Open Access Article
Open Access ArticleRegioselective, one-pot, multi-component, green synthesis of substituted benzo[c]pyrazolo[2,7]naphthyridines†
Abida Ashrafab,
Zahid Shafiq a,
Khalid Mahmooda,
Muhammad Yaqub*a and
Waqar Rauf
a,
Khalid Mahmooda,
Muhammad Yaqub*a and
Waqar Rauf c
c
aInstitute of Chemical Sciences, Bahauddin Zakariya University, Multan, Pakistan. E-mail: mayaqub2@yahoo.com
bDepartment of Chemistry, Kutchery Campus, The Women University Multan, Multan 60000, Pakistan
cNational Institute for Biotechnology and Genetic Engineering (NIBGE), PO Box 577, Faisalabad, Pakistan
First published on 5th February 2020
Abstract
An efficient and environmentally benign synthetic protocol has been developed for the synthesis of benzo[c]pyrazolo[2,7]naphthyridine derivatives through regioselective multi-component “on-water” reaction of isatin, malononitrile and 3-aminopyrazole. The Knoevenagel condensation of isatin with malononitrile resulted in the formation of arylidene, which subsequently underwent Michael addition with 3-aminopyrazole followed by basic hydrolysis, cyclization, decarboxylation and aromatization to give the target naphthyridines in good to excellent yields. The one-pot multi-component protocol was also employed to obtain the said naphthyridines in a lower yield (10–15%) than obtained by basic hydrolysis of spiro-intermediates. The present study shows attractive features such as the use of water as a green solvent, short reaction time, reduced waste products and transition metal free C–C and C–N bond formation. The structures of the synthesized derivatives were established through FTIR, 1H-NMR, 13C-NMR spectroscopy and ESI-mass spectrometry.
Introduction
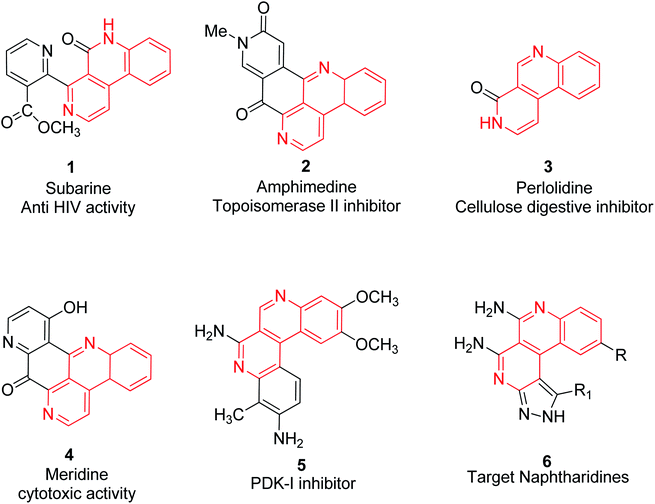
The diversity of oriented synthesis has inspired advances in drug design and the synthesis of stereochemical and structural variants of special molecular motifs resembling natural product skeletons.1 2,7-Naphthyridine scaffolds, known as small bispyridine structures, have recently gained attention in novel drug development. Out of the six isomeric forms of naphthyridines, benzo[c][2,7]naphthyridines are found in a number of biologically important alkaloids2,3 such as subarine 1,4 amphimedine 2,5 perlolidine 3,6 meridine 4 (ref. 7) and PDK-I inhibitor 5,8 as shown in Fig. 1.1,2 Pyrazole derivatives are an important class of nitrogen-containing heterocycles and have gained much interest in the fields of agriculture research and drug discovery.9 The pyrazole containing natural products including L-α-amino-β-(pyrazolyl-N)-propanoic acid,10 withasomnine,11 pyrazofurin,12 formycin,13 oxoformycin B,14 nostocine15,16 and so on, are scaffolds that exhibit a wide range of importance in medicinal chemistry.
Studies in the literature reveal that many of the benzo[c][2,7]naphthyridine derivatives17–21 have been synthesized employing new strategies to investigate the potential of fused five membered heterocycles such as isoxazolo-,22 cyclopenta-,23 pyrazolo-,24 imidazo-25 and furo-26,27 annulated 2,7-naphthyridines. Numerous reported elegant reactions that often involved multistep harsh reaction conditions using organic solvents and homogenous or heterogeneous catalysts.28,29
In particular, transition metal free synthesis is an ideal synthetic route starting from simple and easily available raw materials and carried out under green and standard reaction conditions with a single operation and excellent tolerance of multifunctional groups for further derivatization. The use of green solvents is well accepted and an effective solution for not having to use multistep harsh reaction conditions. Water is the most often used green solvent for organic syntheses and is the first choice of organic chemists as it is nontoxic, readily available, inexpensive, has valuable effects on reaction rates and selectivity of organic transformations.30 Various terminologies such as “on-water,” “in-water”, “in the presence of water” have evolved for when water is used as a reaction medium. Insolubility of several organic reactants in water make the reaction mixture heterogeneous which may cause difficulties in the use of water as a solvent.31 It has been observed that many organic reactions proceed faster in heterogeneous mixtures rather than in homogeneous mixtures. The in-water (homogeneous mixture) reactions usually accelerate the rate via an enforced polarity effect, hydrophobic and hydrogen bonding interactions, whereas in the case of on-water (heterogeneous mixture) reactions, the acceleration of reaction rates has been observed due to the presence of free OH groups on the organic-water interface.32,33 Herein, the development of new eco-friendly and efficient routes for the solid–liquid phase heterogeneous synthesis of benzo[c]pyrazolo[2,7]naphthyridines via an on-water multi-component reaction in the presence of NaOH, are described. Although, as far as is known, 2,7-naphthyridines fused with benzo at side-c and with pyrazole at side-f depicted as naphthyridine 6 (Fig. 1) are hitherto unknown. So this paper reports an on-water green synthetic methodology of benzo[c]naphthyridine derivatives fused with a pyrazole moiety.
