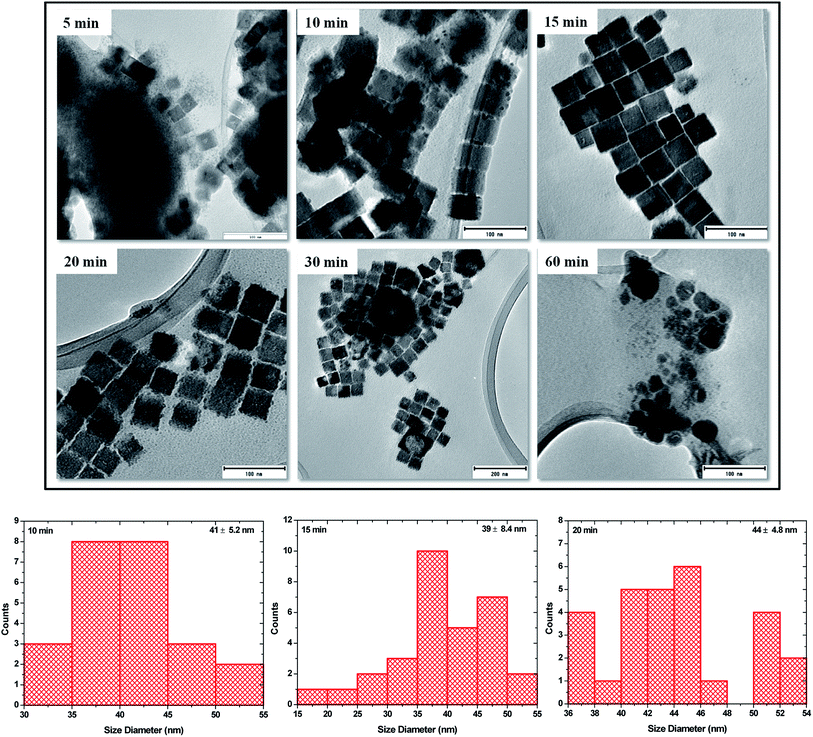 | ||
| Fig. 4 TEM images of Cu3N NPs obtained after 5, 10, 15, 20, 30 and 60 min in ODA and size distribution for 10, 15 and 20 min samples. | ||
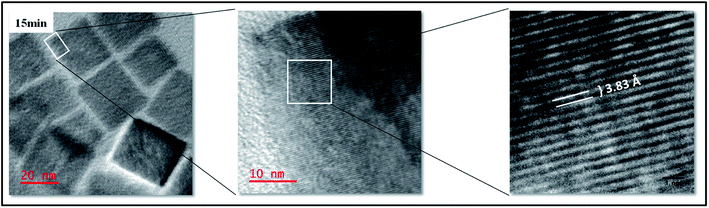
At 10 min, there is a much greater population of the nanocubes and much less of the dot-like NPs and the nanocubes are much more defined and show some degree of self-assembly. The ‘dots’ can still be seen and some are found on and in between the cubes and on the edges of the cubes hence making it slightly difficult to measure the size of the cubes as some of them do not possess well-defined edges, nevertheless, the average size is estimated to be 41 ± 6.2 nm. The 15 min sample brings about a change in composition. The nanocubes are aligned next to each other resembling a pattern of layered bricks. The dot-like NPs are almost non-existent and the edges of the cubes are well-defined with no evidence of dots on them. These are then named ‘perfect nanocubes’ and measurement of their edges gives a size of 39 ± 8.4 nm. At 20 min of synthesis, the ‘perfect nanocubes’ are no longer perfect however, the sizes can still be estimated at 44 ± 4.8 nm. They look like they are crumbling and the edges start having the dot-like NPs again. At this stage, XRD shows the emergence of a peak around 2θ = 50.6° that is assigned to Cu. Attempted measurements of the edges gives a rough estimate of 44 nm and no further measurements are taken thereafter as the nanocubes have no well-defined shapes. The break down of the nanocubes continues and by 30 min, the brokendown cubes seem to affect the neighbouring cubes resulting in the formation of giant cube-like structures with hollow interiors. At this stage, Cu related peaks can be seen on the XRD pattern although they are of very low intensity compared to the Cu3N peaks. By 60 min, no cubes can be seen but polydispersed nanoparticles are obtained and the XRD shows peaks that are all ascribed to Cu and there is no evidence of peaks that can be associated with Cu3N.
Shown in Fig. 5 are the HRTEM images of the perfect nanocubes. The nanocubes have lattice fringes with the d-spacing of 3.83 Å corresponding to the (100) plane of the anti-ReO3 cubic structure of Cu3N. This is consistent with the results obtained in the X-ray diffraction in Fig. 1.
