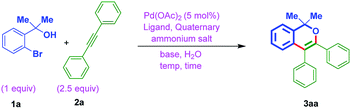Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium mediated domino reaction: synthesis of isochromenes under aqueous medium†
Lavisha Punia,
Karu Ramesh and
Gedu Satyanarayana *
*
Department of Chemistry, Indian Institute of Technology (IIT) Hyderabad, Kandi – 502 285, Sangareddy District, Telangana, India. E-mail: gvsatya@iith.ac.in; Web: https://sites.google.com/site/gsresearchgrouphomepage/home Fax: +91 40 2301 6032
First published on 2nd January 2020
Abstract
Isochromenes have been synthesized using palladium-catalyzed C–C and C–O bond forming reactions starting from ortho-bromo tertiary benzylic alcohols and internal acetylenes. Notably, this domino process is feasible by using the green solvent, water. The protocol exhibited a broad substrate scope and afforded various isochromenes.
Introduction
Domino reactions are effective in the construction of two or more bonds and thereby facilitate obtaining products with reasonable structural complexity, under a particular set of reaction conditions, which may be devoid of additional reagents/catalysts.1 Moreover, in such a reaction, there is no need to isolate the intermediate product(s). Mainly, domino reactions play an important role in achieving complex hetero- and carbo-cyclic products, which are of great importance in the realm of biologically active molecules. Amongst them, fused bicyclic ethers (isochromenes) consisting of an aromatic ring and a pyran moiety are widely available heterocyclic motifs comprising natural products of biological significance.2 They are often identified in bacteria, fungi, plants, etc.3 and exhibit useful biological properties, such as antitumor,4 anticancer,5 antifungal,6 antibacterial, antibiotic,7 and antimicrobial activities.8 Some of the representative examples of natural products are as depicted in Fig. 1.9–12 Due to their interesting structural features and biological properties, isochromenes have captured the attention of chemists.13 As a consequence, a good number of synthetic routes were developed towards the synthesis of isochromenes.14 Especially, palladium-catalyzed annulation using relatively more reactive ortho-iodo tertiary benzylic alcohols with internal alkynes was also reported.15 These annulations were also driven by means of a stable palladacycle catalyst, as reported in ref. 16. Nevertheless, very few examples have been synthesized in these above two reports. In this regard, to the best of our knowledge, there are no reports of, particularly, using relatively less sensitive ortho-bromo tertiary benzylic alcohols catalyzed by a simple palladium-based catalyst and by employing water as the whole solvent medium, for the synthesis of isochromenes. In our efforts to establish metal-catalyzed coupling reactions,17 herein, we describe the synthesis of isochromenes by utilizing the solvent water as the reaction medium.Results and discussion
The optimization was begun with ortho-bromo tertiary benzylic alcohol 1a and diphenylacetylene 2a. In general, quaternary ammonium salts (QX; X = Cl, Br, I and HSO4) can act as a phase transfer catalyst mostly when the solvent is water or also can act as an additive to accelerate the catalytic activity of metal-catalyst.18 With this viewpoint in our mind, firstly, the alcohol 1a and diphenylacetylene 2a were treated at different temperatures using Pd(OAc)2 catalyst (5 mol%), tetrabutylammonium iodide (TBAI) (1 equiv.), base K2CO3 (4 equiv.) and with different ligands (entries 1 to 3, Table 1). Conversely, no progress of the reaction was noted. While treating 1a and 2a by using Xantphos ligand at 140 °C for 36 h, resulted the expected chromene 3aa, although in poor yield (entry 4, Table 1). Gratifyingly, the combination of Pd(OAc)2 and L-proline, TBAI, and base K2CO3 resulted in 76% yield of the desired chromene 3aa (entry 5, Table 1). Whereas other attempts with the combination of tetrabutylammonium iodide (TBAI/Na2CO3, TBAI/Li2CO3) and benzyltriethylammonium chloride (BTEAC)/K2CO3 in the presence of L-proline as the ligand were not that much efficient (entries 6 to 8, Table 1). Further, decreasing the quantity of solvent (0.2 mL of water), gave 3aa in 50% yield (entry 9, Table 1). While with 0.1 mL solvent water (entry 10, Table 1) or under neat reaction conditions (entry 11, Table 1), lead to the decomposition. On the other hand, when Na2CO3 (2 equiv.) was used as the base, there was no initiation in the reaction (entry 12, Table 1). While 30% yield of 3aa was formed with 2 equiv. of K2CO3 (entry 13, Table 1). Treatment of 1a and 2a with 4 equiv. of Et3N, lead to the decomposition (entry 14, Table 1).| Entry | Ligand (20 mol%) | Quaternary ammonium salt (equiv.) & base (equiv.) | Temp. (°C) | Time (h) | Yield of 3aaa (%) |
|---|---|---|---|---|---|
| a Conditions: 1a (54 mg, 0.25 mmol), 2a (111.2 mg, 0.625 mmol), Pd(OAc)2 (2.8 mg, 0.012 mmol), base (1 mmol), ligand (0.05 mmol), quaternary ammonium salt (0.25 mmol), water (0.5 mL), 140 °C, 36 h.b Isolated yields of the product 3aa.c Lead to the decomposition.d Used 0.2 mL of water.e The reaction was performed using 0.1 mL of water.f Used neat reaction conditions.g Starting materials were recovered. TBAI: tetrabutylammonium iodide. BTEAC: benzyltriethylammonium chloride. | |||||
| 1 | BINAP | TBAI (1) + K2CO3 (4) | 140 | 36 | —b |
| 2 | 2,2′-Bipy | TBAI (1) + K2CO3(4) | 120 | 38 | —b |
| 3 | P(Cy)3 | TBAI (1) + K2CO3 (4) | 100 | 42 | —b |
| 4 | Xantphos | TBAI (1) + K2CO3 (4) | 140 | 36 | 30 |
| 5 | L-Proline | TBAI (1) + K2CO3 (4) | 140 | 36 | 76 |
| 6 | L-Proline | TBAI (1) + Na2CO3 (4) | 140 | 38 | 20 |
| 7 | L-Proline | TBAI (1) + Li2CO3 (4) | 140 | 36 | 15 |
| 8 | L-Proline | BTEAC (1) + K2CO3 (4) | 140 | 36 | 15 |
| 9c | L-Proline | TBAI (1) + K2CO3 (4) | 140 | 36 | 50 |
| 10d | L-Proline | TBAI (1) + K2CO3 (4) | 140 | 36 | —b |
| 11e | L-Proline | TBAI (1) + K2CO3 (4) | 140 | 36 | —b |
| 12 | L-Proline | TBAI (1) + Na2CO3 (2) | 140 | 46 | —f |
| 13 | L-Proline | TBAI (1) + K2CO3 (2) | 140 | 42 | 30 |
| 14 | L-Proline | TBAI (1) + NEt3 (4) | 140 | 36 | —b |
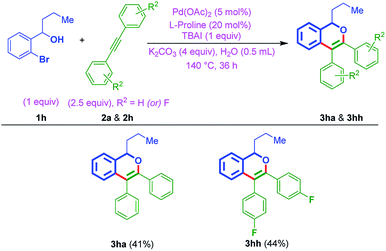
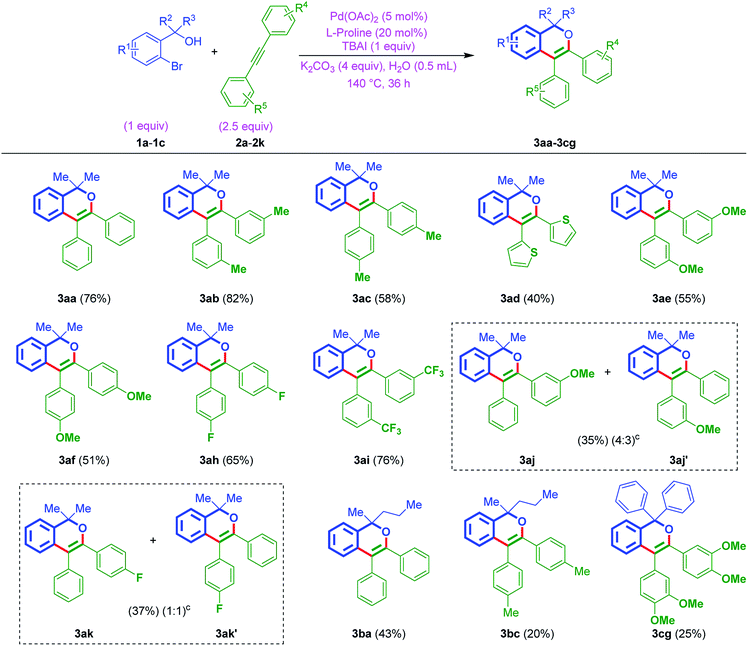
With the above best-optimized conditions (entry 5, Table 1), to test the feasibility of the process, it was decided to test the protocol with other substrates. Thus, ortho-bromo tertiary benzylic alcohols 1a–1c were reacted with different internal alkynes 2a–2k, under standard conditions. Interestingly, this protocol was quite successful and afforded corresponding isochromenes 3aa–3cg (Table 2). In addition, the protocol exhibited good compatibility with various substituents bearing aromatic rings of acetylenes. For example, the reaction was also smooth on internal alkyne flanked to a heteroaromatic ring (3ad, Table 2). Also, furnished the products 3ah and 3ai bearing electron deactivating F and CF3 moieties. Besides, when ortho-bromo tertiary benzylic alcohol 1a was subjected to the reaction using unsymmetrical acetylenes 2j and 2k, as anticipated, furnished regioisomeric mixtures [(3aj![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3aj′ in 4
3aj′ in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) and (3ak
3 ratio) and (3ak![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ak′ in 1
3ak′ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) (Table 2)].
