Open Access Article
Open Access ArticleUsing a visible light-triggered pH switch to activate nanozymes for antibacterial treatment†
Juqun Xiabc,
Jingjing Zhanga,
Xiaodong Qiand,
Lanfang Ana and
Lei Fan *e
*e
aInstitute of Translational Medicine, Department of Pharmacology, School of Medicine, Yangzhou University, Yangzhou, Jiangsu 225001, China
bJiangsu Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment of Senile Diseases, Yangzhou, Jiangsu 225001, China
cJiangsu Co-Innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, College of Veterinary Medicine, Yangzhou, 225009, Jiangsu, China
dDepartment of Cardiology, First Affiliated Hospital of Soochow University, Suzhou, 215006, Jiangsu, China
eSchool of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, Jiangsu, China. E-mail: fanlei@yzu.edu.cn
First published on 3rd January 2020
Abstract
Here, we develop a visible light-triggered platform to activate the biomimetic activity of CuS nanoparticles by incorporating a photoacid generator. Under visible-light illumination, the remarkable pH decrease, caused by the intramolecular photoreaction of the photoacid generator, activates the peroxidase-like activity of the CuS nanoparticles. This visible light-triggered pH switch meets the antibacterial demands of peroxidase mimics perfectly in bacteria-infected wounds. Importantly, the built-in torches of mobile phones are able to replace the visible-light source to activate the peroxidase-mimicking activity of CuS nanoparticles to combat bacteria, which greatly promotes the utility and adaptability of this antibacterial platform.
Introduction
Nanozymes have aroused much interest due to their practical advantages over natural enzymes, including high stability, straightforward preparation, and low cost.1 A growing number of nanomaterials have been found to exhibit enzyme mimetic activities, such as that of oxidase, peroxidase, superoxide dismutase, and catalase.2 These activities of nanozymes have since been applied to bio-analysis,3 disease diagnosis and therapy,4 and environmental chemistry.5 For example, the catalytic reaction of peroxidase, which can convert hydrogen peroxidase (H2O2) into highly toxic hydroxyl radicals (˙OH), has been widely used to kill cancer cells and bacteria.6 For instance, peroxidase mimics (such as Au,7 Fe3O4,8 and graphene9), with the assistance of H2O2, have been widely utilized to treat bacteria-infected wounds. However, similar to horseradish peroxidase (HRP), the activities of peroxidase mimics are pH dependent, with most being inactive at near neutral pH.10 As for wound treatment, the pH of infected sites ranges from 6.5 to 8.5, with higher pH for more severe infections.11 This feature easily decreases the antibacterial effects of peroxidase mimics. Therefore, understanding how to activate and maintain peroxidase-like (POD) activity of nanozymes in bacteria-infected wounds is highly desirable to promote the application of nanozymes in biomedicine.To overcome this problem, we introduced a photoacid generator (PAG) to a nanozyme/H2O2 antibacterial system. PAGs, as photoinduced acid sources,12 attract widely attention due to their wide applications in photolithography, polymerization, and stimuli-responsive systems.13 Especially, PAGs possess the ability to induce proton dissociation under light irradiation, thus changing pH of an aqueous solution.14 Protonated merocyanine (MEH) is reported to be a long-lived PAGs. As shown in Fig. S1,† under visible-light illumination, a proton is rapidly generated through the intramolecular photoreaction of MEH, resulting in a decrease in pH.15 This unique physicochemical property meets the antibacterial demands of peroxidase mimics perfectly.
In this work, we synthesized albumin-stabilized copper sulfide nanoparticles (CuS NPs). CuS, known as a semiconductor, has received wide attention because of its potential application in electronic, optical and catalysis fields.16 Besides the applications as a semiconductor, CuS, with low systemic toxicity, also shows great potential in biomedical fields, such as bio-molecular sensing, cancer therapy, and molecular imaging.17 Here, we found that CuS exhibited obvious activity as an excellent peroxidase (POD) mimic. In particular, with the assistance of MEH and irradiation of visible light, the POD-like activity of the CuS NPs was activated in the wounds, thereby exerting a remarkable antibacterial effect and accelerating the wound-healing process. In addition, the release of Cu2+ from CuS mixed system containing MEH could also be triggered by visible-light irradiation, thus further enhancing the antibacterial effects. It was worth stressing that the built-in torches of mobile phones, including iPhone, HUAWEI, XIAOMI, and OPPO, were able to replace the visible-light source to activate the POD-like activity of the CuS NPs, which greatly promoted the utility and adaptability of our antibacterial platform in practical application.