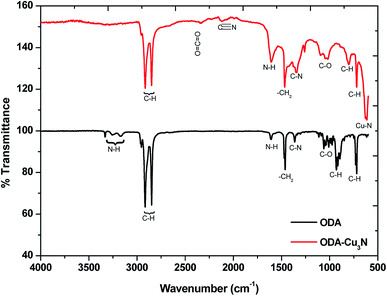
To investigate the coordination of the ligand, Fourier transform infra-red (FTIR) spectroscopic analysis was performed. The spectrum that is obtained from the nanoparticles synthesized in 15 min is compared with that of raw ODA (Fig. 6). Generally, the results show that the Cu3N NPs are capped by the organic ligand, as the corresponding vibrations in ODA are detected on the synthesized NPs. The characteristic C–H stretching vibrations are obtained from 2845 cm−1 to 2959 cm−1 and the –CH2 bending vibration at 1460 cm−1 is slightly shifted from the 1465 cm−1 in ODA showing that the chemical environment has slightly changed due to the coordination. The peak at 2335 cm−1 for the ODA capped Cu3N is due to the atmospheric CO2. Also observed in both ODA and capped particles is the C–O stretching frequency. This suggests the presence of impurities in ODA possibly oxidation and this explains the presence of C–O in the XPS. A sharp peak was obtained at 624.6 cm−1 which is very close to the 639,28 ∼650 (ref. 6) and 653 cm−1 (ref. 5) that were obtained in previous reports ascribed to Cu–N vibrations in copper(I) nitride.29
Wang and Li11 suggested that the ODA does not participate in the reaction but rather acts as a catalyst that facilitates the transfer of electrons from O2− to Cu2+ and N5+ of the nitrate. However, we are of the notion that ODA is most likely a participant in the reaction. In the FTIR, the symmetric and antisymmetric N–H vibration modes found at 3259.5 cm−1 and 3333 cm−1 respectively on the ODA spectrum were not detected on the Cu3N spectrum. Instead, a new peak was spotted at 2120 cm−1 and can be assigned to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration mode. In another study, this peak was detected at about 2092 cm−1 and we agree with the authors who suggested the possibility of the existence of C
N stretching vibration mode. In another study, this peak was detected at about 2092 cm−1 and we agree with the authors who suggested the possibility of the existence of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N due to the high temperature breakdown of ODA.10
N due to the high temperature breakdown of ODA.10
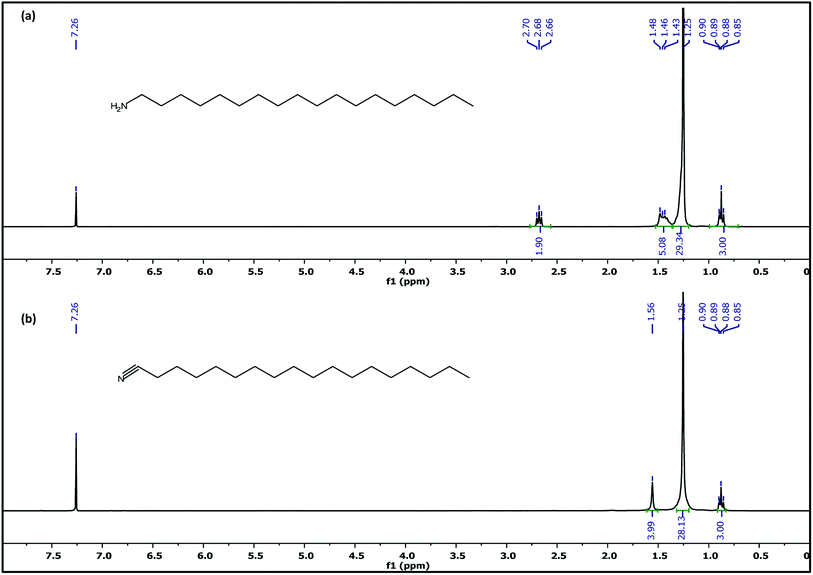
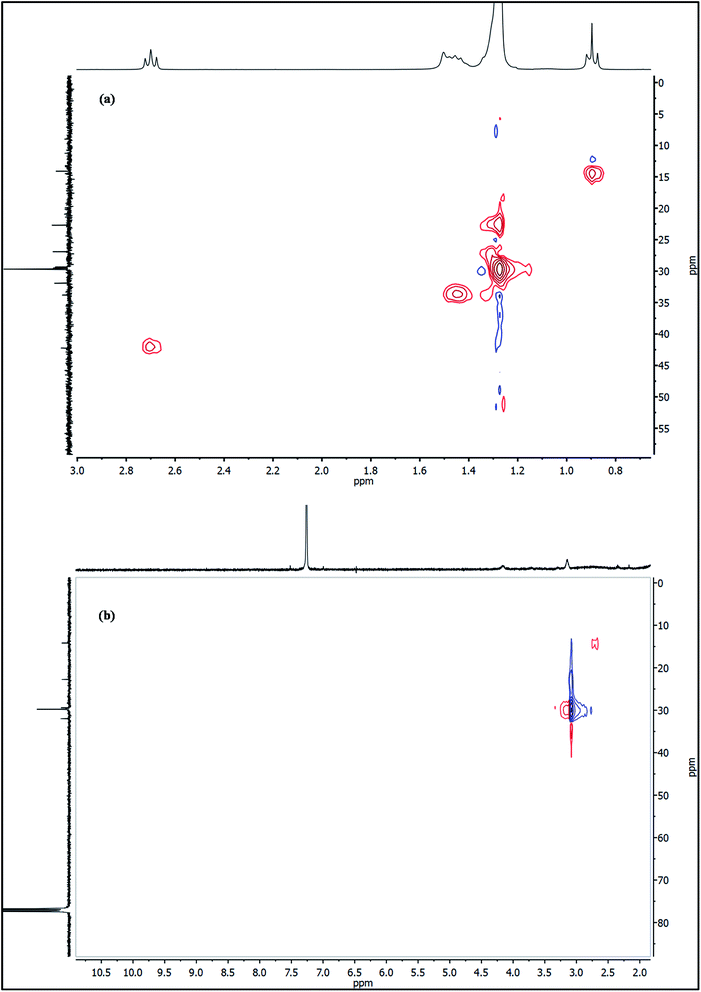
To further probe this theory, the as-synthesized Cu3N nanocubes and raw ODA were analyzed using proton nuclear magnetic resonance (1H NMR). Analysis of the 1H NMR clearly revealed the deprotonation of the ODA (CH3(CH2)16CH2–NH2), resulting in the formation of stearonitrile (CH3(CH2)16C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N). From the 1H NMR spectra in Fig. 7, the resonance for the H3C– protons is centralized around δ 0.88 ppm in both the spectra of raw ODA and the Cu3N NPs. At δ 1.25 and δ 1.26 ppm, a singlet is observed in both spectra and it accounts for protons that are on the CH2 chains respectively. Henceforth, notable differences were observed in the two spectra. In the raw ODA, a slightly broad triplet was observed centralized at δ 1.46 ppm but on the other hand, the spectrum that was obtained from the NPs showed a very broad peak with a peak maximum at δ 1.57 ppm. Thereafter, no further proton peaks were detected on the spectrum of the Cu3N NPs but a triplet was obtained at δ 2.68 ppm on raw ODA and this accounted for the missing 2 H in the –CH2 that is directly bonded to the N on the amine end. These CH2 protons are clearly non-existent in the spectrum of the Cu3N NPs. This is most probably because the H on the H2N–CH2– are all involved in the reaction that leads to the formation of Cu3N NPs and stearonitrile remains as a by-product as depicted in the proposed chemical equation below:
N). From the 1H NMR spectra in Fig. 7, the resonance for the H3C– protons is centralized around δ 0.88 ppm in both the spectra of raw ODA and the Cu3N NPs. At δ 1.25 and δ 1.26 ppm, a singlet is observed in both spectra and it accounts for protons that are on the CH2 chains respectively. Henceforth, notable differences were observed in the two spectra. In the raw ODA, a slightly broad triplet was observed centralized at δ 1.46 ppm but on the other hand, the spectrum that was obtained from the NPs showed a very broad peak with a peak maximum at δ 1.57 ppm. Thereafter, no further proton peaks were detected on the spectrum of the Cu3N NPs but a triplet was obtained at δ 2.68 ppm on raw ODA and this accounted for the missing 2 H in the –CH2 that is directly bonded to the N on the amine end. These CH2 protons are clearly non-existent in the spectrum of the Cu3N NPs. This is most probably because the H on the H2N–CH2– are all involved in the reaction that leads to the formation of Cu3N NPs and stearonitrile remains as a by-product as depicted in the proposed chemical equation below:
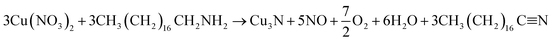 | (1) |
The formation of the stearonitrile is in agreement with the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N vibration modes that were detected at 2120 cm−1 on the FTIR spectrum in Fig. 6. At temperatures above 21.2 °C, NO2 is a reddish-brown gas with an pungent odor and it decomposes at about 160 °C (ref. 30) to give NO and O2 which are both colorless gases. To try and understand the full mechanism of the formation of the Cu3N and the role of the expelled gases and ODA, the following processes are thought to be occurring during the reaction and these account for the overall eqn (1).
N vibration modes that were detected at 2120 cm−1 on the FTIR spectrum in Fig. 6. At temperatures above 21.2 °C, NO2 is a reddish-brown gas with an pungent odor and it decomposes at about 160 °C (ref. 30) to give NO and O2 which are both colorless gases. To try and understand the full mechanism of the formation of the Cu3N and the role of the expelled gases and ODA, the following processes are thought to be occurring during the reaction and these account for the overall eqn (1).
Decomposition of copper nitrate
 | (2) |
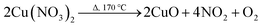 | (3) |
 | (4) |
Oxidation of octadecylamine
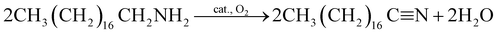 | (5) |
Decomposition of nitrous oxide
 | (6) |
Formation of copper nitride
| 6Cu + N2 → Cu3N | (7) |
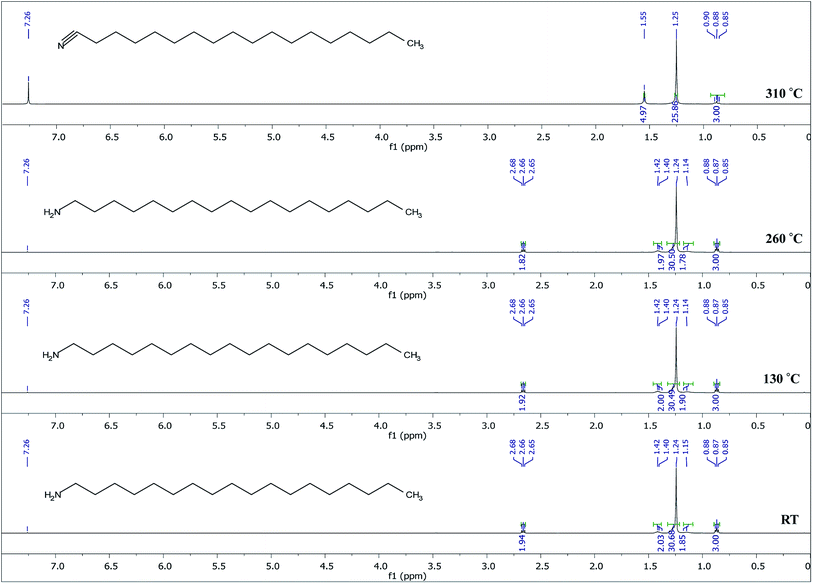
The breaking down of copper nitrate is common knowledge and results in the formation of CuO and NO. As shown on the 1H NMR in Fig. 7, the ODA is converted to stearonitrile. To check if indeed this occurs due to high temperature thermolysis as previously suggested,10 ODA was heated from room temperature (RT) up to 260 °C under nitrogen and then analyzed with 1H NMR. The results are shown in Fig. 8.
The 1H NMR spectra in Fig. 8 show that at temperatures up to the synthetic temperature (260 °C), no oxidation of ODA occurs, however, at higher temperatures (310 °C) and longer reaction times this occurs. This suggests that in the current study, the conversion of ODA to stearonitrile is not thermally driven; however, the oxidation is driven by the presence of a catalyst and oxygen in the reaction system. This is in accordance with other studies where oxidation of a primary amine to nitrile has occurred using a catalyst in a presence of oxygen.31 Herein, we therefore suggest that CuO catalyzes the oxidation of ODA. At the same time, it catalyzes the decomposition of NO to N2 and O2.32 In the process of catalyzing these reactions, the CuO is then reduced to Cu. Finally Cu and N2 react to form Cu3N. The reaction of Cu with N2 has been readily shown in the RF magnetron sputtering of Cu3N,33,34 however ultra-pure nitrogen gas is used. Herein, we therefore suggest that the N2 gas feed is merely to maintain an inert atmosphere and is not reactive. As such the N2 generated from the decomposition of NO is the reactive gas and a limiting reagent in the formation of Cu3N. The process is shown from eqn (2)–(7).