1 ratio) (Table 2)].
| a Conditions: 1a–1c (0.25 mmol, 1 equiv.), 2a–2k (0.625 mmol, 2.5 equiv.), Pd(OAc)2 (2.8 mg, 0.012 mmol, 5 mol%), K2CO3 (138.6 mg, 1 mmol, 4 equiv.), L-proline (5.6 mg, 0.05 mol, 20 mol%), TBAI (tetrabutylammonium iodide) (92.3 mg, 0.25 mmol, 1 equiv.), 140 °C, 36 h.b Yields are isolated pure products 3aa–3cg.c Determined by NMR analysis. |
|---|
 |
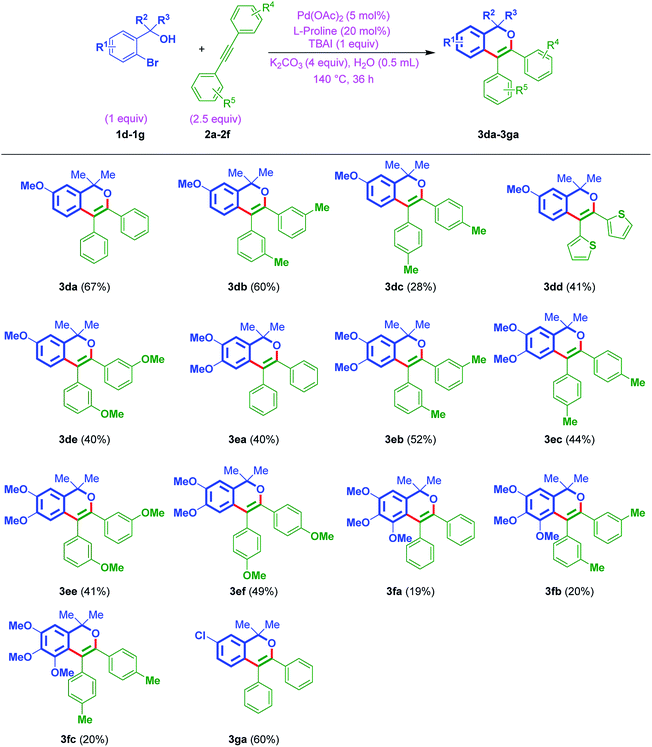
Furthermore, to establish the usefulness of the strategy, this time, the reaction was tested with the aromatic variant of ortho-bromo tertiary benzylic alcohols 1d–1g with internal alkynes 2a–2f. In general, the reaction exhibited a broad substrate scope and delivered the products 3da–3ga (Table 3). The protocol was tolerable to different functionalities of either aromatic rings. Notably, the strategy was found to be smooth with electron-donating OMe groups of ortho-bromo tertiary benzylic alcohols 1d–1f and also electron-withdrawing Cl group of ortho-bromo tertiary benzylic alcohol 1g. On the other hand, amenable to simple phenyl, tolyl, anisyl, and thiophenyl rings of internal alkynes as well.
| a Conditions: 1d–1g (0.25 mmol, 1 equiv.), 2a–2f (0.625 mmol, 2.5 equiv.), Pd(OAc)2 (2.8 mg, 0.012 mmol, 5 mol%), K2CO3 (138.6 mg, 1 mmol, 4 equiv.), L-proline (5.6 mg, 0.05 mol, 20 mol%), TBAI (tetrabutylammonium iodide) (92.3 mg, 0.25 mmol, 1 equiv.), 140 °C, 36 h.b Yields are isolated pure products 3da–3ga. |
|---|
 |
Furthermore, to show the effectiveness of the protocol, the annulation of ortho-bromo secondary benzylic alcohol 1h was attempted with internal alkynes (2a & 2h), using optimized conditions. Notably, as anticipated, afforded the cyclic ethers 3ha and 3hh in 41% and 44% yields, respectively (Table 4).
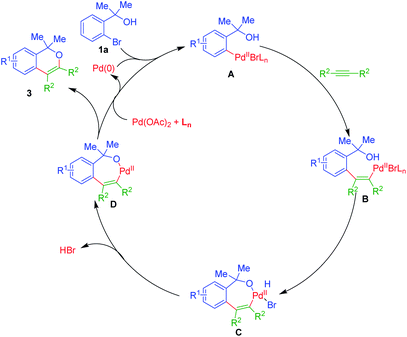
A plausible mechanistic path of this annulation process is as depicted in Scheme 1.
Initially, Pd(II) species A could be formed via oxidative incorporation of active Pd(0) catalyst onto the sigma C–Br bond of ortho-bromo tertiary benzylic alcohols 1. The subsequent, syn-addition reaction of A upon C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond of alkynes 2, gives acyclic Pd(II)-species B, which on subsequent intramolecular coupling with the nucleophilic hydroxyl group, would furnish a seven-membered palladacycle C [Pd(II)]. Removal of HBr from C could yield seven-membered Pd(II)-cycle D. Ultimately, reductive elimination of D, gives isochromenes 3 along with the regeneration of Pd(0) active catalyst and hence, fulfils the overall catalytic path of the reaction.19
C bond of alkynes 2, gives acyclic Pd(II)-species B, which on subsequent intramolecular coupling with the nucleophilic hydroxyl group, would furnish a seven-membered palladacycle C [Pd(II)]. Removal of HBr from C could yield seven-membered Pd(II)-cycle D. Ultimately, reductive elimination of D, gives isochromenes 3 along with the regeneration of Pd(0) active catalyst and hence, fulfils the overall catalytic path of the reaction.19
Conclusions
In summary, a palladium-catalyst promoted annulation between ortho-bromo benzylic alcohols and internal acetylenes is established, for achieving isochromenes. Significantly, this dual bond-forming process was feasible in water solvent and enabled the formation of various isochoromenes.Experimental section
General
IR spectra were recorded on a Bruker Tensor 37 (FTIR) spectrophotometer. 1H NMR spectra were recorded on Bruker Avance 400 (400 MHz) spectrometer at 295 K in CDCl3; chemical shifts (δ ppm) and coupling constants (Hz) are reported in standard fashion with reference to either internal standard tetramethylsilane (TMS) (δH = 0.00 ppm) or CDCl3 (δH = 7.25 ppm). 13C{1H} NMR spectra were recorded on Bruker Avance 400 (100 MHz) spectrometer at RT in CDCl3; chemical shifts (δ ppm) are reported relative to CDCl3 [δC = 77.00 ppm (central line of triplet)]. In the 13C{1H} NMR, the nature of carbons (C, CH, CH2 and CH3) was determined by recording the DEPT-135 spectra, and is given in parentheses and noted as s = singlet (for C), d = doublet (for CH), t = triplet (for CH2) and q = quartet (for CH3). In the 1H-NMR, the following abbreviations were used throughout: s = singlet, d = doublet, t = triplet, q = quartet, qui = quintet, sept = septet, dd = doublet of doublets, m = multiplet and br. s = broad singlet. The assignment of signals was confirmed by 1H, 13C{1H} CPD and DEPT spectra. High-resolution mass spectra (HR-MS) were recorded on an Agilent 6538 UHD Q-TOF electron spray ionization (ESI) mode and atmospheric pressure chemical ionization (APCI) modes. Solvents were distilled prior to use; petroleum ether with a boiling range of 60 to 80 °C was used. Palladium acetate, L-proline, TBAI (tetrabutylammonium iodide) and K2CO3 were purchased from Sigma-Aldrich/local sources and used as received. Acme's silica gel (60–120 mesh) was used for column chromatography (approximately 20 g per one gram of crude material). It is worth noting that these sort of experimental procedures have already been published elsewhere.17GP (general procedure for the synthesis of isochromenes)
An oven dried Screwcap vial was equipped with a magnetic stir bar and Pd(OAc)2 (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.3 mL) were added via syringe. After the addition of solvent, the solution was heated at 140 °C for 10 min for pre catalyst formation and brought to ambient temperature. A second oven dried vial was equipped with the starting materials, ortho-bromobenzyl alcohols 1 (54.0–84.8 mg, 0.25 mmol), internal alkynes 2 (111.2–196.2 mg, 0.625 mmol) and water (0.2 mL) was added at room temperature. This solution was then transferred to the first vial in which active catalyst was there via syringe. The reaction mixture was stirred at 140 °C for 36 h. Reaction mixture was then cooled to room temperature, quenched by the addition of aqueous NaHCO3 solution and extracted by using ethyl acetate (3 × 20 mL). The organic layer was washed with saturated NH4Cl solution, dried by Na2SO4 and then filtered. Evaporation of the solvent(s) under reduced pressure and refinement of the crude mixture by silica gel column chromatography (petroleum ether/ethyl acetate) gave the isochromenes 3 (19–82%) as semi-solid/solid.1,1-Dimethyl-3,4-bis(3-methylphenyl)-1H-isochromene (3ab)
GP was carried out with 2-(2-bromophenyl)propan-2-ol 1a (53.4 mg, 0.25 mmol), 1-methyl-3-[(3-methylphenyl)ethynyl]benzene 2b (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 99
0 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain isochromene 3ab (69.6 mg, 82%) as a light yellow solid compound, melting point = 140 °C, [TLC control (petroleum ether/ethyl acetate 99
1) to obtain isochromene 3ab (69.6 mg, 82%) as a light yellow solid compound, melting point = 140 °C, [TLC control (petroleum ether/ethyl acetate 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Rf (2b) = 0.8, Rf (3ab) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3057, 3018, 1688, 1471, 1355, 1249, 1040, 956, 744, 598 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26–7.17 (m, 3H, Ar-H), 7.16–7.08 (m, 3H, Ar-H), 7.07–6.99 (m, 4H, Ar-H), 6.96 (s, 1H, Ar-H), 6.88 (d, 1H, J = 7.8 Hz, Ar-H), 2.31 (s, 3H, CH3), 2.20 (s, 3H, CH3), 1.78 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 138.0 (s, Ar-C), 137.0 (s, Ar-C), 136.7 (s, Ar-C), 136.4 (s, Ar-C), 135.9 (s, Ar-C), 132.2 (d, 2 × Ar-CH), 129.3 (d, Ar-CH), 128.7 (d, Ar-CH), 128.5 (d, Ar-CH), 128.3 (d, Ar-CH), 127.6 (d, Ar-CH), 127.2 (d, Ar-CH), 127.2 (d, Ar-CH), 126.7 (d, Ar-CH), 126.0 (d, Ar-CH), 123.6 (d, Ar-CH), 122.1 (d, Ar-CH), 115.7 (s, Ar-CH), 77.5 (s, Ar-CH), 27.0 (q, 2 × CH3), 21.4 (q, CH3), 21.4 (q, CH3) ppm. HR-MS (ESI+) m/z calculated for [C25H25O]+ = [M + H]+: 341.1900; found 341.1901.