Results and discussion
Herein, the CuS NPs were prepared by directly mixing CuCl2 and Na2S solution in the presence of bovine serum albumin (BSA) at 90 °C for 15 min.18 BSA was utilized here to control the stability and size of the CuS NPs during synthesis.19 A typical High Resolution Transmission Electron Microscope (HRTEM) image of the CuS NPs was shown in Fig. 1a. Based on statistical analysis (Fig. S2†), the average size of the CuS NPs was ca. 6 nm in diameter. Additionally, the dark field TEM image and the corresponding element mapping images of CuS NPs were shown in Fig. 1b–g. Besides the signals of Cu and S from CuS, we also observed the C, N, O signals from BSA, which indicated that BSA molecules coated uniformly in CuS NPs. Fig. 2a showed the powder X-ray diffraction (XRD) patterns of the CuS NPs, which were indexed to standard CuS (JCPDS No. 06-0464).20 X-ray photoelectron spectroscopy (XPS) spectra (Fig. 2b and S3†) demonstrated the presence of Cu, S, C, O, and N elements in the CuS NPs. The C, N, and O peaks derived from BSA also indicated the successful coating of BSA on the surface of the CuS NPs. The Cu 2p peaks, due to Cu 2p3/2 and Cu 2p1/2, appeared at 932.12 and 952.08 eV (Fig. 2c), respectively, corresponding to Cu(II).21 Moreover, by comparing the TGA (thermogravimetric analysis) curves of BSA and BSA coated CuS (BSA–CuS), the percentage of CuS in BSA–CuS nanocomposites was about 18% (w/w) (Fig. 2d). Over all, these results illustrated the successful synthesis of the BSA coated CuS NPs.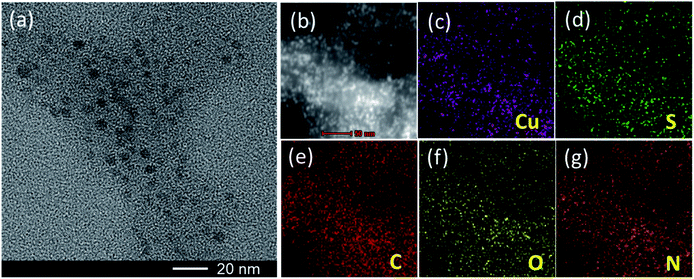 | ||
| Fig. 1 (a) HRTEM image of the CuS NPs. (b) Dark-field TEM image of the CuS NPs. (c–g) TEM elemental mappings of Cu, S, C, O and N in the CuS NPs, respectively. | ||
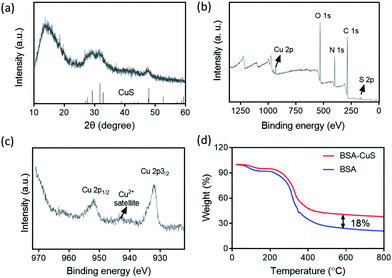 | ||
| Fig. 2 (a) XRD pattern of the CuS NPs. (b) XPS spectrum of the CuS NPs. (c) Cu 2p high-resolution XPS spectrum of CuS NPs. (d) TGA curves of BSA and BSA coated CuS NPs. | ||
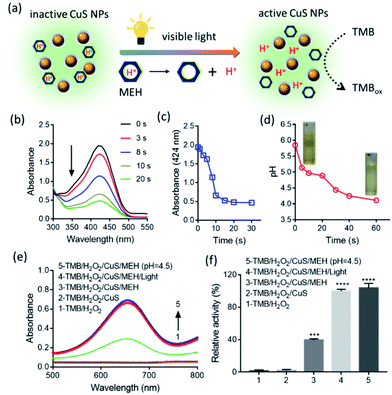
To achieve visible light-activated POD-like activity of the CuS NPs, a photoacid generator plays an important role. The working principle was illustrated in Fig. 3a. Firstly, MEH was synthesized according to previous research and its chemical structure was confirmed by 1H Nuclear Magnetic Resonance (1H NMR) spectroscopy,22 as shown in Fig. S4.† The UV-Vis spectra of MEH (Fig. 3b) before illumination showed a characteristic absorption peak at 424 nm. Upon visible-light irradiation, the absorbance of MEH decreased quickly by 75.5% in 20 s (Fig. 3c), indicating its rapid photoreaction. Moreover, the initial pH of the MEH solution was about 5.8. When the MEH solution was irradiated with visible light, the color of the solution changed from yellow to colorless (Fig. 3d, inset), and the pH decreased to 4.1 within 60 s (Fig. 3d). Moreover, in the dark, the absorbance of MEH slowly returned within 10 min (Fig. S5a and b†), and