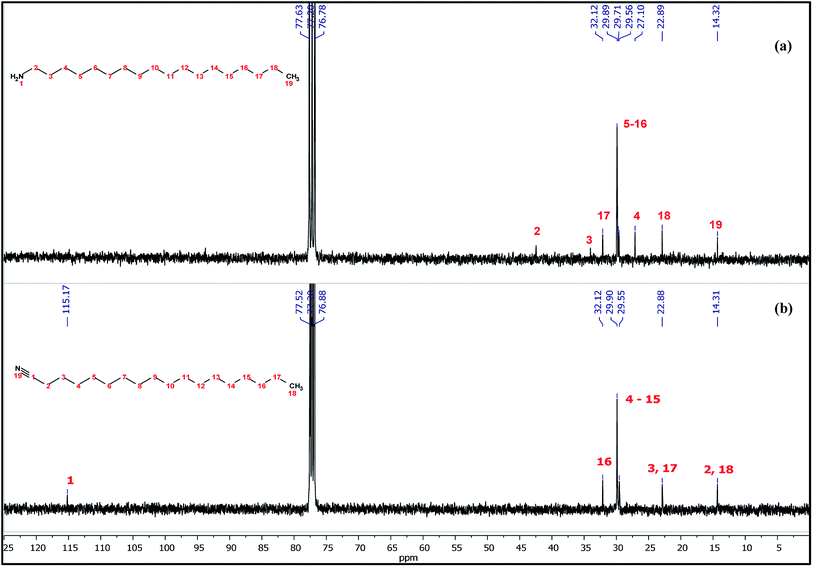
The 13C NMR spectra of raw ODA and ODA-capped Cu3N NPs are shown in Fig. 9. In both spectra, the 13C signals of the methyl and methylene groups are detected around δ 14.32 and 22.89–32.12 ppm respectively. Of great interest is the huge downfield shift of the carbon peak at δ 42.46 in raw ODA to δ 115.17 ppm in the NPs which can be used as a diagnostic signal. The obtained 115.17 ppm in this study is lower than the 119.77 ppm that was obtained by Gunstone35 when he was working with oleyl compounds but fall within the shift ranges of nitriles. This huge downfield shift is due to the deprotonation of the carbon that is bonded to nitrogen, H2C–NH2 to form the nitrile (RCN). The appearance of the carbon peak at δ 115.17 ppm suggests the formation of a quaternary nitrile-bearing center hence, confirms the presence of octadecylnitrile in the Cu3N NPs. Due to the strong influence of the nitrile group, the nearby carbon atoms, C3 and C4 are greatly affected owing to π-electron deshielding and the polar inductive effect36 of the nitrile group. As suggested in the equation above, the synthesis of Cu3N nanoparticles involves the process of deprotonation of octadecylamine to form octadecylnitrile. The nitrile group then forms sigma bonds with the Cu+ ions resulting in end-on coordination hence accounting for the obtained down field shift at 115.17 ppm. If the coordination was side-on through the π system of the triple bond, a farther downfield resonance in the 180–240 ppm range would have been detected.37
The protons are matched to the carbon peaks as shown on the 1H 13C HSQC NMR spectra in Fig. 10a and b for both the ODA and Cu3N nanocubes respectively and all the protons and carbons are accounted for. This confirms that copper is in the oxidation state of 1+ since there are no unpaired electrons in the analyzed Cu3N NPs. If copper existed as Cu0 or Cu2+ in the analyzed Cu3N ‘perfect’ nanocubes that were obtained after 15 min, then the spectrum would not have been clearly resolved due to the unpaired electrons that exist in the 4s and 3d orbitals respectively.
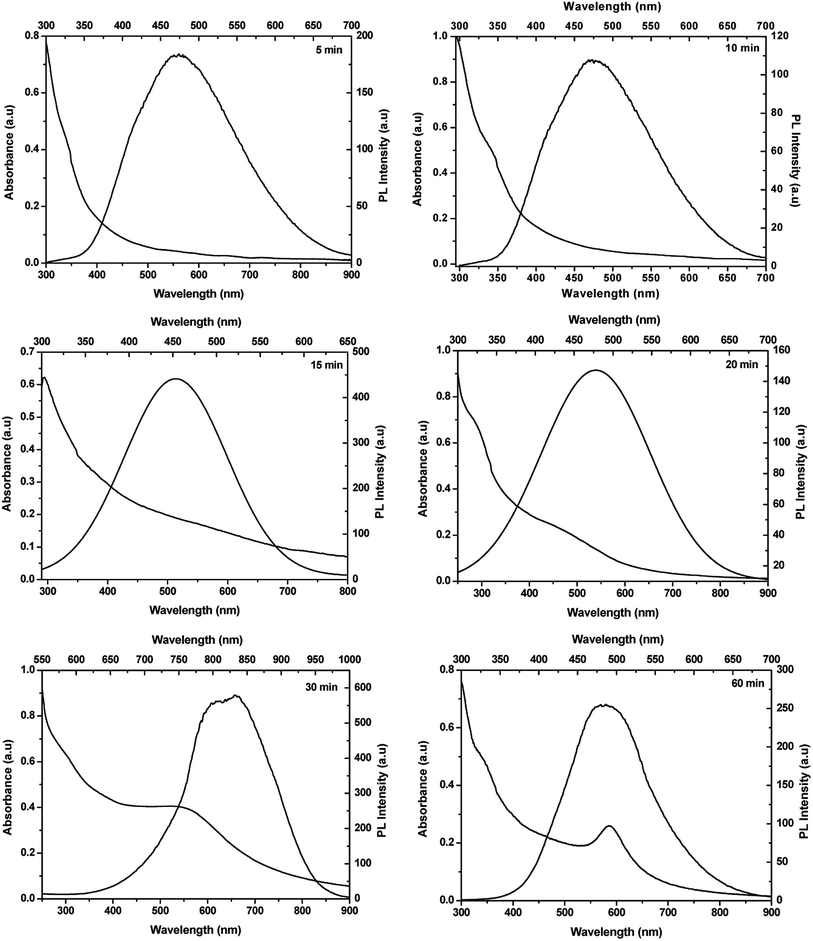
The optical properties of the resultant Cu3N NPs were studied using UV-vis absorption and photoluminescence spectroscopy and the results are depicted in Fig. 11. In literature, there has been controversy with regards to the optical properties of Cu3N. From the varying band gap values obtained both theoretically and experimentally, they are said to vary between 0.23 eV and 2.06 eV, to the nature of the band gap transition whether it is direct or indirect.38,39 Presented in Table 1 is the summary of the optical properties. A single sharp excitonic peak is observed for 5 and 10 min samples whilst a tailing spectrum, also with a single excitonic peak is observed for the 15 min sample. Two peaks are observed for particles synthesized from 20 min to 60 min. From band structure calculations, three transitions have been reported, one indirect and two direct band gaps at the Γ and R points.39 The exciton A is blue-shifted from the reported values and the transition is in the visible region of the electromagnetic spectrum whilst exciton B is consistent with the reported values with the band gap energies of 1.6–2.04 eV. The observed trends could be because of quantum confinement effects in the nanocrystals as most reported results are for bulk Cu3N.38,39 Also possible, is the presence of the localized surface plasmon resonance (LSPR) due to the presence of copper nanoparticles. The LSPR for Cu nanoparticles is usually found around 570 nm and can be blue or red shifted due to the size and shape of the nanoparticles, the photoluminescence is usually weak hence the observed photoluminescence peaks are only associated with the semiconducting Cu3N NPs.40
| Time (min) | Exciton A (nm) | Exciton A (eV) | Exciton B (nm) | Exciton B (eV) | λ max (nm) | λ max (eV) |
|---|---|---|---|---|---|---|
| 5 | 476 | 2.61 | — | — | 476 | 2.61 |
| 10 | 456 | 2.72 | — | — | 476 | 2.61 |
| 15 | 436 | 2.84 | — | — | 455 | 2.73 |
| 20 | 378 | 3.28 | 608 | 2.04 | 478 | 2.59 |
| 30 | 340 | 3.65 | 760 | 1.63 | 832 | 1.49 |