Results and discussion
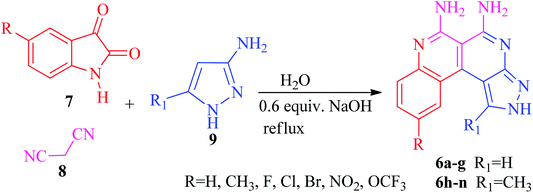
Synthesis of benzo[c]pyrazolo[2,7]naphthyridines
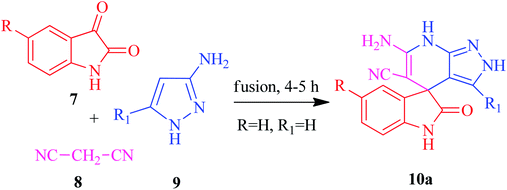
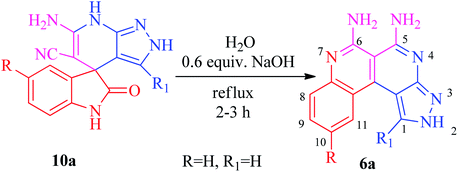
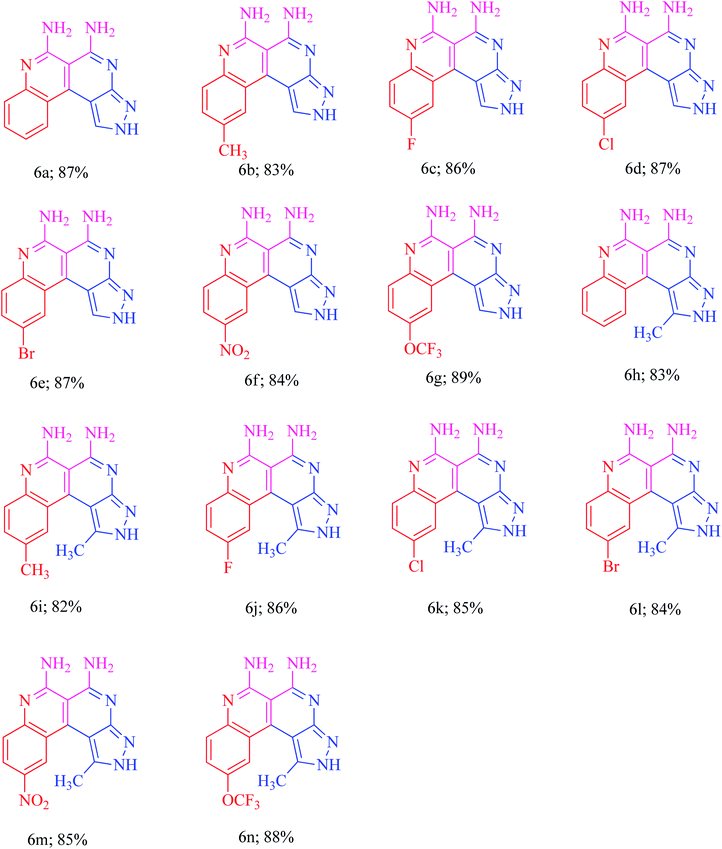
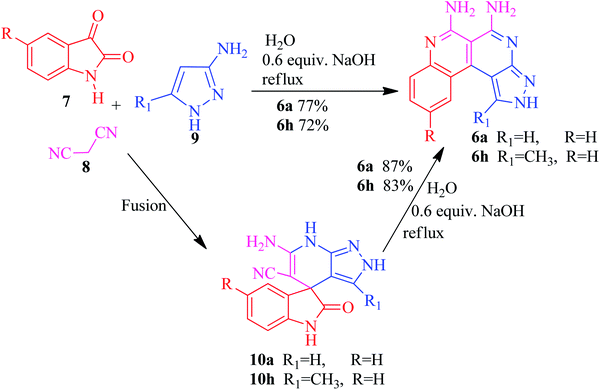
The current research points towards the exploitation of the innate reactivity of substituted benzo[c]pyrazolo[2,7]naphthyridines 6. An effective strategy for the regioselective synthesis of benzo[c]pyrazolo[2,7]naphthyridines 6a–n was established through a one-pot multi-component synthesis in comparison with a two-step synthetic procedure. The one-pot multi-component synthesis of the respective compounds was carried out by using H2O as the solvent. For the said purpose, isatin 7, malononitrile 8 and 3-aminopyrazole 9 were fused, refluxed in H2O for 4–5 h and charged with NaOH for a further 2–3 h to afford the formation of the benzo[c]pyrazolo[2,7]naphthyridines 6a–n (Scheme 1).The synthesis of benzo[c]pyrazolo[2,7]naphthyridines was also established through a two-step procedure via the formation of an intermediate, spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine] followed by alkaline hydrolysis and intramolecular cyclization. Previously, spiroindoline scaffolds were synthesized by the use of various catalysts in different solvents,34 whereas in the present study the scaffolds were synthesized by a catalyst free, on-water fusion method. For this, initially the one-pot, three component reaction of isatins 7, malanonitrile 8 and 3-aminopyrazole 9 was performed by fusing them on a sand bath for an appropriate time without a catalyst to afford the formation of the intermediate, spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine] 10a (Scheme 2).
In order to evaluate the substrate scope, the syntheses of various (un)substituted spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines] 10a–n were accomplished with excellent yields under optimized reaction conditions (Table 1).
| Product | R | R1 | Equiv. H2O | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: isatin 7 (2 mmol), malononitrile 8 (2 mmol), 3-amino-5-methylpyrazole/3-aminopyrazole 9 (2 mmol) and water (2–5 equiv.), fusion at 110 °C.b Isolated yield. | |||||
| 10a | H | H | 2 | 5 | 90 |
| 10b | CH3 | H | 2 | 4 | 81 |
| 10c | F | H | 4 | 3 | 78 |
| 10d | Cl | H | 5 | 3.5 | 79 |
| 10e | Br | H | 5 | 3 | 81 |
| 10f | NO2 | H | 5 | 2 | 84 |
| 10g | OCF3 | H | 4 | 1 | 85 |
| 10h | H | CH3 | 2 | 4 | 93 |
| 10i | CH3 | CH3 | 2 | 3 | 85 |
| 10j | F | CH3 | 3 | 2.5 | 82 |
| 10k | Cl | CH3 | 5 | 2.5 | 84 |
| 10l | Br | CH3 | 5 | 2.5 | 86 |
| 10m | NO2 | CH3 | 5 | 2 | 85 |
| 10n | OCF3 | CH3 | 4 | 1.5 | 87 |
The benzo[c]pyrazolo[2,7]naphthyridines 6a–n were subsequently synthesized by an efficient and eco-friendly protocol involving basic hydrolysis of the synthesized spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines] 10a–n. The solvent also played a significant role in determining the reaction rates and isolated yields so to investigate the efficiency of the basic hydrolysis of the spiroindoline scaffold 10a, the model reaction was carried out using the solvent free grindstone method as well as in various polar and non-polar solvents, i.e., CH2Cl2, DMSO, EtOH, MeOH, and THF. From the data listed in Table 2, water emerged as the best solvent for the basic hydrolysis of isatin, as its structure did not leave free water molecules due to the strong intramolecular hydrogen bonding. It also increased the concentration of hydroxide ions in the medium which enhanced the rate of the hydrolysis of isatin35–38 to form the product with a maximum yield in a shorter reaction time (Scheme 3).
| Entry | Solvent | Base (equiv.) | Time (h) | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | H2O | — | 12 | — |
| 2 | H2O | NaOH (0.2) | 3 | 42 |
| 3 | H2O | NaOH (0.3) | 3 | 54 |
| 4 | H2O | NaOH (0.4) | 3 | 68 |
| 5 | H2O | NaOH (0.5) | 3 | 76 |
| 6 | H2O | NaOH (0.6) | 3 | 87 |
| 7 | H2O | Piperidine (0.6) | 3 | 40 |
| 8 | H2O | DABCO (0.6) | 3 | 32 |
| 9 | Neat | NaOH (0.6) | 3 | 62 |
| 10 | MeOH | NaOH (0.6) | 5 | 30 |
| 11 | EtOH | NaOH (0.6) | 5 | 28 |
| 12 | THF | NaOH (0.6) | 6 | 35 |
| 13 | DMSO | NaOH (0.6) | 5 | 79 |
| 14 | CH2Cl2 | NaOH (0.6) | 6 | 21 |
Furthermore, the reaction was also performed in the presence and absence of different potential bases such as piperidine, DABCO and NaOH to evaluate the efficiency of the basic hydrolysis of the spiroindoline scaffold. The NaOH (0.6 equiv.) was found to be the best to obtain the target naphthyridine 6a (Table 2, entry 6). The optimized reaction conditions were utilized for the required transformations to afford the benzo[c]pyrazolo[2,7]naphthyridines 6a–n in excellent yields. The presence of electron withdrawing and electron donating groups on the isatin ring had an obvious effect on the yields of the products. The yields were higher for the electron withdrawing groups rather than the electron donating groups. The substrate scope reaction results are summarized in Fig. 2.
Comparison of reaction sequences
In order to explore the reaction sequences, the control experiment was performed with two different pathways and the same products were obtained with a variation in yields (Scheme 4).The overall yields of substituted benzo[c]pyrazolo[2,7]naphthyridines 6a–n through one-pot synthesis, were found to have a lower yield (10–15%) than that obtained using hydrolysis of the spiro-intermediate 10a–n.