synchronously, the pH increased gradually and reverted to 5.8 (Fig. S5c†). It was worth stressing that the color fading of MEH (from yellow to colorless) was also triggered by illumination from the built-in torches of mobile phones. As shown in Fig. S6,† the built-in torches of different mobile phones, including iPhone, HUAWEI, XIAOMI, and OPPO, also caused the rapid decrease of MEH absorbance at 424 nm in 30 s, indicating that the proton dissociation of MEH molecules could be triggered simply and conveniently with such devices. Furthermore, during the five on/off cycles of visible-light irradiation, the MEH solution maintained good proton dissociation ability (Fig. S7†), indicating that MEH was a stable, recyclable, and long-lived photoacid.
Next, we evaluated the POD-like activity of the CuS NPs. 3,3′,5,5′-tetramethylbenzidine (TMB) was used as a substrate to yield a TMBox product during catalytic reaction. As shown in Fig. S8,† the CuS NPs efficiently catalyzed the oxidation of TMB in the presence of H2O2 to produce TMBox with a characteristic absorption peak at 652 nm. In contrast, the reaction rarely occurred in the absence of H2O2 or CuS NPs, confirming that the intrinsic POD-like activity was a typical property of the CuS NPs. Analyzing the curves using the Michaelis–Menten equation, we detected the maximum initial velocity (Vmax) and Michaelis–Menten constant (Km), with results shown in Fig. S9 and Table S1.† Similar to HRP, the catalytic activity of the CuS NPs was dependent on pH and temperature (Fig. S10†).23 More precisely, the CuS NPs showed the strongest POD-like activity at pH 4.5. With the increase of pH, the catalytic activity of the CuS NPs decreased gradually. At pH 7.0, the relative POD-like activity of CuS NPs was only about 20% of the maximum activity. This result demonstrated that the CuS NPs hold the pH-dependent POD-like activity, and this activity easily inactivated at near neutral pH.
Then, we investigated whether visible light could activate the POD-like activity of the CuS NPs in the presence of MEH at neutral pH. As shown in Fig. 3e, at pH 7.0, no distinct absorption peak of TMBox was observed at 652 nm in the TMB/H2O2 and TMB/H2O2/CuS groups, suggesting that the POD-like activity of the CuS NPs was inactive. After adding MEH to the TMB/H2O2/CuS system, a weak absorption peak could be detected at 652 nm. This partial activity recovery of the CuS NPs was due to the MEH solution itself being weakly acidic (pH 5.8). Importantly, after visible-light illumination, the TMBox absorption value was further promoted to its maximum value obtained at pH 4.5. The relative activity under different conditions, as shown in Fig. 3f, indicated that the POD-like activity of the CuS NPs nearly returned to its maximum vitality upon visible-light exposure. Thus, the pH decrease induced by irradiation of photosensitive MEH almost entirely activated the POD-like activity of the CuS NPs.