| 60 | 400 | 3.10 | 695 | 1.78 | 487 | 2.55 |
The photoluminescence spectra are red-shifted with respect to the excitonic peak A for samples synthesized for 10–20 min as well as 60 min however for the 30 min sample the emission maximum correlates with the exciton B transition. The photoluminescence spectra confirm that the resultant nanoparticles are direct band gap semiconductors. Evident from the results is the complexity of the optical properties and the need to further probe these. Based on the obtained results, it is quite evident that Cu3N is an interesting and rather complex semiconductor. Nevertheless, herein using TEM, a mechanism for the evolution of Cu3N morphologies can be postulated and it is depicted in Fig. 12. The reaction was started by dissolution of Cu(NO3)2·3H2O in OD and mixed with ODA and then the temperature was raised from room temperature to 110 °C resulting in a deep blue mixture. After an hour, the temperature was slowly increased and at about 165 °C, a very pale yellow clear solution was obtained. This could be a result of the formation of a complex between the Cu(I) ions and octadecylamine as has been observed in a previous study.10 The blue coloration that was caused by the unpaired electron in the Cu2+ suddenly disappeared due to the reduction of Cu2+ to Cu+ in the presence of ODA. Due to complete d orbital, Cu+ does not give off color hence the yellow color is brought about by the complexation that occurs between the Cu(I) ions and the amine. The solution then slowly turned to a clear brown around 200 °C and then to an opaque brown by the time the temperature reached 260 °C. It has been shown that in direct heating methods, high quality crystals are generally obtained at high temperatures that are above 200 °C hence 260 °C was chosen as the reaction temperature in this study. The introduction of the brown color indicates the initial stages of the formation of Cu3N nuclei. An attempt to analyze the samples that were obtained after 30 s at 260 °C proved futile as the aliquots turned greenish blue upon adding organic solvents like ethanol or chloroform in an attempt to clean the NPs. This color change indicated the oxidation of the Cu+ to Cu2+. There was no marked difference between the NPs obtained after 1 min and those obtained at 5 min except that there were more defined nanocubes at 5 min as compared to 1 min as seen in Fig. 12. As such, below 1 min, the nuclei that had formed were not yet stable and reacted rapidly with air and the added solvent. ODA has been reported as having a tendency to bind to the 100 plane of Cu3N NPs hence it slows down the growth process and facilitates the formation of cubes.10 High population of ‘dots’ were detected in the 5 min sample while few cubes had already been formed although not fully developed. The less stable small nanocrystals undergo Oswald ripening41 as they slowly dissolve and recrystallize on the larger particles giving rise to nanocubes. This was observed at 10 min where self-assembly of cubes was noted and fewer but bigger dots were seen on and in-between the cubes. Subsequent ripening or shaping led to the formation of the ‘perfect’ nanocubes with smooth edges and no apparent blemishes at 15 min. Continued heating resulted in further reduction which led to the detected small peak of Cu in the XRD of the 20 min sample. The reduction of the copper ions on the surface of the cubes led to the influx of Cu+ ions from the core to the surface of the nanocubes.42 As such, the smooth surface that had been observed on the ‘perfect’ nanocubes could no longer hold on the NPs that were obtained at 20 min. The cationic movement of Cu+ from the core of the cubes to the edges continued and voids can be seen in the interior of the distorted cubes, consequently resulting in the reemission of nitrogen. As the cubes broke down, they joined together to form larger cubes with void interiors. That is why at this stage, pronounced Cu peaks could be detected at 30 min as Cu3N decomposed and the Cu+ was reduced to Cu0 whilst the N3− was oxidized to nitrogen gas. Finally with time, ‘all’ Cu3N had been reduced to Cu hence no copper nitride peaks could be detected after 60 min.