1), Rf (2b) = 0.8, Rf (3ab) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3057, 3018, 1688, 1471, 1355, 1249, 1040, 956, 744, 598 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26–7.17 (m, 3H, Ar-H), 7.16–7.08 (m, 3H, Ar-H), 7.07–6.99 (m, 4H, Ar-H), 6.96 (s, 1H, Ar-H), 6.88 (d, 1H, J = 7.8 Hz, Ar-H), 2.31 (s, 3H, CH3), 2.20 (s, 3H, CH3), 1.78 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 138.0 (s, Ar-C), 137.0 (s, Ar-C), 136.7 (s, Ar-C), 136.4 (s, Ar-C), 135.9 (s, Ar-C), 132.2 (d, 2 × Ar-CH), 129.3 (d, Ar-CH), 128.7 (d, Ar-CH), 128.5 (d, Ar-CH), 128.3 (d, Ar-CH), 127.6 (d, Ar-CH), 127.2 (d, Ar-CH), 127.2 (d, Ar-CH), 126.7 (d, Ar-CH), 126.0 (d, Ar-CH), 123.6 (d, Ar-CH), 122.1 (d, Ar-CH), 115.7 (s, Ar-CH), 77.5 (s, Ar-CH), 27.0 (q, 2 × CH3), 21.4 (q, CH3), 21.4 (q, CH3) ppm. HR-MS (ESI+) m/z calculated for [C25H25O]+ = [M + H]+: 341.1900; found 341.1901.
1,1-Dimethyl-3,4-dithien-2-yl-1H-isochromene (3ad)
GP was carried out with 2-(2-bromophenyl)propan-2-ol 1a (53.4 mg, 0.25 mmol), 2-(thien-2-ylethynyl)thiophene 2d (118.6 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 99
0 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain isochromene 3ad (32.3 mg, 40%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 99
1) to obtain isochromene 3ad (32.3 mg, 40%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Rf (2d) = 0.8, Rf (3ad) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2981, 1714, 1589, 1445, 1238, 1158, 1093, 1017, 922, 750, 680 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.52 (dd, 1H, J = 5.4 and 1.0 Hz, Ar-H), 7.24–7.13 (m, 5H, Ar-H), 7.05 (dd, 1H, J = 5.4 and 1.0 Hz, Ar-H), 7.00–6.92 (m, 2H, Ar-H), 6.91–6.83 (m, 1H, Ar-H), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 145.5 (s, Ar-C) 138.5 (s, Ar-C) 137.1 (s, Ar-C), 136.0 (s, Ar-C), 132.1 (s, Ar-C), 129.8 (d, Ar-CH), 127.8 (d, Ar-CH), 127.7 (d, Ar-CH), 127.6 (d, Ar-CH), 127.5 (d, Ar-CH) 127.5 (d, Ar-CH), 126.9 (d, Ar-CH), 126.8 (d, Ar-CH), 123.6 (d, Ar-CH), 122.0 (d, Ar-CH), 106.2 (s, Ar-CH), 78.5 (s, Ar-CH), 27.0 (q, 2 × CH3) ppm. HR-MS (ESI+) m/z calculated for [C19H17OS2]+ = [M + H]+: 325.0715; found 325.0705.
1), Rf (2d) = 0.8, Rf (3ad) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2981, 1714, 1589, 1445, 1238, 1158, 1093, 1017, 922, 750, 680 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.52 (dd, 1H, J = 5.4 and 1.0 Hz, Ar-H), 7.24–7.13 (m, 5H, Ar-H), 7.05 (dd, 1H, J = 5.4 and 1.0 Hz, Ar-H), 7.00–6.92 (m, 2H, Ar-H), 6.91–6.83 (m, 1H, Ar-H), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 145.5 (s, Ar-C) 138.5 (s, Ar-C) 137.1 (s, Ar-C), 136.0 (s, Ar-C), 132.1 (s, Ar-C), 129.8 (d, Ar-CH), 127.8 (d, Ar-CH), 127.7 (d, Ar-CH), 127.6 (d, Ar-CH), 127.5 (d, Ar-CH) 127.5 (d, Ar-CH), 126.9 (d, Ar-CH), 126.8 (d, Ar-CH), 123.6 (d, Ar-CH), 122.0 (d, Ar-CH), 106.2 (s, Ar-CH), 78.5 (s, Ar-CH), 27.0 (q, 2 × CH3) ppm. HR-MS (ESI+) m/z calculated for [C19H17OS2]+ = [M + H]+: 325.0715; found 325.0705.
3,4-Bis(3-methoxyphenyl)-1,1-dimethyl-1H-isochromene (3ae)
GP was carried out with 2-(2-bromophenyl)propan-2-ol 1a (53.4 mg, 0.25 mmol), 1-methoxy-3-[(3-methoxyphenyl)ethynyl]benzene 2e (148.7 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3ae (51 mg, 55%) as a light yellow solid compound, melting point = 82 °C, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3ae (51 mg, 55%) as a light yellow solid compound, melting point = 82 °C, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2e) = 0.6, Rf (3ae) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2839, 1596, 1511, 1458, 1249, 1140, 1027, 810, 761 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (ddd, J = 7.8, 78 and 1.0 Hz, 1H, Ar-H), 7.23–7.18 (m, 2H, Ar-H), 7.14–7.10 (m, 1H, Ar-H), 7.06 (ddd, J = 7.8, 78 and 1.0 Hz, 1H, Ar-H), 6.93 (ddd, J = 7.8, 78 and 1.0 Hz, 2H, Ar-H), 6.89–6.77 (m, 4H, Ar-H), 6.76–6.69 (m, 1H, Ar-H), 3.72 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 1.77 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.8 (s, Ar-C), 158.6 (s, Ar-C), 147.6 (s, Ar-C), 138.5 (s, Ar-C), 137.2 (s, Ar-C), 136.3 (s, Ar-C), 131.8 (s, Ar-C), 129.6 (d, Ar-CH), 128.5 (d, Ar-CH), 127.2 (d, Ar-CH), 126.9 (d, Ar-CH), 124.1 (d, Ar-CH), 123.7 (d, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 116.9 (d, Ar-CH), 115.6 (s, Ar-CH), 114.2 (d, Ar-CH), 113.7 (d, Ar-CH), 112.6 (d, Ar-CH), 77.6 (s, Ar-C), 55.2 (q, OCH3), 54.9 (q, OCH3), 27.0 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H25O3]+ = [M + H]+: 373.1798; found 373.1784.
2), Rf (2e) = 0.6, Rf (3ae) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2839, 1596, 1511, 1458, 1249, 1140, 1027, 810, 761 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.26 (ddd, J = 7.8, 78 and 1.0 Hz, 1H, Ar-H), 7.23–7.18 (m, 2H, Ar-H), 7.14–7.10 (m, 1H, Ar-H), 7.06 (ddd, J = 7.8, 78 and 1.0 Hz, 1H, Ar-H), 6.93 (ddd, J = 7.8, 78 and 1.0 Hz, 2H, Ar-H), 6.89–6.77 (m, 4H, Ar-H), 6.76–6.69 (m, 1H, Ar-H), 3.72 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 1.77 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.8 (s, Ar-C), 158.6 (s, Ar-C), 147.6 (s, Ar-C), 138.5 (s, Ar-C), 137.2 (s, Ar-C), 136.3 (s, Ar-C), 131.8 (s, Ar-C), 129.6 (d, Ar-CH), 128.5 (d, Ar-CH), 127.2 (d, Ar-CH), 126.9 (d, Ar-CH), 124.1 (d, Ar-CH), 123.7 (d, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 116.9 (d, Ar-CH), 115.6 (s, Ar-CH), 114.2 (d, Ar-CH), 113.7 (d, Ar-CH), 112.6 (d, Ar-CH), 77.6 (s, Ar-C), 55.2 (q, OCH3), 54.9 (q, OCH3), 27.0 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H25O3]+ = [M + H]+: 373.1798; found 373.1784.
1,1-Dimethyl-3,4-bis[4-(trifluoromethyl)phenyl]-1H-isochromene (3ai)
GP was carried out with 2-(2-bromophenyl)propan-2-ol 1a (53.4 mg, 0.25 mmol), 1-(trifluoromethyl)-4-{[4-(trifluoromethyl)phenyl]ethynyl}benzene 2i (196.2 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3ai (84.9 mg, 76%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3ai (84.9 mg, 76%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2i) = 0.7, Rf (3ai) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2854, 1619, 1444, 1329, 1245, 1166, 1124, 1073, 914, 803, 753, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.65–7.55 (m, 1H, Ar-H), 7.55–7.34 (m, 6H, Ar-H), 7.33–7.21 (m, 3H, Ar-H), 7.21–7.07 (m, 1H, Ar-H), 6.91–6.69 (m, 1H, Ar-H), 1.80 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.5 (s, Ar-C), 137.5 (s, 1 Ar-C), 136.3 (s, Ar-C), 136.2 (s, Ar-C), 135.0 (d, Ar-CH), 131.7 (d, Ar-CH), 131.3 (d, J = 32.3 Hz, Ar-C), 130.8 (d, Ar-CH), 130.1 (d, J = 5.1 Hz, Ar-C), 129.3 (d, Ar-CH), 128.3 (q, Jc–f = 3.7 Hz, Ar-C), 128.1 (d, Ar-CH), 127.7 (d, Ar-CH), 127.5 (d, Ar-CH), 125.6 (q, Jc–f = 3.7 Hz, Ar-C), 124.6 (q, Jc–f = 3.7 Hz, Ar-C), 124.2 (q, Jc–f = 3.7 Hz, Ar-C), 124.0 (q, Jc–f = 272.9 Hz, Ar-CF3), 123.8 (q, Jc–f = 272.9 Hz, Ar-CF3), 123.4 (d, Ar-CH), 122.6 (d, Ar-CH), 115.5 (s, Ar-C), 78.4 (s, Ar-C), 27.2 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H19F6O]+ = [M + H]+: 449.1335; found 449.1338.