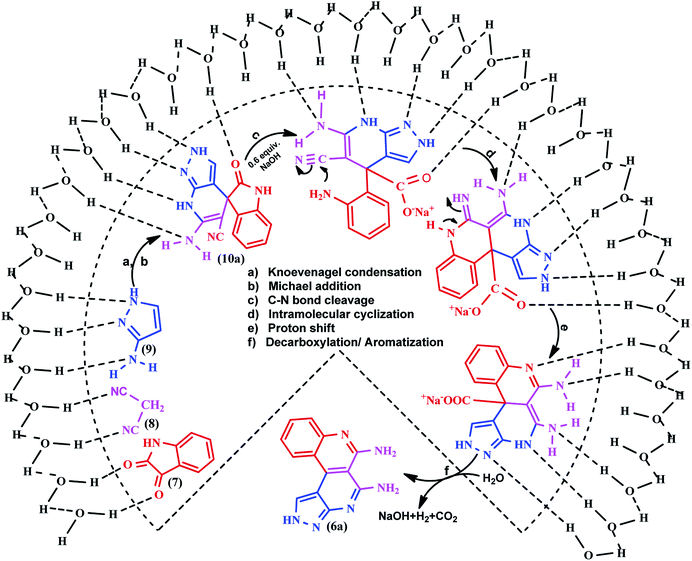
Reaction mechanism using the Jung and Marcus model
The Jung and Marcus model39 was used to explain the efficiency and rate acceleration for the on-water synthesis of benzo[c]pyrazolo[2,7]naphthyridines. The experimental results suggested a tentative mechanistic interpretation that the reaction was initiated at the solid–liquid phase water boundary and stabilized by the hydrogen bonding interactions of the free OH groups of water molecules to deliver the product with an excellent yield. Knoevenagel condensation of isatin 7 with malononitrile 8 form an arylidine intermediate with the loss of a water molecule, followed by Michael addition of pyrazole 9 with arylidine resulted in the formation of the spiroindoline scaffold 10a. The intermediate 10a undergoes ring opening from the amide linkage by basic hydrolysis, followed by ring closure, tautomerization, decarboxylation and aromatization to produce the targeted heterocycle 6a (Scheme 5).Ring annulation of benzo[c]pyrazolo[2,7]naphthyridines
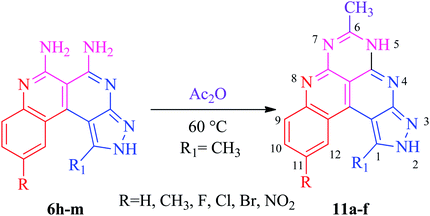
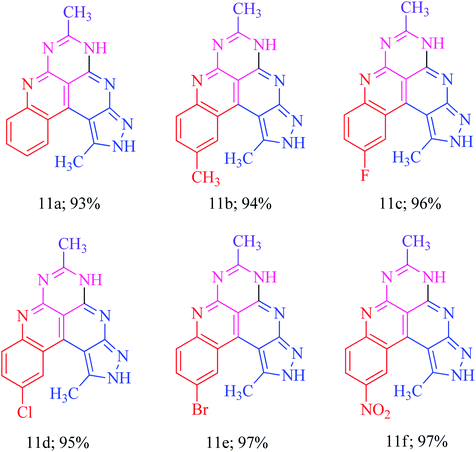
The basis of the synthesis of the benzo[c]pyrazolo[2,7]naphthyridines was the additional ring annulation by using amino groups present at 5 and 6 positions. The treatment of derivatives 6h–m with excess acetic anhydride offered benzo[c]pyrazolo[4,3-f]pyrimido[4,5,6-ij][2,7]naphthyridines 11a–f with a quantitative yield (Scheme 6). The substrate scope reaction results are summarized in Fig. 3.Application of benzo[c]pyrazolo[2,7]naphthyridines as a cationic chemosensor (6a–n)
In recent years, great attention has been devoted to the detection of metal ions in environmental or biological systems. For this purpose, different types of chemosensors have been synthesized and shown to display high selectivity for the detection of targeted metals in a pool of different metal ions. Among the various transition metal ions, Ni2+ is an essential nutrient for living organisms and is involved in different biological processes such as metabolism, biosynthesis and respiration. The deficiency and extensive use of nickel, affects the life of prokaryotic and eukaryotic organisms.40The synthesized compounds 6a–n represent a new class of chemosensors with the presence of benzo[c]pyrazolo[2,7]naphthyridines as a chromophore and two amino groups as binding sites for selective detection of metals by UV-visible spectroscopy. The UV-visible spectra of compound 6a in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) were recorded on addition of different metal cations (Al3+, Cd2+, Co2+, Cr3+, Cu2+, Fe2+, Hg2+, Mn2+, Ni2+, Sn2+ and Sr2+). The spectra of model compound 6a (Fig. S1, ESI†) showed a bathochromic shift of bands at 537 nm upon the addition of Ni2+ ions and no shifting of bands was observed for other competing cations. The initial spectroscopic studies of benzo[c]pyrazolo[2,7]naphthyridines showed a selective colorimetric response for Ni2+ ions.
5 v/v) were recorded on addition of different metal cations (Al3+, Cd2+, Co2+, Cr3+, Cu2+, Fe2+, Hg2+, Mn2+, Ni2+, Sn2+ and Sr2+). The spectra of model compound 6a (Fig. S1, ESI†) showed a bathochromic shift of bands at 537 nm upon the addition of Ni2+ ions and no shifting of bands was observed for other competing cations. The initial spectroscopic studies of benzo[c]pyrazolo[2,7]naphthyridines showed a selective colorimetric response for Ni2+ ions.