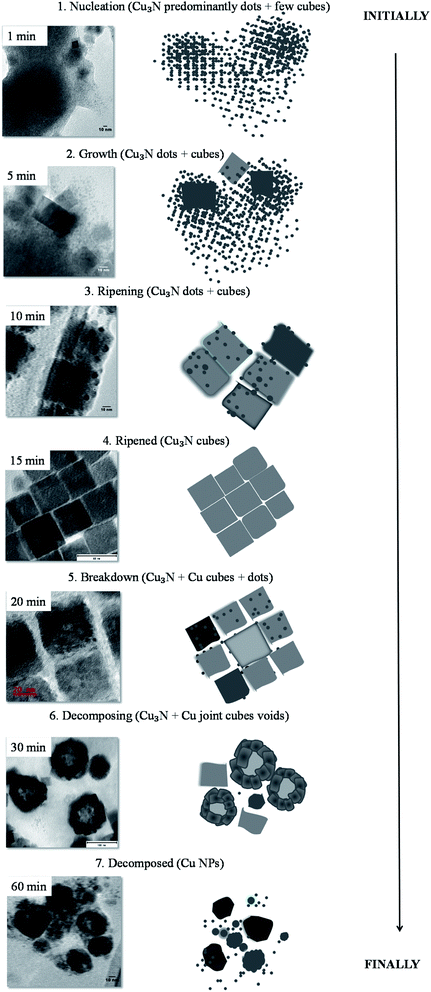 | ||
| Fig. 12 Steps followed in the colloidal thermolysis of Cu(NO3)2 in ODA to form Cu3N NPs and eventually Cu. | ||
To determine if this is a unique feature to octadecylamine, the coordinating ligand was changed from ODA to hexadecylamine (HDA). The same synthetic procedure was followed and the resultant X-ray diffractograms are shown in Fig. 13.
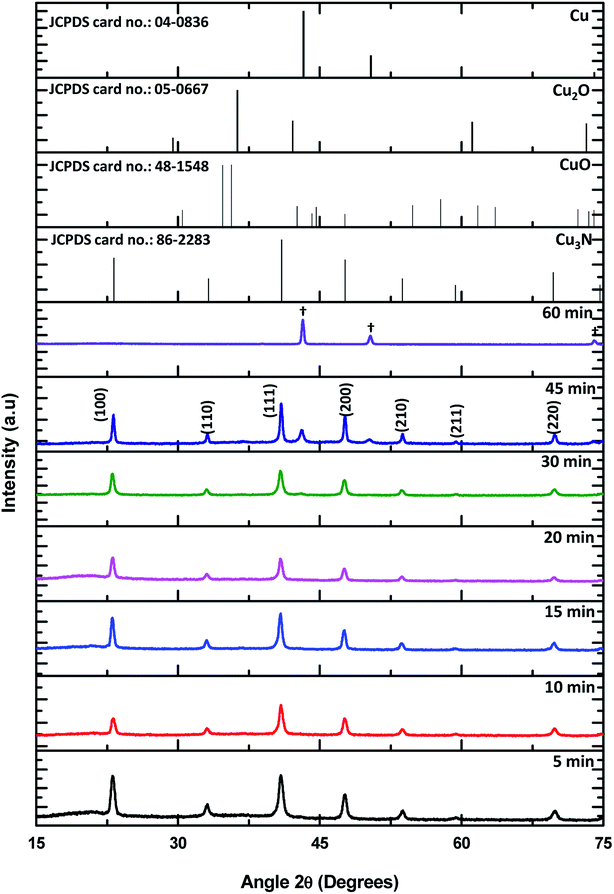 | ||
| Fig. 13 Powder XRD of Cu3N NPs obtained after (a) 5, 10, 15, 20, 30, 45 and 60 min at 260 °C in HDA. The symbol (†) shows peaks for Cu decomposition product of the capping agent. | ||
The XRD diffractograms showed a similar pattern with the one obtained in ODA, the main difference being the initial detection of Cu occurring after 30 min whilst in ODA it was detected at 20 min. This difference was seen in the TEM images of the NPs that were obtained in HDA. ‘Perfect’ nanocubes could be seen at 15 min but not much variation was detected in the 20 min sample. Mixed morphology of nanocubes and tiny dot-like NPs were clearly seen in the 5 and 10 min samples and breaking down of the nanocubes was only detected after 30 min. Just like in ODA, compact Cu NPs were obtained after 60 min and no nanocubes could be seen (Fig. 14). The 1H NMR and 13C NMR of HDA shown in Fig. 2S and 3S† respectively were similar to the ODA NMR and therefore the mechanism was consistent with ODA.