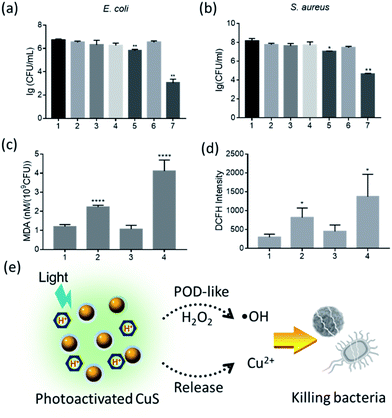
Subsequently, we tested the antibacterial activity of CuS NPs/H2O2 system under different pH conditions. As shown in Fig. S11,† under weakly acidic conditions (pH = 4.5), the combination of CuS/H2O2 was effective at suppressing the growth of both Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). At near neutral pH (7.0), however, bacterial growth was not observably inhibited. We also noted that the CuS NPs without H2O2 at pH 4.5 partially inhibited the growth of E. coli. This antibacterial activity could be attributed to the release of Cu2+ ions from the CuS NPs under weakly acidic conditions (Fig. S12†), and Cu2+ ions were proved to process the antibacterial ability.24 Just as shown in Fig. S13,† the Cu2+ derived from CuCl2 exhibited relatively strong antibacterial activity against E. coli, but less effective activity against S. aureus. Overall, our results indicated that the antibacterial nature of the CuS NPs (based on their POD-like activity) was pH dependent and was only activated under acidic conditions.
Regarding the pH dependence of antibacterial ability, we expected that visible-light illumination was able to activate the antibacterial activity of the CuS NPs in the presence of a photoacid. Exclusion experiments, including irradiation time and concentration of MEH, were first carried out. As shown in Fig. S14,† with the increase of irradiation time (0–15 min) or MEH concentration (0–500 μM), the bacterial viability (S. aureus and E. coli) was well maintained, indicating that exposure to visible light or MEH alone slightly affected bacterial viability. In the following tests, we fixed the concentration of MEH at 500 μM and the powder density at 375 mW cm−1 for 15 min. As shown in Fig. 4a for E. coli, the CuS/MEH/light and CuS/H2O2/MEH/light groups showed significant differences to the control group. No distinct antibacterial activity was observed for the other groups at pH 7.0. Moreover, the CuS/H2O2/MEH/light group was able to kill E. coli with −3.6 log-reduction, demonstrating a 630-fold increase in antibacterial activity compared with the CuS/MEH/light group. Similar antibacterial effects were shown towards S. aureus (Fig. 4b).
To further investigate the antibacterial mechanism of the CuS NPs, we assessed oxidative state in the bacterial strains. As reported,24,25 the antibacterial activity of Cu2+ was mainly resulted from the lipid peroxidation. Here, the lipid peroxidation was tested using a malondialdehyde (MDA) assay. Taking E. coli as an example, compared with the control, MDA levels increased by four-fold and two-fold in the CuS/H2O2/MEH/light and CuS/MEH/light groups, respectively. These results indicated that after adding H2O2 into the group of CuS/MEH/light, the POD-like activity of CuS NPs was able to convert H2O2 into free radicals to induce more serious lipid peroxidation, further promoting the antibacterial activity of CuS NPs (Fig. 4c). Moreover, the fluorescent intensity of 2,7-Dichlorodihydrofluorescein diacetate (DCFH, a probe of reactive oxygen species) also increased obviously in CuS/H2O2/MEH/light (Fig. 4d), confirming the production of free radicals through POD-like catalytic reaction. The morphology and structural integrity of the bacteria treated by CuS/H2O2/MEH/light were also severely affected (Fig. S15†). Thus, visible-light irradiation was able to activate the antibacterial activity of the CuS/H2O2 system in the presence of a photoacid (Fig. 4e). This antibacterial activity was mainly correlated with two factors: the released-Cu2+ and the free radicals produced by the POD-like activity of the CuS NPs. Importantly, these two capacities were both pH dependent and could be simultaneously activated by visible light in the presence of a photoacid. In previous reports,19,26 CuS NPs exhibited significant antibacterial activity due to their photothermal conversion performance. In our experiments, we demonstrated that CuS NPs could also showed the excellent antibacterial capability through exerting their enzyme mimicking activity. Although these two antibacterial modes were different, the results showed that CuS NPs could be applied as a potential antibacterial agent.