Characterization of the synthesized compounds
The structures of the synthesized compounds 10a–n, 6a–n and 11a–f were established on the basis of their spectroscopic data, i.e., IR, 1H-NMR, 13C-NMR spectroscopy, and mass spectrometry (MS). The physical and spectral data of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine] derivatives 10h–i, and 10k–m were in agreement with previously reported data.34Characterization by IR and NMR
The IR spectra of benzo[c]pyrazolo[2,7]naphthyridines 6a–n showed N–H stretching of the pyrazole and amino groups in the 3421–3088 cm−1 regions. The 1H-NMR spectra showed NH2 moieties which resonated at 6.50–7.45 ppm and exhibited a singlet pyrazole N2–H at δ 12.82–13.32 ppm in the naphthyridine derivatives. Similarly, the 13C-NMR spectra of compounds 6a–n also agreed with the IR and 1H-NMR results. The IR spectra of benzo[c]pyrazolopyrimido[2,7]naphthyridines 11a–f showed N–H stretching of pyrazole in the 3394–3048 cm−1 region. The 1H-NMR spectra exhibited a broad singlet for pyrimido N5–H at 12.41–12.67 ppm and a singlet for pyrazolo N2–H at 13.38–13.65 ppm in the benzo[c]pyrazolopyrimido[2,7]naphthyridines derivatives. The 13C-NMR spectra of the compounds 11a–f were also in agreement with the IR and 1H-NMR results.Characterization by MS (ESI)
The electron-spray ionization (ESI) spectra of all these novel heterocyclic compounds exhibited molecular ions of varied intensity which authenticated their molecular weights. The MS/MS spectra of 6a–n showed the loss of ammonia (NH3) and nitrogen (N2) as the main fragmentation pathway due to the breakage of C–NH, NH bonds that validated the formation of benzo[c]pyrazolo[2,7]naphthyridines. The MS/MS spectra of 11a–f showed the loss of N2 as a main fragmentation pathway. The proposed fragmentation pattern of 6k is illustrated Fig. S2 (ESI†).Comparison of 1H-NMR and 13C-NMR spectra
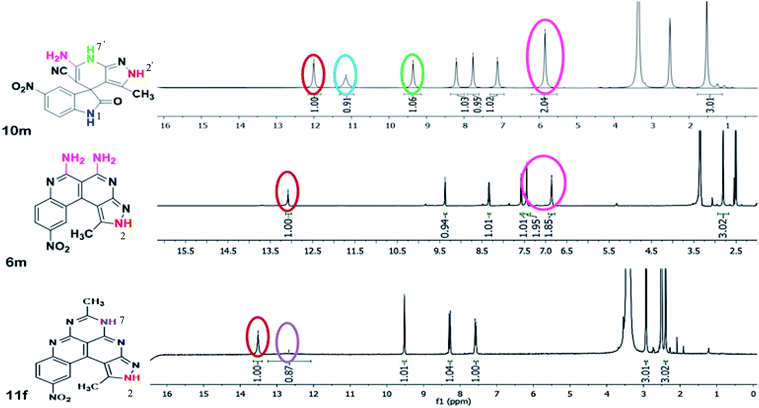
A comparison of the 1H-NMR spectra of the synthesized compounds 10m, 6m, 11f is shown in Fig. 4. The 1H-NMR spectra of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine] 10m has four separate singlets of protons attached to four different nitrogen atoms at δ 5.84 ppm for NH2, δ 9.36 ppm for pyridine N7′–H, 11.14 ppm for indole N1–H and δ 12.00 ppm for pyrazole N2′–H protons, respectively. After the formation of benzo[c]pyrazolo[2,7]naphthyridine 6m, the two peaks of the protons attached to pyridine N7′–H and indole N1–H disappeared and new peaks of NH2 moieties appeared at 6.86 ppm and 7.45 ppm and the downfield shifting of signals for pyrazole N2–H at δ 13.08 ppm was observed. After ring expansion of benzo[c]pyrazolo[2,7]naphthyridine 6m to benzo[c]pyrazolopyrimido[2,7]naphthyridine 11f, the peaks of the NH2 moieties in 6m disappeared and a new peak of pyrimido N5–H was observed at 12.67 ppm. More downfield shifting of signals for pyrazole N2–H at δ 13.51 ppm was also observed.The 13C-NMR spectra of the spiroindoline scaffold 10m showed the peaks of CN, C–NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups at 110.31, 155.27 and 180.16 ppm, respectively, which disappeared in 6m and 11f due to the transformation of these functionalities into new C–C and C–N bond (Fig. S3, ESI†).
O groups at 110.31, 155.27 and 180.16 ppm, respectively, which disappeared in 6m and 11f due to the transformation of these functionalities into new C–C and C–N bond (Fig. S3, ESI†).
Conclusion
A novel multi-component, efficient and eco-friendly strategy was established using H2O as the solvent, in the absence of a transition metal catalyst, which exhibited enhanced efficiency and rate of reaction to synthesize the spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine] derivatives which were further transformed by basic hydrolysis into new benzo[c][2,7]naphthyridines as the target products. The chemical modification involved in the new C–C and C–N bond formation was achieved by Knoevenagel condensation, Michael addition, intramolecular cyclization, decarboxylation and aromatization sequences. These hitherto unknown compounds were not only prepared by multi-component reactions but a one-pot multi-component protocol was also employed to synthesize the required naphthyridines. The scope of the reaction was further enhanced by additional ring expansion of benzo[c][2,7]naphthyridine to form benzo[c]pyrazolopyrimido[2,7]naphthyridine. The resulting benzo[c]pyrazolo[2,7]naphthyridine heterocycles have a number of potent functionalities for further chemical modifications to develop new precursors as medically important heterocycles.Experimental
General
All the chemicals were used as received from Sigma-Aldrich. All the melting points were determined using a Fisher-Johns melting point apparatus and were uncorrected. The IR spectra were recorded using KBr pellets on a Shimadzu Prestige-21 spectrophotometer. The 1H-NMR and 13C-NMR spectra were recorded on Bruker Avance spectrometer at 300 MHz and 500 MHz, respectively, using DMSO-d6. The mass spectra were recorded on a Thermo Scientific LTQ XL system fitted with an ESI source, a Jeol 600 MS Route, and a Jeol HX-110 high-resolution mass spectrometer (EI-HR). Pre-coated aluminum sheets of silica gel 60 GF254 (Merck) were used as TLC plates to check the purity of compounds.General procedure for the synthesis of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine] (10a–n)
A mixture of isatin 7 (2 mmol), malononitrile 8 (2 mmol), 3-amino-5-methylpyrazole/3-aminopyrazole 9 (2 mmol) and water (2–5 equiv.) were fused under stirring for an appropriate time (4–5 h) in a sand bath at 110 °C. After cooling, the products were washed with hot water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product 10a–n. Further purification was done by recrystallization in methanol.
1) to give the desired product 10a–n. Further purification was done by recrystallization in methanol.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1693 (C
N), 1693 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1643, 1623, 1589, 1506, 1471, 937, 923, 748, 727; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.68 (s, 2H, NH2), 6.85 (d, J = 7.80 Hz, 1H, indole C7–H), 6.93 (d, J = 7.20 Hz, 1H, indole C4–H), 6.99 (t, J = 6.90 Hz, 1H, indole C5–H), 7.05 (s, 1H, pyrazole
O), 1643, 1623, 1589, 1506, 1471, 937, 923, 748, 727; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.68 (s, 2H, NH2), 6.85 (d, J = 7.80 Hz, 1H, indole C7–H), 6.93 (d, J = 7.20 Hz, 1H, indole C4–H), 6.99 (t, J = 6.90 Hz, 1H, indole C5–H), 7.05 (s, 1H, pyrazole  ), 7.18 (t, J = 6.60 Hz, 1H, indole C6–H), 9.29 (s, 1H, pyridine NH), 10.25 (s, 1H, indole NH), 12.14 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 48.98 (C), 54.08 (C–CN), 101.78 (C), 109.94 (C
), 7.18 (t, J = 6.60 Hz, 1H, indole C6–H), 9.29 (s, 1H, pyridine NH), 10.25 (s, 1H, indole NH), 12.14 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 48.98 (C), 54.08 (C–CN), 101.78 (C), 109.94 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 121.52 (C), 122.58 (CH), 124.93 (CH), 125.21 (CH), 128.61 (CH), 136.78 (CH), 141.54 (C), 146.85 (C), 155.17 (C–NH2), 180.33 (C
N), 121.52 (C), 122.58 (CH), 124.93 (CH), 125.21 (CH), 128.61 (CH), 136.78 (CH), 141.54 (C), 146.85 (C), 155.17 (C–NH2), 180.33 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 277.08 [M − H]−. Anal. calcd for C14H10N6O (%): C, 60.43; H, 3.62; N, 30.20; found (%): C, 60.36; H, 3.59; N, 30.17.