 | ||
| Fig. 14 TEM images of Cu3N NPs obtained after 5, 10, 15, 20 and 30 min and Cu at 60 min in HDA at 260 °C. | ||
4. Conclusion
The thermal decomposition of Cu(NO3)2 at 260 °C in ODA lead to the formation of dot-like nuclei which resulted in the formation of Cu3N nanocubes. A cloud of Cu3N nuclei with developing nanocubes was obtained after 5 min. Aligned nanocubes were seen at 10 min with the presence of bigger but fewer ‘dots’. With time, well-defined Cu3N nanocubes were obtained after 15 min and this was observed in both ODA and HDA. Further heating resulted in the breaking down of the nanocubes and XRD indicated the presence of both Cu3N and Cu at 30 min. By 60 min, the Cu3N had completely decomposed to Cu. Furthermore, results from the FTIR and NMR spectroscopy suggested the formation of a nitrile (RCN) as a product in the synthesis of Cu3N in ODA and that the particles are possibly capped by the nitrile. The proposed mechanism suggests that the Cu3N NPs dorms from the reaction of Cu with N2, however this requires further experimental probing. The optical studies revealed that the as-synthesized nanoparticles were semiconducting with two transitions observed in the UV-vis absorption spectra. The synthesized Cu3N NPs can potentially be applied as microscopic metallic links due to its metallization at relatively low temperatures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the National Research Foundation (South Africa) and the University of the Witwatersrand. The authors' gratitude is duly directed to the Microscopy Unit (MMU) at Wits for the TEM and XRD analysis.References
-
Z. Cao, Thin film growth: physics, materials science and applications, Woodheal Publishing Limited, 2011 Search PubMed
.
- T. Nakamura, N. Hiyoshi, H. Hayashi and T. Ebina, Preparation of plate-like copper nitride nanoparticles from a fatty acid copper (II) salt and detailed observations by high resolution transmission electron microscopy and high-angle annular dark-field scanning transmission electron microscopy, Mater. Lett., 2015, 139, 271–274 CrossRef CAS
.
- T. Nakamura, H. Hayashi, T.-A. Hanaoka and T. Ebina, Preparation of copper nitride (Cu3N) nanoparticles in long-chain alcohols at 130 – 200 °C and nitridation mechanism, Inorg. Chem., 2013, 53, 710–715 CrossRef PubMed
.
- M. D. Reichert, M. A. White, M. J. Thompson, G. J. Miller and J. Vela, Preparation and instability of nanocrystalline cuprous nitride, Inorg. Chem., 2015, 54, 6356–6362 CrossRef CAS PubMed
.
- R. Szczęsny, E. Szłyk, M. A. Wiśniewski, T. K. Hoang and D. H. Gregory, Facile preparation of copper nitride powders and nanostructured films, J. Mater. Chem. C, 2016, 4, 5031–5037 RSC
.
- J. Choi and E. Gillan, Solvothermal synthesis of nanocrystalline copper nitride from an energetically unstable copper azide precursor, Inorg. Chem., 2005, 44, 7385 CrossRef CAS PubMed
.
- S. Mondal and C. R. Raj, Copper nitride nanostructure for the electrocatalytic reduction of oxygen: kinetics and reaction pathway, J. Mater. Chem. C, 2018, 122, 18468–18475 CAS
.
- T. Nakamura, H. Hayashi and T. Ebina, Preparation of copper nitride nanoparticles using urea as a nitrogen source in a long-chain alcohol, J. Nanoparticle Res., 2014, 16, 2699 CrossRef
.
- Z.-Q. Liang, T.-T. Zhuang, A. Seifitokaldani, J. Li, C.-W. Huang, C.-S. Tan, Y. Li, P. De Luna, C. T. Dinh and Y. Hu, Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2, Nat. Commun., 2018, 9, 1–8 CrossRef CAS PubMed
.
- H. Wu and W. Chen, Copper nitride nanocubes: size-controlled synthesis and application as cathode catalyst in alkaline fuel cells, J. Am. Chem. Soc., 2011, 133, 15236–15239 CrossRef CAS PubMed
.
- D. Wang and Y. Li, Controllable synthesis of Cu-based nanocrystals in ODA solvent, Chem. Commun., 2011, 47, 3604–3606 RSC
.
- R. K. Sithole, L. F. Machogo, M. A. Airo, S. S. Gqoba, M. J. Moloto, P. Shumbula, J. Van Wyk and N. Moloto, Synthesis and characterization of Cu3N nanoparticles using pyrrole-2-carbaldpropyliminato Cu (II) complex and Cu (NO3)2 as single-source precursors: the search for an ideal precursor, New J. Chem., 2018, 42, 3042–3049 RSC
.
- P. Xi, Z. Xu, D. Gao, F. Chen, D. Xue, C.-L. Tao and Z.-N. Chen, Solvothermal synthesis of magnetic copper nitride nanocubes with highly electrocatalytic reduction properties, RSC Adv., 2014, 4, 14206–14209 RSC
.
- D. S. Wang, T. Xie, Q. Peng, S. Y. Zhang, J. Chen and Y. D. Li, Direct thermal decomposition of metal nitrates in octadecylamine to metal oxide nanocrystals, Chem. - Eur. J., 2008, 14, 2507–2513 CrossRef CAS PubMed
.
- T. Maruyama and T. Morishita, Copper nitride and tin nitride thin films for write-once optical recording media, Appl. Phys. Lett., 1996, 69, 890–891 CrossRef CAS
.
- R. Cremer, M. Witthaut, D. Neuschütz, C. Trappe, M. Laurenzis, O. Winkler and H. Kurz, Deposition and Characterization of Metastable Cu3N Layers for Applications in Optical Data Storage, Microchim. Acta, 2000, 133, 299–302 CrossRef CAS
.
- A. Majumdar, S. Drache, H. Wulff, A. K. Mukhopadhyay, S. Bhattacharyya, C. A. Helm and R. Hippler, Strain effects by surface oxidation of Cu3N thin films deposited by DC magnetron sputtering, Coatings, 2017, 7, 64 CrossRef
.
- X. Fan, Z. Wu, H. Li, B. Geng, C. Li and P. Yan, Morphology and thermal stability of Ti-doped copper nitride films, J. Phys. D: Appl. Phys., 2007, 40, 3430 CrossRef CAS
.
- A. Fallberg, M. Ottosson and J. O. Carlsson, CVD of copper(I) nitride, Chem. Vap. Deposition, 2009, 15, 300–305 CrossRef CAS
.