2), Rf (2i) = 0.7, Rf (3ai) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2854, 1619, 1444, 1329, 1245, 1166, 1124, 1073, 914, 803, 753, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.65–7.55 (m, 1H, Ar-H), 7.55–7.34 (m, 6H, Ar-H), 7.33–7.21 (m, 3H, Ar-H), 7.21–7.07 (m, 1H, Ar-H), 6.91–6.69 (m, 1H, Ar-H), 1.80 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.5 (s, Ar-C), 137.5 (s, 1 Ar-C), 136.3 (s, Ar-C), 136.2 (s, Ar-C), 135.0 (d, Ar-CH), 131.7 (d, Ar-CH), 131.3 (d, J = 32.3 Hz, Ar-C), 130.8 (d, Ar-CH), 130.1 (d, J = 5.1 Hz, Ar-C), 129.3 (d, Ar-CH), 128.3 (q, Jc–f = 3.7 Hz, Ar-C), 128.1 (d, Ar-CH), 127.7 (d, Ar-CH), 127.5 (d, Ar-CH), 125.6 (q, Jc–f = 3.7 Hz, Ar-C), 124.6 (q, Jc–f = 3.7 Hz, Ar-C), 124.2 (q, Jc–f = 3.7 Hz, Ar-C), 124.0 (q, Jc–f = 272.9 Hz, Ar-CF3), 123.8 (q, Jc–f = 272.9 Hz, Ar-CF3), 123.4 (d, Ar-CH), 122.6 (d, Ar-CH), 115.5 (s, Ar-C), 78.4 (s, Ar-C), 27.2 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H19F6O]+ = [M + H]+: 449.1335; found 449.1338.
3-(3-Methoxyphenyl)-1,1-dimethyl-4-phenyl-1H-isochromene (3aj) and 4-(3-methoxyphenyl)-1,1-dimethyl-3-phenyl-1H-isochromene (3aj′)
GP was carried out with 2-(2-bromophenyl)propan-2-ol 1a (53.4 mg, 0.25 mmol), 1-methoxy-3-(phenylethynyl)benzene 2j (130 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 99
0 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain isochromene 3aj + 3aj′ (29.8 mg, 35%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 100
1) to obtain isochromene 3aj + 3aj′ (29.8 mg, 35%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), Rf (2j) = 0.7, Rf (3aj + 3aj′) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2975, 2927, 1695, 1591, 1481, 1253, 1158, 1044, 761, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.41–7.33 (m, 2H, Ar-H), 7.33–7.22 (m, 6H, Ar-H), 7.22–7.18 (m, 4H, Ar-H), 7.18–7.10 (m, 5H, Ar-H), 7.06 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 6.97–6.87 (m, 3H, Ar-H), 6.86–6.81 (m, 2H, Ar-H), 6.79–6.76 (m, 1H, Ar-H), 6.75–6.72 (m, 1H, Ar-H), 6.72–6.66 (m, 1H, Ar-H), 3.73 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 1.79 (s, 12H, 4 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.7 (s, Ar-C), 158.5 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 138.4 (s, Ar-C), 137.2 (s, Ar-C), 136.3 (s, Ar-C), 136.2 (s, Ar-C), 136.0 (s, Ar-C), 131.9 (s, Ar-C), 131.8 (s, Ar-C), 131.6 (d, 3 × Ar-CH), 129.5 (d, Ar-CH), 128.6 (d, 4 × Ar-CH), 128.4 (d, Ar-CH), 127.8 (d, Ar-CH), 127.5 (d, 3 × Ar-CH), 127.2 (d, Ar-CH), 127.2 (d, Ar-CH), 126.9 (d, Ar-CH), 126.9 (d, Ar-CH), 126.9 (d, Ar-CH), 124.2 (d, Ar-CH), 123.6 (d, Ar-CH), 123.6 (d, Ar-CH), 122.2 (d, 2 × Ar-CH), 121.2 (d, Ar-CH), 117.0 (d, Ar-CH), 115.8 (s, Ar-C), 115.5 (s, Ar-C), 114.2 (d, Ar-CH), 113.9 (d, Ar-CH), 112.6 (d, Ar-CH), 77.7 (s, Ar-C), 55.2 (q, OCH3), 54.9 (q, OCH3), 27.08 (q, 2 × CH3) 27.05 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H23O2]+ = [M + H]+: 343.1693; found 343.1693.
0), Rf (2j) = 0.7, Rf (3aj + 3aj′) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2975, 2927, 1695, 1591, 1481, 1253, 1158, 1044, 761, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.41–7.33 (m, 2H, Ar-H), 7.33–7.22 (m, 6H, Ar-H), 7.22–7.18 (m, 4H, Ar-H), 7.18–7.10 (m, 5H, Ar-H), 7.06 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 6.97–6.87 (m, 3H, Ar-H), 6.86–6.81 (m, 2H, Ar-H), 6.79–6.76 (m, 1H, Ar-H), 6.75–6.72 (m, 1H, Ar-H), 6.72–6.66 (m, 1H, Ar-H), 3.73 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 1.79 (s, 12H, 4 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.7 (s, Ar-C), 158.5 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 138.4 (s, Ar-C), 137.2 (s, Ar-C), 136.3 (s, Ar-C), 136.2 (s, Ar-C), 136.0 (s, Ar-C), 131.9 (s, Ar-C), 131.8 (s, Ar-C), 131.6 (d, 3 × Ar-CH), 129.5 (d, Ar-CH), 128.6 (d, 4 × Ar-CH), 128.4 (d, Ar-CH), 127.8 (d, Ar-CH), 127.5 (d, 3 × Ar-CH), 127.2 (d, Ar-CH), 127.2 (d, Ar-CH), 126.9 (d, Ar-CH), 126.9 (d, Ar-CH), 126.9 (d, Ar-CH), 124.2 (d, Ar-CH), 123.6 (d, Ar-CH), 123.6 (d, Ar-CH), 122.2 (d, 2 × Ar-CH), 121.2 (d, Ar-CH), 117.0 (d, Ar-CH), 115.8 (s, Ar-C), 115.5 (s, Ar-C), 114.2 (d, Ar-CH), 113.9 (d, Ar-CH), 112.6 (d, Ar-CH), 77.7 (s, Ar-C), 55.2 (q, OCH3), 54.9 (q, OCH3), 27.08 (q, 2 × CH3) 27.05 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H23O2]+ = [M + H]+: 343.1693; found 343.1693.
3-(4-Fluorophenyl)-1,1-dimethyl-4-phenyl-1H-isochromene (3ak) and 4-(4-fluorophenyl)-1,1-dimethyl-3-phenyl-1H-isochromene (3ak′)
GP was carried out with 2-(2-bromophenyl)propan-2-ol 1a (53.4 mg, 0.25 mmol), 1-fluoro-4-(phenylethynyl)benzene 2k (122.5 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3ak + 3ak′ (30.4 mg, 37%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3ak + 3ak′ (30.4 mg, 37%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2k) = 0.8, Rf (3ak + 3ak′) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3062, 2978, 2925, 1695, 1610, 1507, 1230, 1155, 1099, 962, 834, 760, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42–7.28 (m, 4H, Ar-H), 7.28–7.18 (m, 12H, Ar-H), 7.17–7.10 (m, 4H, Ar-H), 7.06–7.00 (m, 2H, Ar-H), 6.93–6.75 (m, 4H, Ar-H), 1.79 (s, 12H, 4 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 162.0 (d, J = 245.0 Hz, Ar-C), 160.8 (s, Ar-C), 147.1 (s, Ar-C), 136.9 (s, Ar-C), 136.2 (s, Ar-C), 135.9 (s, Ar-C), 133.3 (d, Ar-CH), 133.2 (d, Ar-CH), 131.8 (s, Ar-CH), 131.6 (d, 4 × Ar-CH), 130.7 (d, Ar-CH), 130.6 (d, Ar-CH), 128.7 (d, 3 × Ar-CH), 128.6 (d, 5 × Ar-CH), 127.9 (d, Ar-CH), 127.5 (d, 3 × Ar-CH), 127.3 (d, Ar-CH), 127.0 (d, 3 × Ar-CH), 127.0 (d, Ar-CH), 126.9 (d, Ar-CH), 123.5 (d, Ar-CH), 123.3 (d, Ar-CH), 122.3 (d, Ar-CH), 122.2 (d, Ar-CH), 115.7 (d, Ar-CH), 115.4 (d, Ar-CH), 114.5 (d, Ar-CH), 114.3 (d, Ar-CH), 77.8 (s, Ar-C), 27.1 (q, 4 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C23H20FO]+ = [M + H]+: 331.1493; found 331.1494.
2), Rf (2k) = 0.8, Rf (3ak + 3ak′) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3062, 2978, 2925, 1695, 1610, 1507, 1230, 1155, 1099, 962, 834, 760, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42–7.28 (m, 4H, Ar-H), 7.28–7.18 (m, 12H, Ar-H), 7.17–7.10 (m, 4H, Ar-H), 7.06–7.00 (m, 2H, Ar-H), 6.93–6.75 (m, 4H, Ar-H), 1.79 (s, 12H, 4 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 162.0 (d, J = 245.0 Hz, Ar-C), 160.8 (s, Ar-C), 147.1 (s, Ar-C), 136.9 (s, Ar-C), 136.2 (s, Ar-C), 135.9 (s, Ar-C), 133.3 (d, Ar-CH), 133.2 (d, Ar-CH), 131.8 (s, Ar-CH), 131.6 (d, 4 × Ar-CH), 130.7 (d, Ar-CH), 130.6 (d, Ar-CH), 128.7 (d, 3 × Ar-CH), 128.6 (d, 5 × Ar-CH), 127.9 (d, Ar-CH), 127.5 (d, 3 × Ar-CH), 127.3 (d, Ar-CH), 127.0 (d, 3 × Ar-CH), 127.0 (d, Ar-CH), 126.9 (d, Ar-CH), 123.5 (d, Ar-CH), 123.3 (d, Ar-CH), 122.3 (d, Ar-CH), 122.2 (d, Ar-CH), 115.7 (d, Ar-CH), 115.4 (d, Ar-CH), 114.5 (d, Ar-CH), 114.3 (d, Ar-CH), 77.8 (s, Ar-C), 27.1 (q, 4 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C23H20FO]+ = [M + H]+: 331.1493; found 331.1494.