O) ppm; MS (ESI) m/z: 277.08 [M − H]−. Anal. calcd for C14H10N6O (%): C, 60.43; H, 3.62; N, 30.20; found (%): C, 60.36; H, 3.59; N, 30.17.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1687 (C
N), 1687 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1642, 1620, 1580, 1503, 1426, 1321, 1195, 1144, 1072, 1049, 922, 818, 708, 634; 1H-NMR (DMSO-d6, 300 MHz): δ = 2.21 (s, 3H, C5–CH3), 5.68 (s, 2H, NH2), 6.73 (d, J = 7.5 Hz, 1H, indole, C7–H), 6.97 (d, J = 7.5 Hz, 1H, indole, C6–H), 6.81 (s, 1H, indole, C4–H), 7.04 (s, 1H, pyrazole
O), 1642, 1620, 1580, 1503, 1426, 1321, 1195, 1144, 1072, 1049, 922, 818, 708, 634; 1H-NMR (DMSO-d6, 300 MHz): δ = 2.21 (s, 3H, C5–CH3), 5.68 (s, 2H, NH2), 6.73 (d, J = 7.5 Hz, 1H, indole, C7–H), 6.97 (d, J = 7.5 Hz, 1H, indole, C6–H), 6.81 (s, 1H, indole, C4–H), 7.04 (s, 1H, pyrazole  ), 9.28 (s, 1H, pyridine NH), 10.15 (s, 1H, indole NH), 12.14 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 21.10 (CH3), 49.03 (C), 54.24 (C–CN), 101.88 (C), 109.68 (C
), 9.28 (s, 1H, pyridine NH), 10.15 (s, 1H, indole NH), 12.14 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 21.10 (CH3), 49.03 (C), 54.24 (C–CN), 101.88 (C), 109.68 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 121.61 (C), 125.26 (CH), 125.45 (C), 128.89 (CH), 131.36 (CH), 137.01 (C), 139.00 (CH), 146.76 (C), 155.09 (C–NH2), 180.31 (C
N), 121.61 (C), 125.26 (CH), 125.45 (C), 128.89 (CH), 131.36 (CH), 137.01 (C), 139.00 (CH), 146.76 (C), 155.09 (C–NH2), 180.31 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 291.17 [M − H]−. Anal. calcd for C15H12N6O (%): C, 61.64; H, 4.14; N, 28.75; found (%): C, 61.55; H, 4.09; N, 28.71.
O) ppm; MS (ESI) m/z: 291.17 [M − H]−. Anal. calcd for C15H12N6O (%): C, 61.64; H, 4.14; N, 28.75; found (%): C, 61.55; H, 4.09; N, 28.71.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1708 (C
N), 1708 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1642, 1622, 1581, 1504, 1485, 1178, 788, 711; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.74 (s, 2H, NH2), 6.84 (dd, J = 9 Hz, 3.9 Hz, 1H, indole C7–H), 6.87 (d, J = 2.7 Hz, 1H, indole C4–H), 7.02 (td, J = 9 Hz, 2.7 Hz, 1H, indole C6–H), 7.12 (s, 1H, pyrazole
O), 1642, 1622, 1581, 1504, 1485, 1178, 788, 711; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.74 (s, 2H, NH2), 6.84 (dd, J = 9 Hz, 3.9 Hz, 1H, indole C7–H), 6.87 (d, J = 2.7 Hz, 1H, indole C4–H), 7.02 (td, J = 9 Hz, 2.7 Hz, 1H, indole C6–H), 7.12 (s, 1H, pyrazole  ), 9.33 (s, 1H, pyridine NH), 10.28 (s, 1H, indole NH), 12.19 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.56 (C), 53.56 (C–CN), 101.22 (C), 110.74 (CH), 110.84 (CH), 112.32 (CH), 112.64 (CH), 114.92 (CH), 115.23 (CH), 121.41 (C
), 9.33 (s, 1H, pyridine NH), 10.28 (s, 1H, indole NH), 12.19 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.56 (C), 53.56 (C–CN), 101.22 (C), 110.74 (CH), 110.84 (CH), 112.32 (CH), 112.64 (CH), 114.92 (CH), 115.23 (CH), 121.41 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 125.32 (C), 137.77 (CH), 138.31 (C), 138.41 (C), 146.80 (C), 155.27 (C–NH2), 157.30 (C), 160.45 (C), 180.36 (C
N), 125.32 (C), 137.77 (CH), 138.31 (C), 138.41 (C), 146.80 (C), 155.27 (C–NH2), 157.30 (C), 160.45 (C), 180.36 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 295.25 [M − H]−. Anal. calcd for C14H9FN6O (%): C, 56.76; H, 3.06; N, 28.37; found (%): C, 56.79; H, 3.06; N, 28.39.
O) ppm; MS (ESI) m/z: 295.25 [M − H]−. Anal. calcd for C14H9FN6O (%): C, 56.76; H, 3.06; N, 28.37; found (%): C, 56.79; H, 3.06; N, 28.39.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1693 (C
N), 1693 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1639, 1623, 1581, 1504, 1475, 1431, 1217, 819, 723; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.77 (s, 2H, NH2), 6.87 (d, J = 8.1 Hz, 1H, indole C7–H), 7.00 (d, J = 1.8 Hz, 1H, indole C4–H), 7.15 (s, 1H, pyrazole
O), 1639, 1623, 1581, 1504, 1475, 1431, 1217, 819, 723; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.77 (s, 2H, NH2), 6.87 (d, J = 8.1 Hz, 1H, indole C7–H), 7.00 (d, J = 1.8 Hz, 1H, indole C4–H), 7.15 (s, 1H, pyrazole  ), 7.24 (dd, J = 8.1 Hz, 2.1 Hz, 1H, indole C6–H), 9.35 (s, 1H, pyridine NH), 10.41 (s, 1H, indole NH), 12.22 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.35 (C), 53.41 (C–CN), 101.03 (C), 111.53 (C
), 7.24 (dd, J = 8.1 Hz, 2.1 Hz, 1H, indole C6–H), 9.35 (s, 1H, pyridine NH), 10.41 (s, 1H, indole NH), 12.22 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.35 (C), 53.41 (C–CN), 101.03 (C), 111.53 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 121.40 (C), 124.91 (CH), 125.39 (C), 126.51 (CH), 128.65 (CH), 138.74 (CH), 140.51 (C), 146.77 (C), 155.27 (C–NH2), 180.03 (C
N), 121.40 (C), 124.91 (CH), 125.39 (C), 126.51 (CH), 128.65 (CH), 138.74 (CH), 140.51 (C), 146.77 (C), 155.27 (C–NH2), 180.03 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 311.17 [M − H]−. Anal. calcd for C14H9ClN6O (%): C, 53.77; H, 2.90; N, 26.87; found (%): C, 53.69; H, 2.90; N, 26.89.
O) ppm; MS (ESI) m/z: 311.17 [M − H]−. Anal. calcd for C14H9ClN6O (%): C, 53.77; H, 2.90; N, 26.87; found (%): C, 53.69; H, 2.90; N, 26.89.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1692 (C
N), 1692 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1613, 1589, 1508, 1473, 1412, 1301, 1221, 1161, 1077, 1023, 823, 717, 689; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.77 (s, 2H, NH2), 6.83 (d, J = 8.4 Hz, 1H, indole, C7–H), 7.10 (s, 1H, indole, C4–H), 7.15 (s, 1H, pyrazole
O), 1613, 1589, 1508, 1473, 1412, 1301, 1221, 1161, 1077, 1023, 823, 717, 689; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.77 (s, 2H, NH2), 6.83 (d, J = 8.4 Hz, 1H, indole, C7–H), 7.10 (s, 1H, indole, C4–H), 7.15 (s, 1H, pyrazole  ), 7.36 (d, J, 8.1 Hz, 1H, indole, C6–H), 9.36 (s, 1H, pyridine NH), 10.42 (s, 1H, indole NH), 12.22 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.31 (C), 5342 (C–CN), 101.00 (C), 112.09 (C
), 7.36 (d, J, 8.1 Hz, 1H, indole, C6–H), 9.36 (s, 1H, pyridine NH), 10.42 (s, 1H, indole NH), 12.22 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.31 (C), 5342 (C–CN), 101.00 (C), 112.09 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 114.21 (C), 121.41 (CH), 125.43 (C), 127.59 (CH), 131.49 (CH), 139.16 (C), 140.91 (CH), 146.72 (C), 155.26 (C–NH2), 179.76 (C
N), 114.21 (C), 121.41 (CH), 125.43 (C), 127.59 (CH), 131.49 (CH), 139.16 (C), 140.91 (CH), 146.72 (C), 155.26 (C–NH2), 179.76 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 355.08 [M − H]−. Anal. calcd for C14H9BrN6O (%): C, 47.08; H, 2.54; N, 23.53; found (%): C, 47.02; H, 2.51; N, 23.50.