- C. Navio, M. Capitan, J. Alvarez, F. Yndurain and R. Miranda, Intrinsic surface band bending in Cu3N (100) ultrathin films, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 085105 CrossRef
.
- L.-C. Wang, B.-H. Liu, C.-Y. Su, W.-s. Liu, C.-C. Kei, K.-W. Wang and T.-P. Perng, Electronic band structure and electrocatalytic performance of Cu3N nanocrystals, ACS Appl. Nano Mater., 2018, 1(7), 3673–3681 CrossRef CAS
.
- M. Trivedi, G. Singh, A. Kumar and N. P. Rath, A cyano and end-to-end azido bridged 3D copper (II)–copper (I) mixed-valence coordination polymer and its transformation to copper nitride nanoparticles, RSC Adv., 2014, 4, 34110–34116 RSC
.
- A. Miura, T. Takei and N. Kumada, Synthesis of Cu3N from CuO and NaNH2, J. Asian Ceram. Soc., 2014, 2, 326–328 CrossRef
.
-
A. K. Mukhopadhyay, A. Roy, S. C. Das, H. Wulff, R. Hippler and A. Majumdar, Self-buckled effect of cubic Cu3N film: Surface stoichiometry, in AIP Conference Proceedings, AIP Publishing, 2018, p. 100078 Search PubMed
.
- O. Akhavan, R. Azimirad, S. Safa and E. Hasani, CuO/Cu(OH)2 hierarchical nanostructures as bactericidal photocatalysts, J. Mater. Chem., 2011, 21, 9634–9640 RSC
.
- M. Younas, J. Shen, M. He, R. Lortz, F. Azad, M. Akhtar, A. Maqsood and F. C. Ling, Role of multivalent Cu, oxygen vacancies and CuO nanophase in the ferromagnetic properties of ZnO: Cu thin films, RSC Adv., 2015, 5, 55648–55657 RSC
.
- J. Wang, Q. Deng, M. Li, K. Jiang, J. Zhang, Z. Hu and J. Chu, Copper ferrites@ reduced graphene oxide anode materials for advanced lithium storage applications, Sci. Rep., 2017, 7, 8903 CrossRef PubMed
.
- R. Deshmukh, G. Zeng, E. Tervoort, M. Staniuk, D. Wood and M. Niederberger, Ultrasmall Cu3N nanoparticles: surfactant-free solution-phase synthesis, nitridation mechanism, and application for lithium storage, Chem. Mater., 2015, 27, 8282–8288 CrossRef CAS
.
- J. Choi and E. G. Gillan, Solvothermal metal azide decomposition routes to nanocrystalline metastable nickel, iron, and manganese nitrides, Inorg. Chem., 2009, 48, 4470–4477 CrossRef CAS PubMed
.
-
R. P. Pohanish, Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens, William Andrew, 2008 Search PubMed
.
- Q. Sun, Z. Wang, D. Wang, Z. Hong, M. Zhou and X. Li, A review on the catalytic decomposition of NO to N2 and O2: catalysts and processes, Catal. Sci. Technol., 2018, 8, 4563–4575 RSC
.
- O. D. Pavel, P. Goodrich, L. Cristian, S. M. Coman, V. I. Pârvulescum and C. Hardacre, Direct oxidation of amines to nitriles in the presence of ruthenium-terpyridyl complex immobilized on ILs/SILP, Catal. Sci., 2015, 5, 2696–2704 RSC
.
- J. Wang, J. T. Chen, X. M. Yuan, Z. G. Wu, B. B. Miao and P. X. Yan, Copper nitride (Cu3N) thin films deposited by RF magnetron sputtering, J. Cryst. Growth, 2006, 286, 407–412 CrossRef CAS
.
- C. M. Caskey, R. M. Richards, D. S. Ginley and A. Zakutayev, Thin film synthesis and properties of copper nitride, a metastable semiconductor, Mater. Horiz., 2014, 1, 424–430 RSC
.
- F. Gunstone, High-resolution 13C NMR spectra of long-chain acids, methyl esters, glycerol esters, wax esters, nitriles, amides, alcohols and acetates, Chem. Phys. Lipids, 1993, 66, 189–193 CrossRef CAS
.
- R. J. Abraham and M. Reid, Proton chemical shifts in NMR. Part 15-proton chemical shifts in nitriles and the electric field and π-electron effects of the cyano group, Magn. Reson. Chem., 2000, 38, 570–579 CrossRef CAS
.
-
A. B. Jackson, Nitrile reduction and carbon monoxide replacement in tungsten(II) bis(acetylacetonate) complexes, PhD dissertation, The University of North Carolina at Chapel Hill, 2008
.
- U. Hahn and W. Weber, Electronic structure and chemical-bonding mechanism of Cu3N, CuI3NPd, and related Cu (I) compounds, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, 12684 CrossRef CAS PubMed
.
- M. Mikula, D. Búc and E. Pincik, Electrical and optical properties of copper nitride thin films prepared by reactive DC magnetron sputtering, Acta Phys. Slovaca, 2000, 51, 35–43 Search PubMed
.
- O. A. Yeshchenko, I. S. Bondarchuk and M. Yu. Losytskyy, Surface plasmon enhanced photoluminescence from copper nanoparticles: Influence of temperature, J. Appl. Phys., 2014, 116, 054309 CrossRef
.
- M. Mlambo, M. J. Moloto, N. Moloto and P. S. Mdluli, Influence of temperature and precursor concentration on the synthesis of HDA-capped Ag2Se nanoparticles, Mater. Res. Bull., 2013, 48(6), 2196–2200 CrossRef CAS
.
- A. Bruckman and G. Simkovich, Concerning the mechanism of scale growth due to cation diffusion in Fe2O3 and CuS, Corros. Sci., 1972, 12, 595–601 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09546b |
| This journal is © The Royal Society of Chemistry 2020 |