1-Methyl-3,4-diphenyl-1-propyl-1H-isochromene (3ba)
GP was carried out with 2-(2-bromophenyl)pentan-2-ol 1b (60.5 mg), (phenylethynyl)benzene 2a (111.2 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 99
0 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain isochromene 3ba (36.5 mg, 43%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 100
1) to obtain isochromene 3ba (36.5 mg, 43%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), Rf (2a) = 0.8, Rf (3ba) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3055, 2947, 1702, 1605, 1477, 1445, 1348, 1278, 1231, 1026, 703 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.37–7.27 (m, 3H, Ar-H), 7.27–7.19 (m, 4H, Ar-H), 7.19–7.05 (m, 6H, Ar-H), 6.85 (dd, J = 7.3 and 1.0 Hz, 1H, Ar-H), 2.22–1.99 (m, 2H, CH2), 1.74 (s, 3H, CH3), 1.58–1.39 (m, 2H, CH2), 0.93 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.0 (s, Ar-C), 137.2 (s, Ar-C), 136.1 (s, Ar-C), 135.3 (s, Ar-C), 132.4 (s, Ar-C), 131.7 (d, 2 × Ar-CH), 128.7 (d, 3 × Ar-CH), 128.5 (d, Ar-CH), 127.6 (d, Ar-CH), 127.4 (d, 2 × Ar-CH), 127.0 (d, Ar-CH), 126.9 (d, Ar-CH), 126.5 (d, Ar-CH), 123.5 (d, Ar-CH), 122.9 (d, Ar-CH), 115.1 (s, Ar-C), 80.1 (s, Ar-C), 41.8 (t, CH2), 25.7 (q, CH3), 17.4 (t, CH2), 14.6 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H25O]+ = [M + H]+: 341.1900; found 341.1906.
0), Rf (2a) = 0.8, Rf (3ba) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3055, 2947, 1702, 1605, 1477, 1445, 1348, 1278, 1231, 1026, 703 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.37–7.27 (m, 3H, Ar-H), 7.27–7.19 (m, 4H, Ar-H), 7.19–7.05 (m, 6H, Ar-H), 6.85 (dd, J = 7.3 and 1.0 Hz, 1H, Ar-H), 2.22–1.99 (m, 2H, CH2), 1.74 (s, 3H, CH3), 1.58–1.39 (m, 2H, CH2), 0.93 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.0 (s, Ar-C), 137.2 (s, Ar-C), 136.1 (s, Ar-C), 135.3 (s, Ar-C), 132.4 (s, Ar-C), 131.7 (d, 2 × Ar-CH), 128.7 (d, 3 × Ar-CH), 128.5 (d, Ar-CH), 127.6 (d, Ar-CH), 127.4 (d, 2 × Ar-CH), 127.0 (d, Ar-CH), 126.9 (d, Ar-CH), 126.5 (d, Ar-CH), 123.5 (d, Ar-CH), 122.9 (d, Ar-CH), 115.1 (s, Ar-C), 80.1 (s, Ar-C), 41.8 (t, CH2), 25.7 (q, CH3), 17.4 (t, CH2), 14.6 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H25O]+ = [M + H]+: 341.1900; found 341.1906.
1-Methyl-3,4-bis(4-methylphenyl)-1-propyl-1H-isochromene (3bc)
GP was carried out with 2-(2-bromophenyl)pentan-2-ol 1b (60.5 mg, 0.25 mmol), 1-methyl-4-[(4-methylphenyl)ethynyl]benzene 2c (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 99
0 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain isochromene 3bc (18.4 mg, 20%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 99
1) to obtain isochromene 3bc (18.4 mg, 20%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Rf (2c) = 0.7, Rf (3bc) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3025, 2920, 1670, 1507, 1446, 1261, 1181, 1108, 1033, 946, 819, 732 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.20–6.99 (m, 8H, Ar-H), 6.94 (d, J = 1.0 Hz, 1H, Ar-H), 6.93(dd, J = 7.8 and 1.0 Hz, 1H, Ar-H), 6.84 (dd, J = 7.8 and 1.0 Hz, 1H, Ar-H), 6.64 (d, J = 2.0 Hz, 1H, Ar-H), 2.36 (s, 3H, CH3), 2.24 (s, 3H, CH3), 2.15–1.87 (m, 2H, CH2), 1.54 (s, 3H, CH3), 1.52–1.36 (m, 2H, CH2), 0.91 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 137.4 (s, Ar-C), 136.3 (s, Ar-C), 135.3 (s, Ar-C), 134.2 (s, Ar-C), 133.3 (s, Ar-C), 132.7 (s, Ar-C), 131.5 (d, 2 × Ar-CH), 131.3 (d, Ar-CH) 129.3 (d, 2 × Ar-CH), 128.6 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 127.0 (d, Ar-CH), 126.3 (d, Ar-CH), 123.5 (d, Ar-CH), 122.8 (s, Ar-C), 79.8 (s, Ar-C), 41.8 (t, CH2), 25.6 (q, CH3), 21.3 (q, CH3), 21.2 (q, CH3), 17.4 (t, CH2), 14.6 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O]+ = [M + H]+: 369.2213; found 369.2218.
1), Rf (2c) = 0.7, Rf (3bc) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3025, 2920, 1670, 1507, 1446, 1261, 1181, 1108, 1033, 946, 819, 732 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.20–6.99 (m, 8H, Ar-H), 6.94 (d, J = 1.0 Hz, 1H, Ar-H), 6.93(dd, J = 7.8 and 1.0 Hz, 1H, Ar-H), 6.84 (dd, J = 7.8 and 1.0 Hz, 1H, Ar-H), 6.64 (d, J = 2.0 Hz, 1H, Ar-H), 2.36 (s, 3H, CH3), 2.24 (s, 3H, CH3), 2.15–1.87 (m, 2H, CH2), 1.54 (s, 3H, CH3), 1.52–1.36 (m, 2H, CH2), 0.91 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 137.4 (s, Ar-C), 136.3 (s, Ar-C), 135.3 (s, Ar-C), 134.2 (s, Ar-C), 133.3 (s, Ar-C), 132.7 (s, Ar-C), 131.5 (d, 2 × Ar-CH), 131.3 (d, Ar-CH) 129.3 (d, 2 × Ar-CH), 128.6 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 127.0 (d, Ar-CH), 126.3 (d, Ar-CH), 123.5 (d, Ar-CH), 122.8 (s, Ar-C), 79.8 (s, Ar-C), 41.8 (t, CH2), 25.6 (q, CH3), 21.3 (q, CH3), 21.2 (q, CH3), 17.4 (t, CH2), 14.6 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O]+ = [M + H]+: 369.2213; found 369.2218.
3,4-Bis(3,4-dimethoxyphenyl)-1,1-diphenyl-1H-isochromene (3cg)
GP was carried out with (2-bromophenyl)(diphenyl)methanol 1c (84.5 mg, 0.25 mmol), 4-[(3,4-dimethoxyphenyl)ethynyl]-1,2-dimethoxybenzene 2g (186.3 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 to 70
27 to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain isochromene 3cg (34.7 mg, 25%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 70
30) to obtain isochromene 3cg (34.7 mg, 25%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), Rf (2g) = 0.5, Rf (3cg) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2925, 2850, 1511, 1459, 1254, 1151, 1028, 763, 702 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.37–7.26 (m, 10H, Ar-H), 7.23 (ddd, J = 7.8, 7.8 and 1.5 Hz, 1H, Ar-H), 7.11 (ddd, J = 7.8, 7.8 and 1.5 Hz, 1H, Ar-H), 7.08–6.99 (m, 2H, Ar-H), 6.81 (d, J = 8.3 Hz, 1H, Ar-H), 6.74–6.62 (m, 3H, Ar-H) 6.56 (dd, J = 8.3 and 2.0 Hz, 1H, Ar-H), 6.50 (d, J = 2.0 Hz, 1H, Ar-H), 3.85 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.68 (s, 3H, OCH3), 3.48 (s, 3H, OCH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 149.1 (s, Ar-C), 148.5 (s, Ar-C), 148.0 (s, Ar-C), 147.9 (s, Ar-C), 147.4 (s, Ar-C), 144.0 (d, Ar-CH), 134.2 (s, Ar-C), 133.5 (s, Ar-C), 131.5 (s, Ar-C), 130.0 (s, Ar-C), 129.8 (s, Ar-C), 128.8 (d, 3 × Ar-CH), 128.2 (s, Ar-CH), 127.9 (d, Ar-CH), 127.7 (d, 2 × Ar-CH), 127.5 (d, 4 × Ar-CH), 126.8 (s, Ar-C), 126.0 (d, Ar-CH), 123.6 (d, Ar-CH), 123.1 (d, Ar-CH), 121.3 (d, Ar-CH), 116.5 (s, Ar-C), 114.5 (d, Ar-CH), 111.9 (d, Ar-CH), 111.4 (d, Ar-CH), 110.0 (d, Ar-CH), 86.7 (s, Ar-C), 55.9 (q, OCH3), 55.8 (q, OCH3), 55.6 (q, OCH3), 55.3 (q, OCH3) ppm. HR-MS (APCI+) m/z calculated for [C37H33O5]+ = [M + H]+: 557.2323; found 557.2331.
30), Rf (2g) = 0.5, Rf (3cg) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2925, 2850, 1511, 1459, 1254, 1151, 1028, 763, 702 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.37–7.26 (m, 10H, Ar-H), 7.23 (ddd, J = 7.8, 7.8 and 1.5 Hz, 1H, Ar-H), 7.11 (ddd, J = 7.8, 7.8 and 1.5 Hz, 1H, Ar-H), 7.08–6.99 (m, 2H, Ar-H), 6.81 (d, J = 8.3 Hz, 1H, Ar-H), 6.74–6.62 (m, 3H, Ar-H) 6.56 (dd, J = 8.3 and 2.0 Hz, 1H, Ar-H), 6.50 (d, J = 2.0 Hz, 1H, Ar-H), 3.85 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.68 (s, 3H, OCH3), 3.48 (s, 3H, OCH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 149.1 (s, Ar-C), 148.5 (s, Ar-C), 148.0 (s, Ar-C), 147.9 (s, Ar-C), 147.4 (s, Ar-C), 144.0 (d, Ar-CH), 134.2 (s, Ar-C), 133.5 (s, Ar-C), 131.5 (s, Ar-C), 130.0 (s, Ar-C), 129.8 (s, Ar-C), 128.8 (d, 3 × Ar-CH), 128.2 (s, Ar-CH), 127.9 (d, Ar-CH), 127.7 (d, 2 × Ar-CH), 127.5 (d, 4 × Ar-CH), 126.8 (s, Ar-C), 126.0 (d, Ar-CH), 123.6 (d, Ar-CH), 123.1 (d, Ar-CH), 121.3 (d, Ar-CH), 116.5 (s, Ar-C), 114.5 (d, Ar-CH), 111.9 (d, Ar-CH), 111.4 (d, Ar-CH), 110.0 (d, Ar-CH), 86.7 (s, Ar-C), 55.9 (q, OCH3), 55.8 (q, OCH3), 55.6 (q, OCH3), 55.3 (q, OCH3) ppm. HR-MS (APCI+) m/z calculated for [C37H33O5]+ = [M + H]+: 557.2323; found 557.2331.