O) ppm; MS (ESI) m/z: 355.08 [M − H]−. Anal. calcd for C14H9BrN6O (%): C, 47.08; H, 2.54; N, 23.53; found (%): C, 47.02; H, 2.51; N, 23.50.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1717 (C
N), 1717 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1643, 1625, 1581, 1504, 1342, 1217, 715; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.90 (s, 2H, NH2), 7.09 (d, J = 8.7 Hz, 1H, indole C7–H), 7.22 (s, 1H, pyrazole
O), 1643, 1625, 1581, 1504, 1342, 1217, 715; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.90 (s, 2H, NH2), 7.09 (d, J = 8.7 Hz, 1H, indole C7–H), 7.22 (s, 1H, pyrazole  ), 7.77 (d, J = 2.4 Hz, 1H, indole C4–H), 8.19 (dd, J = 8.7 Hz, 2.4 Hz, 1H indole C6–H), 9.52 (s, 1H, pyridine NH), 11.03 (s, 1H, indole NH), 12.30 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.22 (C), 52.73 (C–CN), 100.20 (C), 110.50 (C
), 7.77 (d, J = 2.4 Hz, 1H, indole C4–H), 8.19 (dd, J = 8.7 Hz, 2.4 Hz, 1H indole C6–H), 9.52 (s, 1H, pyridine NH), 11.03 (s, 1H, indole NH), 12.30 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.22 (C), 52.73 (C–CN), 100.20 (C), 110.50 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 120.20 (CH), 121.20 (C), 125.70 (CH), 126.18 (CH), 137.74 (CH), 143.08 (C), 146.72 (C), 148.25 (C), 155.49 (C–NH2), 180.69 (C
N), 120.20 (CH), 121.20 (C), 125.70 (CH), 126.18 (CH), 137.74 (CH), 143.08 (C), 146.72 (C), 148.25 (C), 155.49 (C–NH2), 180.69 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 322.25 [M − H]−. Anal. calcd for C14H9N7O3 (%): C, 52.02; H, 2.81; N, 30.33; found (%): C, 51.97; H, 2.79; N, 30.29.
O) ppm; MS (ESI) m/z: 322.25 [M − H]−. Anal. calcd for C14H9N7O3 (%): C, 52.02; H, 2.81; N, 30.33; found (%): C, 51.97; H, 2.79; N, 30.29.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1767 (C
N), 1767 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1699, 1623, 1585, 1530, 1488, 1280, 1269, 1166, 925, 725; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.78 (s, 2H, NH2), 6.94 (s, 1H, indole C4–H), 6.96 (s, 1H, pyrazole
O), 1699, 1623, 1585, 1530, 1488, 1280, 1269, 1166, 925, 725; 1H-NMR (DMSO-d6, 300 MHz): δ = 5.78 (s, 2H, NH2), 6.94 (s, 1H, indole C4–H), 6.96 (s, 1H, pyrazole  ), 7.16 (s, 1H, indole C7–H), 7.20 (dd, J = 8.1 Hz, 1.2 Hz, 1H, indole C6–H), 9.36 (s, 1H, pyridine NH), 10.47 (s, 1H, indole NH), 12.22 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.50 (C), 53.31 (C–CN), 100.97 (C), 110.92 (C
), 7.16 (s, 1H, indole C7–H), 7.20 (dd, J = 8.1 Hz, 1.2 Hz, 1H, indole C6–H), 9.36 (s, 1H, pyridine NH), 10.47 (s, 1H, indole NH), 12.22 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 75 MHz) δ = 49.50 (C), 53.31 (C–CN), 100.97 (C), 110.92 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 118.40 (CH), 118.92 (CH), 121.28 (C), 122.03 (CH), 122.30 (CH), 125.41 (CH), 138.33 (CH), 140.80 (C), 144.02 (C), 144.04 (C), 146.79 (C), 155.30 (C–NH2), 180.36 (C
N), 118.40 (CH), 118.92 (CH), 121.28 (C), 122.03 (CH), 122.30 (CH), 125.41 (CH), 138.33 (CH), 140.80 (C), 144.02 (C), 144.04 (C), 146.79 (C), 155.30 (C–NH2), 180.36 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 361.25 [M − H]−. Anal. calcd for C15H9F3N6O2 (%): C, 49.73; H, 2.50; N, 23.20; found (%): C, 49.79; H, 2.53; N, 23.23.
O) ppm; MS (ESI) m/z: 361.25 [M − H]−. Anal. calcd for C15H9F3N6O2 (%): C, 49.73; H, 2.50; N, 23.20; found (%): C, 49.79; H, 2.53; N, 23.23.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1702 (C
N), 1702 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1640, 1580, 1470, 936, 835, 746, 720. Anal. calcd for C15H12N6O (%): C, 61.64; H, 4.14; N, 28.75; found (%): C, 61.59; H, 4.12; N, 28.71.
O), 1640, 1580, 1470, 936, 835, 746, 720. Anal. calcd for C15H12N6O (%): C, 61.64; H, 4.14; N, 28.75; found (%): C, 61.59; H, 4.12; N, 28.71.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1680 (C
N), 1680 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1640, 1588, 1500, 1420, 1190, 1075, 925, 801, 708, 630. Anal. calcd for C16H14N6O (%): C, 62.74; H, 4.61; N, 27.44; found (%): C, 62.70; H, 4.57; N, 27.39.
O), 1640, 1588, 1500, 1420, 1190, 1075, 925, 801, 708, 630. Anal. calcd for C16H14N6O (%): C, 62.74; H, 4.61; N, 27.44; found (%): C, 62.70; H, 4.57; N, 27.39.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1705 (C
N), 1705 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1620, 1520, 1480, 1439, 818, 740, 726; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.51 (s, 3H,
O), 1620, 1520, 1480, 1439, 818, 740, 726; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.51 (s, 3H,  ), 5.70 (s, 2H, NH2), 6.86 (s, 2H, indole C4–H, C7–H), 7.03 (s, 1H, indole C6–H), 9.22 (s, 1H, pyridine NH), 10.39 (s, 1H, indole NH), 11.90 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.13 (CH3), 49.66 (C), 53.62 (C–CN), 98.10 (C), 110.61 (CH), 110.67 (CH), 112.46 (CH), 112.65 (CH), 115.08 (CH), 115.27 (CH), 121.41 (C
), 5.70 (s, 2H, NH2), 6.86 (s, 2H, indole C4–H, C7–H), 7.03 (s, 1H, indole C6–H), 9.22 (s, 1H, pyridine NH), 10.39 (s, 1H, indole NH), 11.90 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.13 (CH3), 49.66 (C), 53.62 (C–CN), 98.10 (C), 110.61 (CH), 110.67 (CH), 112.46 (CH), 112.65 (CH), 115.08 (CH), 115.27 (CH), 121.41 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 134.57 (C), 137.19 (C), 137.25 (C), 137.82 (C), 146.98 (C), 155.10 (C–NH2), 158.02 (C), 159.91 (C), 179.79 (C
N), 134.57 (C), 137.19 (C), 137.25 (C), 137.82 (C), 146.98 (C), 155.10 (C–NH2), 158.02 (C), 159.91 (C), 179.79 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 309.17 [M − H]−. Anal. calcd for C15H11FN6O (%): C, 58.06; H, 3.57; N, 27.08; found (%): C, 58.01; H, 3.58; N, 27.03.