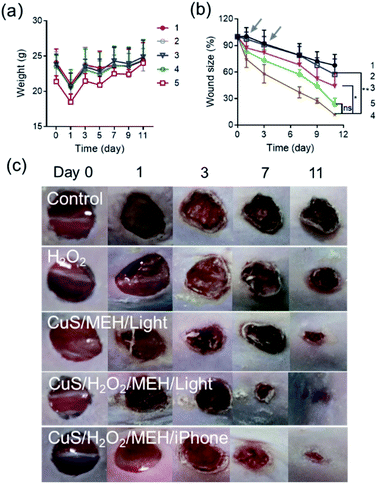
We next used an injury-infection model to further evaluate the antibacterial ability of our visible light-activated CuS nanozymes. As shown in Fig. 5a, each mouse exhibited marked weight loss after S. aureus infection for 24 h, indicating that the wound tissue was seriously infected. After treatment, we found that, compared with the control and other groups (Fig. 5b, c, and S16†), the CuS/H2O2/MEH/light group facilitated wound healing, whereas treatment with either CuS/MEH/light or H2O2 alone only induced moderate reduction in wound area. The wound closure rates on day 11 were 42.6%, 56.0%, and 88.1% for the H2O2, CuS/MEH/light, and CuS/H2O2/MEH/light-treated groups, respectively. The differences in the degrees of wound healing in the CuS/MEH and CuS/H2O2/MEH groups with and without light irradiation also indicated the importance of pH decrease induced by light illumination in the wound sites. Upon visible light illumination, a proton was rapidly produced through the intramolecular photoreaction of MEH, causing pH decrease in bacteria-infected wound sites, which activated the POD-like activity of CuS NPs to produce high levels of free radicals, thus facilitating for killing bacteria and accelerating wound healing. Moreover, the pH decrease could trigger the Cu2+ release from CuS NPs, which further promoted the antibacterial performance. Notably, when we used the built-in torch of an iPhone to replace the visible light, a satisfactory outcome in wound healing was achieved, with a wound closure rate of 76.4% on day 11 (Fig. 5c). Together, these results demonstrated the effectiveness of a light-activated peroxidase mimic, in combination with released Cu2+, reducing the burden of bacterial infections in vivo.
Conclusions
In summary, we developed a simple platform to activate the biomimetic activity of CuS NPs to kill bacteria. Considering that the POD-like activity of CuS NPs was pH-dependent and was deactivated easily in the bacteria-infected wounds. Thus, we introduced a photoacid into the system of CuS/H2O2. Upon light irradiation, the intramolecular photoreaction of MEH induced the pH decrease in wound sites, thus activating the activity of CuS NPs to produce high levels of free radicals, finally facilitating for killing bacteria and accelerating wound healing. Moreover, the pH decrease could also trigger the Cu2+ release from CuS NPs, which further promoted the antibacterial performance. Importantly, the built-in torches of mobile phones were able to replace the visible-light source, which greatly promotes the utility and adaptability of this antibacterial platform.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by the National Natural Science Foundation of China (No. 21703198). The authors also gratefully acknowledge financial support from the Priority Academic Program Development of Jiangsu Higher Education Institutions and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions.Notes and references
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- X. Wang, L. Qin, M. Zhou, Z. Lou and H. Wei, Anal. Chem., 2018, 90, 11696–11702 CrossRef CAS PubMed.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- K. Korschelt, M. N. Tahir and W. Tremel, Chemistry, 2018, 24, 9703–9713 CrossRef CAS PubMed.
- Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao, Z. Chen, J. Ren and X. Qu, Nat. Commun., 2018, 9, 3334 CrossRef PubMed.