7-Methoxy-1,1-dimethyl-3,4-diphenyl-1H-isochromene (3da)
GP was carried out with 2-(2-bromo-5-methoxyphenyl)propan-2-ol 1d (61 mg, 0.25 mmol), (phenylethynyl)benzene 2a (111.2 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 99
0 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain isochromene 3da (57.2 mg, 67%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 100
1) to obtain isochromene 3da (57.2 mg, 67%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), Rf (2a) = 0.8, Rf (3da) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2925, 2364, 1743, 1694, 1511, 1301, 1069, 758, 699 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.39–7.28 (m, 2H, Ar-H), 7.28–7.19 (m, 4H, Ar-H), 7.17–7.08 (m, 3H, Ar-H), 6.89–6.75 (m, 3H, Ar-H), 6.67 (dd, J = 8.6 and 2.7 Hz, 1H, Ar-H), 3.81 (s, 3H, OCH3), 1.77 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.8 (s, Ar-C), 146.0 (s, Ar-C), 138.3 (s, Ar-C), 137.3 (s, Ar-C), 136.1 (s, Ar-C), 131.6 (d, 2 × Ar-CH), 131.4 (d, Ar-CH), 128.6 (d, Ar-CH), 128.5 (d, 2 × Ar-CH), 127.4 (d, Ar-CH), 127.4 (d, Ar-CH), 126.9 (d, Ar-CH), 126.5 (d, Ar-CH), 125.3 (s, Ar-C), 125.1 (d, Ar-CH), 115.6 (s, Ar-C), 111.3 (d, Ar-CH), 109.0 (d, Ar-CH), 77.4 (s, Ar-C), 55.4 (q, OCH3), 26.9 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H23O2]+ = [M + H]+: 343.1693; found 343.1686.
0), Rf (2a) = 0.8, Rf (3da) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2925, 2364, 1743, 1694, 1511, 1301, 1069, 758, 699 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.39–7.28 (m, 2H, Ar-H), 7.28–7.19 (m, 4H, Ar-H), 7.17–7.08 (m, 3H, Ar-H), 6.89–6.75 (m, 3H, Ar-H), 6.67 (dd, J = 8.6 and 2.7 Hz, 1H, Ar-H), 3.81 (s, 3H, OCH3), 1.77 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.8 (s, Ar-C), 146.0 (s, Ar-C), 138.3 (s, Ar-C), 137.3 (s, Ar-C), 136.1 (s, Ar-C), 131.6 (d, 2 × Ar-CH), 131.4 (d, Ar-CH), 128.6 (d, Ar-CH), 128.5 (d, 2 × Ar-CH), 127.4 (d, Ar-CH), 127.4 (d, Ar-CH), 126.9 (d, Ar-CH), 126.5 (d, Ar-CH), 125.3 (s, Ar-C), 125.1 (d, Ar-CH), 115.6 (s, Ar-C), 111.3 (d, Ar-CH), 109.0 (d, Ar-CH), 77.4 (s, Ar-C), 55.4 (q, OCH3), 26.9 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H23O2]+ = [M + H]+: 343.1693; found 343.1686.
7-Methoxy-1,1-dimethyl-3,4-bis(3-methylphenyl)-1H-isochromene (3db)
GP was carried out with 2-(2-bromo-5-methoxyphenyl)propan-2-ol 1d (61 mg, 0.25 mmol), 1-methyl-3-[(3-methylphenyl)ethynyl]benzene 2b (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3db (55.5 mg, 60%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3db (55.5 mg, 60%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2b) = 0.7, Rf (3db) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2364, 1691, 1611, 1506, 1295, 1223, 1043, 785, 704 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.28–7.19 (m, 1H, Ar-H), 7.18–7.09 (m, 2H, Ar-H), 7.09–6.98 (m, 4H, Ar-H), 6.95 (m, 1H, Ar-H), 6.81 (dd, J = 8.3 and 2.5 Hz, 1H, Ar-H), 6.76 (d, J = 2.5 Hz, 1H, Ar-H), 6.67 (dd, J = 8.3 and 2.5 Hz, 1H, Ar-H), 3.81 (s, 3H, OCH3), 2.32 (s, 3H, CH3), 2.21 (s, 3H, CH3), 1.77 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.7 (s, Ar-C), 145.8 (s, Ar-C), 138.4 (s, Ar-C), 138.0 (s, Ar-C), 137.2 (s, Ar-C), 136.8 (s, Ar-C), 136.0 (s, Ar-C), 132.0 (d, Ar-CH), 129.1 (d, Ar-CH), 128.6 (d, Ar-CH), 128.3 (d, Ar-CH), 128.2 (d, Ar-CH), 127.5 (d, Ar-CH), 127.2 (d, Ar-CH), 125.8 (d, Ar-CH), 125.5 (d, Ar-CH), 125.1 (d, Ar-CH), 115.6 (s, Ar-C), 111.3 (d, Ar-CH), 108.9 (d, Ar-CH), 77.2 (s, Ar-C), 55.3 (q, OCH3), 26.9 (q, 2 × CH3), 21.4 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O2]+ = [M + H]+: 371.2006; found 371.1992.
2), Rf (2b) = 0.7, Rf (3db) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2364, 1691, 1611, 1506, 1295, 1223, 1043, 785, 704 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.28–7.19 (m, 1H, Ar-H), 7.18–7.09 (m, 2H, Ar-H), 7.09–6.98 (m, 4H, Ar-H), 6.95 (m, 1H, Ar-H), 6.81 (dd, J = 8.3 and 2.5 Hz, 1H, Ar-H), 6.76 (d, J = 2.5 Hz, 1H, Ar-H), 6.67 (dd, J = 8.3 and 2.5 Hz, 1H, Ar-H), 3.81 (s, 3H, OCH3), 2.32 (s, 3H, CH3), 2.21 (s, 3H, CH3), 1.77 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.7 (s, Ar-C), 145.8 (s, Ar-C), 138.4 (s, Ar-C), 138.0 (s, Ar-C), 137.2 (s, Ar-C), 136.8 (s, Ar-C), 136.0 (s, Ar-C), 132.0 (d, Ar-CH), 129.1 (d, Ar-CH), 128.6 (d, Ar-CH), 128.3 (d, Ar-CH), 128.2 (d, Ar-CH), 127.5 (d, Ar-CH), 127.2 (d, Ar-CH), 125.8 (d, Ar-CH), 125.5 (d, Ar-CH), 125.1 (d, Ar-CH), 115.6 (s, Ar-C), 111.3 (d, Ar-CH), 108.9 (d, Ar-CH), 77.2 (s, Ar-C), 55.3 (q, OCH3), 26.9 (q, 2 × CH3), 21.4 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O2]+ = [M + H]+: 371.2006; found 371.1992.
7-Methoxy-1,1-dimethyl-3,4-bis(4-methylphenyl)-1H-isochromene (3dc)
GP was carried out with 2-(2-bromo-5-methoxyphenyl)propan-2-ol 1d (61 mg, 0.25 mmol), 1-methyl-4-[(4-methylphenyl)ethynyl]benzene 2c (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.67 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3dc (25.9 mg, 28%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3dc (25.9 mg, 28%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2c) = 0.7, Rf (3dc) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2856, 1701, 1614, 1498, 1295, 1222, 1044, 964, 816, 756 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.20–7.04 (m, 6H, Ar-H), 6.94 (dd, J = 7.8 and 1.0 Hz, 2H, Ar-H), 6.81 (d, J = 8.8 Hz, 1H, Ar-H), 6.76 (d, J = 2.9 Hz, 1H, Ar-H), 6.69–6.58 (m, 1H, Ar-H), 3.80 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.25 (s, 3H, CH3), 1.74 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.6 (s, Ar-C), 145.8 (s, Ar-C), 138.3 (s, Ar-C), 137.1 (s, Ar-C), 136.3 (s, Ar-C), 134.3 (s, Ar-C), 133.3 (s, Ar-C), 131.3 (d, 2 × Ar-CH), 129.3 (d, 2 × Ar-CH), 128.4 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 125.6 (s, Ar-C), 125.0 (d, Ar-CH), 115.0 (s, Ar-C), 111.3 (d, Ar-CH), 108.9 (d, Ar-CH), 77.2 (s, Ar-C), 55.3 (q, OCH3) 26.9 (q, 2 × CH3) 21.3 (q, CH3), 21.2 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O2]+ = [M + H]+: 371.2006; found 371.1991.
2), Rf (2c) = 0.7, Rf (3dc) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2856, 1701, 1614, 1498, 1295, 1222, 1044, 964, 816, 756 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.20–7.04 (m, 6H, Ar-H), 6.94 (dd, J = 7.8 and 1.0 Hz, 2H, Ar-H), 6.81 (d, J = 8.8 Hz, 1H, Ar-H), 6.76 (d, J = 2.9 Hz, 1H, Ar-H), 6.69–6.58 (m, 1H, Ar-H), 3.80 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.25 (s, 3H, CH3), 1.74 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.6 (s, Ar-C), 145.8 (s, Ar-C), 138.3 (s, Ar-C), 137.1 (s, Ar-C), 136.3 (s, Ar-C), 134.3 (s, Ar-C), 133.3 (s, Ar-C), 131.3 (d, 2 × Ar-CH), 129.3 (d, 2 × Ar-CH), 128.4 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 125.6 (s, Ar-C), 125.0 (d, Ar-CH), 115.0 (s, Ar-C), 111.3 (d, Ar-CH), 108.9 (d, Ar-CH), 77.2 (s, Ar-C), 55.3 (q, OCH3) 26.9 (q, 2 × CH3) 21.3 (q, CH3), 21.2 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O2]+ = [M + H]+: 371.2006; found 371.1991.