O) ppm; MS (ESI) m/z: 309.17 [M − H]−. Anal. calcd for C15H11FN6O (%): C, 58.06; H, 3.57; N, 27.08; found (%): C, 58.01; H, 3.58; N, 27.03.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1703 (C
N), 1703 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1620, 1520, 1479, 1440, 818, 748, 727; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.52 (s, 3H,
O), 1620, 1520, 1479, 1440, 818, 748, 727; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.52 (s, 3H,  ), 5.72 (s, 2H, NH2), 6.88 (d, J = 10 Hz, 1H, indole C7–H), 6.98 (s, 1H, indole C4–H), 7.25 (d, J = 10 Hz, 1H, indole C6–H), 9.23 (s, 1H, pyridine NH), 10.52 (s, 1H, indole NH), 11.92 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.17 (CH3), 49.43 (C), 53.49 (C–CN), 97.96 (C), 111.36 (C
), 5.72 (s, 2H, NH2), 6.88 (d, J = 10 Hz, 1H, indole C7–H), 6.98 (s, 1H, indole C4–H), 7.25 (d, J = 10 Hz, 1H, indole C6–H), 9.23 (s, 1H, pyridine NH), 10.52 (s, 1H, indole NH), 11.92 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.17 (CH3), 49.43 (C), 53.49 (C–CN), 97.96 (C), 111.36 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 121.39 (C), 124.99 (CH), 126.69 (C), 128.75 (CH), 134.57 (C), 137.56 (CH), 140.52 (C), 146.95 (C), 155.10 (C–NH2), 179.47 (C
N), 121.39 (C), 124.99 (CH), 126.69 (C), 128.75 (CH), 134.57 (C), 137.56 (CH), 140.52 (C), 146.95 (C), 155.10 (C–NH2), 179.47 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; anal. calcd for C15H11ClN6O (%): C, 55.14; H, 3.39; N, 25.72; found (%): C, 55.19; H, 3.42; N, 25.78.
O) ppm; anal. calcd for C15H11ClN6O (%): C, 55.14; H, 3.39; N, 25.72; found (%): C, 55.19; H, 3.42; N, 25.78.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1702, 1613, 1575, 1470, 132, 1215, 1160, 1066, 1021, 715, 679. Anal. calcd for C15H11BrN6O (%): C, 48.54; H, 2.99; N, 22.64; found (%): C, 48.48; H, 2.97; N, 22.59.
N), 1702, 1613, 1575, 1470, 132, 1215, 1160, 1066, 1021, 715, 679. Anal. calcd for C15H11BrN6O (%): C, 48.54; H, 2.99; N, 22.64; found (%): C, 48.48; H, 2.97; N, 22.59.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1708 (C
N), 1708 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610, 1513, 1502, 1340, 1130; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.53 (s, 3H,
O), 1610, 1513, 1502, 1340, 1130; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.53 (s, 3H,  ), 5.84 (s, 2H, NH2), 7.11 (s, 1H, indole C7–H), 7.76 (s, 1H, indole C4–H), 8.20 (s 1H, indole C6–H), 9.36 (s, 1H, pyridine NH), 11.15 (s, 1H, indole NH), 12.00 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.25 (CH3), 49.30 (C), 52.86 (C–CN), 97.20 (C), 110.31 (C
), 5.84 (s, 2H, NH2), 7.11 (s, 1H, indole C7–H), 7.76 (s, 1H, indole C4–H), 8.20 (s 1H, indole C6–H), 9.36 (s, 1H, pyridine NH), 11.15 (s, 1H, indole NH), 12.00 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.25 (CH3), 49.30 (C), 52.86 (C–CN), 97.20 (C), 110.31 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 120.31 (CH), 121.15 (C), 126.24 (CH), 134.74 (C), 136.56 (CH), 143.26 (C), 146.97 (C), 148.03 (C), 155.27 (C–NH2), 180.16 (C
N), 120.31 (CH), 121.15 (C), 126.24 (CH), 134.74 (C), 136.56 (CH), 143.26 (C), 146.97 (C), 148.03 (C), 155.27 (C–NH2), 180.16 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; anal. calcd for C15H11N7O3 (%): C, 53.41; H, 3.29; N, 29.07; found (%): C, 53.36; H, 3.27; N, 29.03.
O) ppm; anal. calcd for C15H11N7O3 (%): C, 53.41; H, 3.29; N, 29.07; found (%): C, 53.36; H, 3.27; N, 29.03.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1711 (C
N), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1623, 1586, 1514, 1488, 1269, 1166, 734; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.48 (s, 3H,
O), 1623, 1586, 1514, 1488, 1269, 1166, 734; 1H-NMR (DMSO-d6, 500 MHz): δ = 1.48 (s, 3H,  ), 5.74 (s, 2H, NH2), 6.95 (s, 1H, indole C4–H), 6.97 (s, 1H, indole C7–H), 7.22 (dd, J = 7.5 Hz, 2.1 Hz, 1H, indole C6–H), 9.25 (s, 1H, pyridine NH) 10.57 (s, 1H, indole NH), 11.93 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.06 (CH3), 49.54 (C), 53.34 (C–CN), 97.86 (C), 110.79 (C
), 5.74 (s, 2H, NH2), 6.95 (s, 1H, indole C4–H), 6.97 (s, 1H, indole C7–H), 7.22 (dd, J = 7.5 Hz, 2.1 Hz, 1H, indole C6–H), 9.25 (s, 1H, pyridine NH) 10.57 (s, 1H, indole NH), 11.93 (s, 1H, pyrazole NH) ppm; 13C-NMR (DMSO-d6, 125 MHz) δ = 9.06 (CH3), 49.54 (C), 53.34 (C–CN), 97.86 (C), 110.79 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 118.53 (CH), 119.61 (CH), 121.28 (CH), 121.64 (CH), 122.14 (C), 134.55 (C), 137.04 (C), 140.88 (C), 144.15 (C), 144.16 (C), 147.02 (C), 155.19 (C–NH2), 179.82 (C
N), 118.53 (CH), 119.61 (CH), 121.28 (CH), 121.64 (CH), 122.14 (C), 134.55 (C), 137.04 (C), 140.88 (C), 144.15 (C), 144.16 (C), 147.02 (C), 155.19 (C–NH2), 179.82 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm; MS (ESI) m/z: 375.17 [M − H]−. Anal. calcd for C16H11F3N6O2 (%): C, 51.07; H, 2.95; N, 22.33; found (%): C, 51.10; H, 2.97; N, 22.37.
O) ppm; MS (ESI) m/z: 375.17 [M − H]−. Anal. calcd for C16H11F3N6O2 (%): C, 51.07; H, 2.95; N, 22.33; found (%): C, 51.10; H, 2.97; N, 22.37.General procedure for one-pot multi-component synthesis of naphthyridines (6a–n)
A mixture of isatin 7 (2 mmol), malononitrile 8 (2 mmol), 3-amino-5-methylpyrazole/3-aminopyrazole 9 (2 mmol) were fused, refluxed in 5 ml of H2O for an appropriate time (4–5 h) to form intermediate spiroindoline scaffolds. The progress of the reaction was monitored by TLC. After consumption of the reactant, as indicated by TLC, NaOH (0.6 equiv.) was added to the crude product. The reaction mixture was further refluxed for 2–3 h. The reaction was allowed to cool down to room temperature. The precipitate formed was filtered by suction and washed thoroughly with water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the crude product. The resulting residue was recrystallized in methanol to obtain pure heterocycles 6a–n.
1) to obtain the crude product. The resulting residue was recrystallized in methanol to obtain pure heterocycles 6a–n.
General procedure for the synthesis of naphthyridines (6a–n) from spiroindolines (10a–n)
To the suspension of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridine] 10a–n (2 mmol) in 5 ml of H2O, NaOH (0.6 equiv.) was added and the mixture was refluxed for (2–3 h). The progress of the reaction was monitored by TLC. After completion of the reaction as indicated by TLC, the reaction mixture was allowed to cool at room temperature, the precipitate formed was filtered by suction and washed thoroughly with water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the crude product. The resulting residue was recrystallized in methanol to obtain pure heterocycles 6a–n.