- C. Wang, C. Liu, J. Luo, Y. Tian and N. Zhou, Anal. Chim. Acta, 2016, 936, 75–82 CrossRef CAS PubMed.
- P. C. Naha, Y. Liu, G. Hwang, Y. Huang, S. Gubara, V. Jonnakuti, A. Simon-Soro, D. Kim, L. Gao, H. Koo and D. P. Cormode, ACS Nano, 2019, 13, 4960–4971 CrossRef CAS PubMed.
- H. Sun, N. Gao, K. Dong, J. Ren and X. Qu, ACS Nano, 2014, 8, 6202–6210 CrossRef CAS.
- H. Wang, K. Wan and X. Shi, Adv. Mater., 2018, e1805368 Search PubMed.
- P. Mostafalu, A. Tamayol, R. Rahimi, M. Ochoa, A. Khalilpour, G. Kiaee, I. K. Yazdi, S. Bagherifard, M. R. Dokmeci, B. Ziaie, S. R. Sonkusale and A. Khademhosseini, Small, 2018, e1703509 CrossRef PubMed.
- R. Li, T. Nakashima, R. Kanazawa, O. Galangau and T. Kawai, Chem.–Eur. J., 2016, 22, 16250–16257 CrossRef CAS PubMed.
- W. H. Zhou, K. L. Braun, T. Y. Yu, J. K. Cammack, C. K. Ober, J. W. Perry and S. R. Marder, Science, 2002, 296, 1106–1109 CrossRef CAS PubMed.
- R. M. D. Nunes, M. Pineiro and L. G. Arnaut, J. Am. Chem. Soc., 2009, 131, 9456–9462 CrossRef CAS PubMed.
- Z. Shi, P. Peng, D. Strohecker and Y. Liao, J. Am. Chem. Soc., 2011, 133, 14699–14703 CrossRef CAS PubMed.
- H. Lee, S. W. Yoon, E. J. Kim and J. Park, Nano Lett., 2007, 7, 778–784 CrossRef CAS.
- S. Goel, F. Chen and W. Cai, Small, 2014, 10, 631–645 CrossRef CAS PubMed.
- J. Huang, J. Zhou, J. Zhuang, H. Gao, D. Huang, L. Wang, W. Wu, Q. Li, D. P. Yang and M. Y. Han, ACS Appl. Mater. Interfaces, 2017, 9, 36606–36614 CrossRef CAS PubMed.
- Y. Qiao, Y. Ping, H. Zhang, B. Zhou, F. Liu, Y. Yu, T. Xie, W. Li, D. Zhong, Y. Zhang, K. Yao, H. A. Santos and M. Zhou, ACS Appl. Mater. Interfaces, 2019, 11, 3809–3822 CrossRef CAS PubMed.
- T. P. Mofokeng, M. J. Moloto, P. M. Shumbula and P. Tetyana, Anal. Biochem., 2019, 580, 36–41 CrossRef CAS PubMed.
- Q. Feng, Y. Xu, B. Hu, L. An, J. Lin, Q. Tian and S. Yang, Chem. Commun., 2018, 54, 10962–10965 RSC.
- Y. Xu, J. Fei, G. Li, T. Yuan, Y. Li, C. Wang, X. Li and J. Li, Angew. Chem., Int. Ed., 2017, 56, 12903–12907 CrossRef CAS PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- X. Li, W. L. Yang, H. J. He, S. H. Wu, Q. Zhou, C. P. Yang, G. M. Zeng, L. Luo and W. Lou, Bioresour. Technol., 2018, 251, 274–279 CrossRef CAS PubMed.
- Q. Zhou, X. Li, Y. Lin, C. P. Yang, W. C. Tang, S. H. Wu, D. H. Li and W. Lou, Water Res., 2019, 158, 171–181 CrossRef CAS PubMed.
- M. Li, X. Liu, L. Tan, Z. Cui, X. Yang, Z. Li, Y. Zheng, K. Yeung, P. K. Chue and S. Wu, Biomater. Sci., 2018, 6, 2110–2121 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09343e |
| This journal is © The Royal Society of Chemistry 2020 |