7-Methoxy-1,1-dimethyl-3,4-dithien-2-yl-1H-isochromene (3dd)
GP was carried out with 2-(2-bromo-5-methoxyphenyl)propan-2-ol 1d (61 mg, 0.25 mmol), 2-(thien-2-ylethynyl)thiophene 2d (118.6 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3dd (36.2 mg, 41%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3dd (36.2 mg, 41%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2d) = 0.6, Rf (3dd) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3041, 2923, 1712, 1595, 1482, 1446, 1264, 1209, 1167, 1081, 989, 907, 745, 695 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.51 (dd, J = 5.4 and 1.5 Hz, 1H, Ar-H), 7.23–7.15 (m, 2H, Ar-H), 7.04 (dd, J = 3.4 and 1.0 Hz, 1H, Ar-H), 6.96–6.85 (m, 3H, Ar-H), 6.75 (d, J = 2.9 Hz, 1H, Ar-H), 6.70 (dd, J = 8.8 and 3.0 Hz, 1H, Ar-H), 3.80 (s, 3H, OCH3), 1.73 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.9 (s, Ar-C), 143.5 (s, Ar-C), 138.7 (s, Ar-C), 138.0 (s, Ar-C), 137.3 (s, Ar-C), 129.6 (d, Ar-CH), 127.7 (d, Ar-CH), 127.4 (d, Ar-CH), 127.1 (d, Ar-CH), 126.9 (d, Ar-CH), 126.7 (d, Ar-CH), 125.4 (s, Ar-C), 125.1 (d, Ar-CH), 111.6 (d, Ar-CH), 108.9 (d, Ar-CH), 106.3 (s, Ar-C), 78.2 (s, Ar-C), 55.4 (q, OCH3), 26.8 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C20H19O2S2]+ = [M + H]+: 355.0821; found 355.0818.
2), Rf (2d) = 0.6, Rf (3dd) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3041, 2923, 1712, 1595, 1482, 1446, 1264, 1209, 1167, 1081, 989, 907, 745, 695 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.51 (dd, J = 5.4 and 1.5 Hz, 1H, Ar-H), 7.23–7.15 (m, 2H, Ar-H), 7.04 (dd, J = 3.4 and 1.0 Hz, 1H, Ar-H), 6.96–6.85 (m, 3H, Ar-H), 6.75 (d, J = 2.9 Hz, 1H, Ar-H), 6.70 (dd, J = 8.8 and 3.0 Hz, 1H, Ar-H), 3.80 (s, 3H, OCH3), 1.73 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.9 (s, Ar-C), 143.5 (s, Ar-C), 138.7 (s, Ar-C), 138.0 (s, Ar-C), 137.3 (s, Ar-C), 129.6 (d, Ar-CH), 127.7 (d, Ar-CH), 127.4 (d, Ar-CH), 127.1 (d, Ar-CH), 126.9 (d, Ar-CH), 126.7 (d, Ar-CH), 125.4 (s, Ar-C), 125.1 (d, Ar-CH), 111.6 (d, Ar-CH), 108.9 (d, Ar-CH), 106.3 (s, Ar-C), 78.2 (s, Ar-C), 55.4 (q, OCH3), 26.8 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C20H19O2S2]+ = [M + H]+: 355.0821; found 355.0818.
7-Methoxy-3,4-bis(3-methoxyphenyl)-1,1-dimethyl-1H-isochromene (3de)
GP was carried out with 2-(2-bromo-5-methoxyphenyl)propan-2-ol 1d (61 mg, 0.25 mmol), 1-methoxy-3-[(3-methoxyphenyl)ethynyl]benzene 2e (148.7 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3de (40.2 mg, 40%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3de (40.2 mg, 40%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2e) = 0.6, Rf (3de) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3087, 2974, 2838, 1608, 1487, 1425, 1297, 1216, 1043, 834, 701 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.27 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 7.06 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 6.92 (dt, J = 7.8 and 1.0 Hz, 1H, Ar-H), 6.88–6.82 (m, 3H, Ar-H), 6.82–6.72 (m, 3H, Ar-H), 6.67 (m, 2H, Ar-H), 3.80 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.8 (s, Ar-C), 158.9 (s, Ar-C), 158.6 (s, Ar-C), 145.5 (s, Ar-C), 138.8 (s, Ar-C), 138.3 (s, Ar-C), 137.3 (s, Ar-C), 129.6 (d, Ar-CH), 128.4 (d, Ar-CH), 125.1 (d, Ar-CH), 125.1 (s, Ar-C), 124.0 (d, Ar-CH), 120.9 (d, Ar-CH), 116.8 (d, Ar-CH), 115.6 (s, Ar-C), 113.9 (q, Ar-CH), 113.5 (d, Ar-CH), 112.7 (d, Ar-CH), 111.4 (d, Ar-CH), 109.0 (d, Ar-CH), 77.4 (s, Ar-C), 55.3 (q, OCH3), 55.2 (q, OCH3), 54.9 (q, OCH3), 26.9 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O4]+ = [M + H]+: 403.1904; found 403.1901.
2), Rf (2e) = 0.6, Rf (3de) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3087, 2974, 2838, 1608, 1487, 1425, 1297, 1216, 1043, 834, 701 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.27 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 7.06 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 6.92 (dt, J = 7.8 and 1.0 Hz, 1H, Ar-H), 6.88–6.82 (m, 3H, Ar-H), 6.82–6.72 (m, 3H, Ar-H), 6.67 (m, 2H, Ar-H), 3.80 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.8 (s, Ar-C), 158.9 (s, Ar-C), 158.6 (s, Ar-C), 145.5 (s, Ar-C), 138.8 (s, Ar-C), 138.3 (s, Ar-C), 137.3 (s, Ar-C), 129.6 (d, Ar-CH), 128.4 (d, Ar-CH), 125.1 (d, Ar-CH), 125.1 (s, Ar-C), 124.0 (d, Ar-CH), 120.9 (d, Ar-CH), 116.8 (d, Ar-CH), 115.6 (s, Ar-C), 113.9 (q, Ar-CH), 113.5 (d, Ar-CH), 112.7 (d, Ar-CH), 111.4 (d, Ar-CH), 109.0 (d, Ar-CH), 77.4 (s, Ar-C), 55.3 (q, OCH3), 55.2 (q, OCH3), 54.9 (q, OCH3), 26.9 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O4]+ = [M + H]+: 403.1904; found 403.1901.
6,7-Dimethoxy-1,1-dimethyl-3,4-diphenyl-1H-isochromene (3ea)
GP was carried out with 2-(2-bromo-4,5-dimethoxyphenyl)propan-2-ol 1e (68.5 mg, 0.25 mmol), (phenylethynyl)benzene 2a (111.2 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 to 80
15 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain isochromene 3ea (37.2 mg, 40%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 80
20) to obtain isochromene 3ea (37.2 mg, 40%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), Rf (2a) = 0.6, Rf (3ea) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2942, 2843, 1603, 1505, 1452, 1242, 1171, 1026, 825, 733 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42–7.17 (m, 7H, Ar-H), 7.17–7.00 (m, 3H, Ar-H), 6.73 (s, 1H, Ar-H), 6.45 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.64 (s, 3H, OCH3), 1.76 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.0 (s, Ar-C), 147.9 (s, Ar-C), 146.6 (s, Ar-C), 137.2 (s, Ar-C), 136.0 (s, Ar-C), 131.5 (d, 3 × Ar-CH), 129.0 (s, Ar-C), 128.6 (d, Ar-CH), 128.6 (d, 2 × Ar-CH), 127.5 (d, 2 × Ar-CH), 127.4 (d, Ar-CH), 126.9 (d, Ar-CH), 125.3 (s, Ar-C), 115.6 (s, Ar-C), 107.6 (d, Ar-CH), 106.2 (d, Ar-CH), 77.3 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 27.1 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H25O3]+ = [M + H]+: 373.1798; found 373.1780.
20), Rf (2a) = 0.6, Rf (3ea) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2942, 2843, 1603, 1505, 1452, 1242, 1171, 1026, 825, 733 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42–7.17 (m, 7H, Ar-H), 7.17–7.00 (m, 3H, Ar-H), 6.73 (s, 1H, Ar-H), 6.45 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.64 (s, 3H, OCH3), 1.76 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.0 (s, Ar-C), 147.9 (s, Ar-C), 146.6 (s, Ar-C), 137.2 (s, Ar-C), 136.0 (s, Ar-C), 131.5 (d, 3 × Ar-CH), 129.0 (s, Ar-C), 128.6 (d, Ar-CH), 128.6 (d, 2 × Ar-CH), 127.5 (d, 2 × Ar-CH), 127.4 (d, Ar-CH), 126.9 (d, Ar-CH), 125.3 (s, Ar-C), 115.6 (s, Ar-C), 107.6 (d, Ar-CH), 106.2 (d, Ar-CH), 77.3 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 27.1 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C25H25O3]+ = [M + H]+: 373.1798; found 373.1780.
6,7-Dimethoxy-1,1-dimethyl-3,4-bis(3-methylphenyl)-1H-isochromene (3eb)
GP was carried out with 2-(2-bromo-4,5-dimethoxyphenyl)propan-2-ol 1e (68.5 mg, 0.25 mmol), 1-methyl-3-[(3-methylphenyl)ethynyl]benzene 2b (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 90
7 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain isochromene 3eb (52 mg, 52%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 90
10) to obtain isochromene 3eb (52 mg, 52%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), Rf (2b) = 0.6, Rf (3eb) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3055, 2933, 1595, 1485, 1448, 1244, 1072, 1027, 747, 697 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.22 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 7.16–7.07 (m, 2H, Ar-H), 7.07–7.01 (m, 2H, Ar-H), 7.01–6.89 (m, 3H, Ar-H), 6.72 (s, 1H, Ar-H), 6.46 (s, 1H, Ar-H), 3.90 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 2.30 (s, 3H, CH3), 2.20 (s, 3H, CH3), 1.76 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 147.9 (s, Ar-C), 146.5 (s, Ar-C), 138.0 (s, Ar-C), 137.1 (s, Ar-C), 136.8 (s, Ar-C), 136.0 (s, Ar-C), 132.0 (d, Ar-CH), 129.1 (d, Ar-CH), 128.6 (d, Ar-CH), 128.3 (d, Ar-CH), 128.2 (d, Ar-CH), 127.6 (d, Ar-CH), 127.2 (d, Ar-CH), 125.8 (d, Ar-CH), 125.6 (s, Ar-C), 115.6 (s, Ar-C), 107.8 (d, Ar-CH), 106.2 (d, Ar-CH), 77.2 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 27.0 (q, 2 × CH3), 21.4 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O3]+ = [M + H]+: 401.2111; found 401.2101.