1) to obtain the crude product. The resulting residue was recrystallized in methanol to obtain pure heterocycles 6a–n.
General procedure for the synthesis of benzo[c]pyrazolo[4,3-f]pyrimido[4,5,6-ij][2,7]naphthyridines (11a–f)
A solution of benzo[c]pyrazolo[2,7]naphthyridines 6h–m (2 mmol) in acetic anhydride (6 ml) was heated at 60 °C for 2–3 h. The completion of the reaction was indicated by TLC, the precipitate formed was filtered and washed with water. The crude product was recrystallized in methanol to obtain pure heterocycles 11a–f.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. Y. is thankful to B. Z. University for providing financial support to PhD students. Z. Shafiq is thankful to Higher Education Commission of Pakistan for financial support vide Project No. NRPU-I/6975.References
- J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300–1308 CrossRef CAS.
- A. Madaan, R. Verma, V. Kumar, A. T. Singh, S. K. Jain and M. Jaggi, Arch. Pharm., 2015, 348, 837–860 CrossRef CAS PubMed.
- K. Goutham, V. Kadiyala, B. Sridhar and G. V. Karunakar, Org. Biomol. Chem., 2017, 15, 7813–7818 RSC.
- M. Lotter and F. Bracher, Sci. Pharm., 2009, 77, 1–8 CrossRef CAS.
- F. J. Schmitz, S. K. Agarwal, S. P. Gunasekera, P. G. Schmidt and J. N. Shoolery, J. Am. Chem. Soc., 1983, 105, 4835–4836 CrossRef CAS.
- I. Reifer and E. White, N. Z. J. Sci. Technol. B Gen, 1945, 27, 242 CAS.
- F. J. Schmitz, F. S. DeGuzman, M. B. Hossain and D. Van der Helm, J. Org. Chem., 1991, 56, 804–808 CrossRef CAS.
- T. Nittoli, R. G. Dushin, C. Ingalls, K. Cheung, M. B. Floyd, H. Fraser, A. Olland, Y. Hu, G. Grosu and X. Han, Eur. J. Med. Chem., 2010, 45, 1379–1386 CrossRef CAS PubMed.
- V. Kumar, K. Kaur, G. K. Gupta and A. K. Sharma, Eur. J. Med. Chem., 2013, 69, 735–753 CrossRef CAS PubMed.
- F. Noe and L. Fowden, Nature, 1959, 184, BA 69 CrossRef.
- A. A. Wube, E.-M. Wenzig, S. Gibbons, K. Asres, R. Bauer and F. Bucar, Phytochemistry, 2008, 69, 982–987 CrossRef CAS PubMed.
- C. R. Petrie III, G. R. Revankar, N. K. Dalley, R. D. George, P. A. McKernan, R. L. Hamill and R. K. Robins, J. Med. Chem., 1986, 29, 268–278 CrossRef PubMed.
- J. G. Buchanan, A. R. Edgar, R. J. Hutchison, A. Stobie and R. H. Wightman, J. Chem. Soc., Chem. Commun., 1980, 237–238, 10.1039/C39800000237.
- R. P. Ojha, M. Roychoudhury and N. K. Sanyal, J. Biosci., 1987, 12, 311–320 CrossRef CAS.
- K. Hirata, S. Yoshitomi, S. Dwi, O. Iwabe, A. Mahakhant, J. Polchai and K. Miyamoto, J. Biosci. Bioeng., 2003, 95, 512–517 CrossRef CAS PubMed.
- K. Hirata, S. Yoshitomi, S. Dwi, O. Iwabe, A. Mahakant, J. Polchai and K. Miyamoto, J. Biotechnol., 2004, 110, 29–35 CrossRef CAS PubMed.
- V. P. Litvinov, S. V. Roman and V. D. Dyachenko, Russ. Chem. Rev., 2001, 70, 299–320 CrossRef CAS.
- V. P. Litvinov, S. V. Roman and V. D. Dyachenko, Russ. Chem. Rev., 2000, 69, 201–220 CrossRef CAS.
- A. Noravyan, E. Paronikyan and S. Vartanyan, Khim.-Farm. Zh., 1985, 19, 790–800 CAS.
- H. C. Van Der Plas, M. Woźniak and H. J. Van Den Haak, in Advances in Heterocyclic Chemistry, Elsevier, 1983, vol. 33, pp. 95–146 Search PubMed.
- W. W. Paudler and R. M. Sheets, in Advances in Heterocyclic Chemistry, Elsevier, 1983, vol. 33, pp. 147–184 Search PubMed.
- G. Winters, A. Sala, A. De Paoli and V. Ferri, Synthesis, 1984, 1984, 1052–1054 CrossRef.
- M. Wanner, G. Koomen and U. Pandit, Tetrahedron, 1982, 38, 2741–2748 CrossRef CAS.
- M. Winn, J. Heterocycl. Chem., 1975, 12, 523–524 CrossRef CAS.
- A. V. Tverdokhlebov, E. V. Resnyanska, A. V. Zavada, A. A. Tolmachev, A. N. Kostyuk and A. N. Chernega, Tetrahedron, 2004, 60, 5777–5783 CrossRef CAS.
- K. Goerlitzer and P. M. Dobberkau, Pharmazie, 1996, 51(6), 386–391 CAS.
- A. V. Tverdokhlebov, A. V. Zavada, A. A. Tolmachev, A. N. Kostyuk, A. N. Chernega and E. Rusanov, Synthesis of furo[2,3-c]-2,7-naphthyridine derivatives via domino heterocyclization reaction, Tetrahedron, 2005, 61, 9618–9623 CrossRef CAS.
- K. Goutham, V. Kadiyala, B. Sridhar and G. V. Karunakar, Org. Biomol. Chem., 2017, 15, 7813–7818 RSC.
- R. A. Mekheimer, M. A. Al-Sheikh, H. Y. Medrasi and G. A. Bahatheg, Mol. Diversity, 2018, 22, 159–171 CrossRef CAS PubMed.
- M. B. Gawande, V. D. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522–5551 RSC.
- S. Narayan, J. Muldoon, M. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS PubMed.
- D. Guo, D. Zhu, X. Zhou and B. Zheng, Langmuir, 2015, 31, 13759–13763 CrossRef CAS PubMed.
- F. Noruzian, A. Olyaei and R. Hajinasiri, Res. Chem. Intermed., 2019, 1–12 Search PubMed.
- A. Dandia, A. k. Laxkar and R. Singh, Tetrahedron Lett., 2012, 53, 3012–3017 CrossRef CAS.
- L. A. Casey, R. Galt and M. I. Page, J. Chem. Soc., Perkin Trans. 2, 1993, 23–28 RSC.
- H. M. A. El-Nader and M. N. Moussa, Chem. Pharm. Bull., 1996, 44, 1641–1646 CrossRef CAS.
- M. F. Fathalla and A. M. Ismail, Indian J. Chem., 2006, 45, 901–904 Search PubMed.
- A. Ismail and A. Zaghloul, Int. J. Chem. Kinet., 1998, 30, 463–469 CrossRef CAS.
- Y. Jung and R. Marcus, J. Am. Chem. Soc., 2007, 129, 5492–5502 CrossRef CAS PubMed.
- J. Prabhu, K. Velmurugan, A. Raman, N. Duraipandy, M. Kiran, S. Easwaramoorthi and R. Nandhakumar, Sens. Actuators, B, 2017, 238, 306–317 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09148c |
| This journal is © The Royal Society of Chemistry 2020 |