10), Rf (2b) = 0.6, Rf (3eb) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3055, 2933, 1595, 1485, 1448, 1244, 1072, 1027, 747, 697 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.22 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 7.16–7.07 (m, 2H, Ar-H), 7.07–7.01 (m, 2H, Ar-H), 7.01–6.89 (m, 3H, Ar-H), 6.72 (s, 1H, Ar-H), 6.46 (s, 1H, Ar-H), 3.90 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 2.30 (s, 3H, CH3), 2.20 (s, 3H, CH3), 1.76 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 147.9 (s, Ar-C), 146.5 (s, Ar-C), 138.0 (s, Ar-C), 137.1 (s, Ar-C), 136.8 (s, Ar-C), 136.0 (s, Ar-C), 132.0 (d, Ar-CH), 129.1 (d, Ar-CH), 128.6 (d, Ar-CH), 128.3 (d, Ar-CH), 128.2 (d, Ar-CH), 127.6 (d, Ar-CH), 127.2 (d, Ar-CH), 125.8 (d, Ar-CH), 125.6 (s, Ar-C), 115.6 (s, Ar-C), 107.8 (d, Ar-CH), 106.2 (d, Ar-CH), 77.2 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 27.0 (q, 2 × CH3), 21.4 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O3]+ = [M + H]+: 401.2111; found 401.2101.
6,7-Dimethoxy-1,1-dimethyl-3,4-bis(4-methylphenyl)-1H-isochromene (3ec)
GP was carried out with 2-(2-bromo-4,5-dimethoxyphenyl)propan-2-ol 1e (68.5 mg, 0.25 mmol), 1-methyl-4-[(4-methylphenyl)ethynyl]benzene 2c (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 90
7 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain isochromene 3ec (44 mg, 44%) as a light yellow solid compound, melting point = 88 °C, [TLC control (petroleum ether/ethyl acetate 90
10) to obtain isochromene 3ec (44 mg, 44%) as a light yellow solid compound, melting point = 88 °C, [TLC control (petroleum ether/ethyl acetate 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), Rf (2c) = 0.6, Rf (3ec) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3094, 2975, 2936, 2841, 1703, 1609, 1490, 1419, 1290, 1219, 1044, 835, 703 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.18–7.11 (m, 6H, Ar-H), 6.93 (dd, J = 7.8 and 1.0 Hz, 2H, Ar-H), 6.72 (s, 1H, Ar-H), 6.47 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.25 (s, 3H, CH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 147.8 (s, Ar-C), 146.5 (s, Ar-C), 137.2 (s, Ar-C), 136.3 (s, Ar-C), 134.2 (s, Ar-C), 133.3 (s, Ar-C), 131.3 (d, 2 × Ar-CH), 129.3 (d, 2 × Ar-CH), 129.1 (s, Ar-C), 128.5 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 125.7 (s, Ar-C), 114.9 (s, Ar-C), 107.6 (d, Ar-CH), 106.2 (d, Ar-CH), 77.1 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 27.0 (q, 2 × CH3), 21.3 (q, CH3), 21.2 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O3]+ = [M + H]+: 401.2111; found 401.2107.
10), Rf (2c) = 0.6, Rf (3ec) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3094, 2975, 2936, 2841, 1703, 1609, 1490, 1419, 1290, 1219, 1044, 835, 703 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.18–7.11 (m, 6H, Ar-H), 6.93 (dd, J = 7.8 and 1.0 Hz, 2H, Ar-H), 6.72 (s, 1H, Ar-H), 6.47 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 2.37 (s, 3H, CH3), 2.25 (s, 3H, CH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 147.9 (s, Ar-C), 147.8 (s, Ar-C), 146.5 (s, Ar-C), 137.2 (s, Ar-C), 136.3 (s, Ar-C), 134.2 (s, Ar-C), 133.3 (s, Ar-C), 131.3 (d, 2 × Ar-CH), 129.3 (d, 2 × Ar-CH), 129.1 (s, Ar-C), 128.5 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 125.7 (s, Ar-C), 114.9 (s, Ar-C), 107.6 (d, Ar-CH), 106.2 (d, Ar-CH), 77.1 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 27.0 (q, 2 × CH3), 21.3 (q, CH3), 21.2 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O3]+ = [M + H]+: 401.2111; found 401.2107.
6,7-Dimethoxy-3,4-bis(3-methoxyphenyl)-1,1-dimethyl-1H-isochromene (3ee)
GP was carried out with 2-(2-bromo-4,5-dimethoxyphenyl)propan-2-ol 1e (68.5 mg, 0.25 mmol), 1-methoxy-3-[(3-methoxyphenyl)ethynyl]benzene 2e (148.7 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 to 70
27 to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain isochromene 3ee (44.2 mg, 41%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 70
30) to obtain isochromene 3ee (44.2 mg, 41%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), Rf (2e) = 0.5, Rf (3ee) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2944, 2834, 1597, 1504, 1452, 1237, 1163, 1105, 1020, 825, 741 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.28 (ddd, J = 7.8, 7.8 and 2.4 Hz, 1H, Ar-H), 7.06 (ddd, J = 7.8, 7.8 and 2.4 Hz, 1H, Ar-H), 6.94–6.88 (m, 2H, Ar-H), 6.87–6.82 (m, 1H, Ar-H), 6.82–6.76 (m, 2H, Ar-H), 6.72 (s, 1H, Ar-H), 6.71–6.66 (m, 1H, Ar-H), 6.49 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.9 (s, Ar-C), 158.6 (s, Ar-C), 148.1 (s, Ar-C), 147.9 (s, Ar-C), 146.2 (s, Ar-C), 138.7 (s, Ar-C), 137.3 (s, Ar-C), 129.6 (d, Ar-CH), 129.1 (s, Ar-C), 128.4 (d, Ar-CH), 125.1 (s, Ar-C), 123.9 (d, Ar-CH), 121.0 (d, Ar-CH), 116.7 (d, Ar-CH), 115.5 (s, Ar-C), 114.0 (d, Ar-CH), 113.5 (d, Ar-CH), 112.8 (d, Ar-CH), 107.7 (d, Ar-CH), 106.3 (d, Ar-CH), 77.3 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 55.2 (q, OCH3), 54.9 (q, OCH3), 27.1 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O5]+ = [M + H]+: 433.2010; found 433.2016.
30), Rf (2e) = 0.5, Rf (3ee) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2944, 2834, 1597, 1504, 1452, 1237, 1163, 1105, 1020, 825, 741 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.28 (ddd, J = 7.8, 7.8 and 2.4 Hz, 1H, Ar-H), 7.06 (ddd, J = 7.8, 7.8 and 2.4 Hz, 1H, Ar-H), 6.94–6.88 (m, 2H, Ar-H), 6.87–6.82 (m, 1H, Ar-H), 6.82–6.76 (m, 2H, Ar-H), 6.72 (s, 1H, Ar-H), 6.71–6.66 (m, 1H, Ar-H), 6.49 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 159.9 (s, Ar-C), 158.6 (s, Ar-C), 148.1 (s, Ar-C), 147.9 (s, Ar-C), 146.2 (s, Ar-C), 138.7 (s, Ar-C), 137.3 (s, Ar-C), 129.6 (d, Ar-CH), 129.1 (s, Ar-C), 128.4 (d, Ar-CH), 125.1 (s, Ar-C), 123.9 (d, Ar-CH), 121.0 (d, Ar-CH), 116.7 (d, Ar-CH), 115.5 (s, Ar-C), 114.0 (d, Ar-CH), 113.5 (d, Ar-CH), 112.8 (d, Ar-CH), 107.7 (d, Ar-CH), 106.3 (d, Ar-CH), 77.3 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 55.2 (q, OCH3), 54.9 (q, OCH3), 27.1 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O5]+ = [M + H]+: 433.2010; found 433.2016.
6,7-Dimethoxy-3,4-bis(4-methoxyphenyl)-1,1-dimethyl-1H-isochromene (3ef)
GP was carried out with 2-(2-bromo-4,5-dimethoxyphenyl)propan-2-ol 1e (68.5 mg, 0.25 mmol), 1-methoxy-4-[(4-methoxyphenyl)ethynyl]benzene 2f (148.7 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 to 70
27 to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain isochromene 3ef (52.9 mg, 49%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 70
30) to obtain isochromene 3ef (52.9 mg, 49%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), Rf (3f) = 0.5, Rf (3ef) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3090, 1792, 1652, 1529, 1409, 1266, 1198, 834, 697 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.23–7.12 (m, 4H, Ar-H), 6.94–6.84 (m, 2H, Ar-H), 6.71 (s, 1H, Ar-H), 6.70–6.61 (m, 2H, Ar-H), 6.47 (s, 1H, Ar-H), 3.90 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 1.74 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.7 (s, Ar-C), 158.4 (s, Ar-C), 147.9 (s, Ar-C), 147.7 (s, Ar-C), 146.4 (s, Ar-C), 132.5 (d, 2 × Ar-CH), 129.9 (d, 2 × Ar-CH), 129.6 (s, Ar-C), 128.9 (s, Ar-C), 128.7 (s, Ar-C), 125.9 (s, Ar-C), 114.0 (d, 2 × Ar-CH), 113.9 (s, Ar-C), 112.8 (d, 2 × Ar-CH), 107.5 (d, Ar-CH), 106.3 (d, Ar-CH), 77.1 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 55.1 (q, OCH3), 55.0 (q, OCH3), 27.0 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O5]+ = [M + H]+: 433.2010; found 433.2018.
30), Rf (3f) = 0.5, Rf (3ef) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3090, 1792, 1652, 1529, 1409, 1266, 1198, 834, 697 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.23–7.12 (m, 4H, Ar-H), 6.94–6.84 (m, 2H, Ar-H), 6.71 (s, 1H, Ar-H), 6.70–6.61 (m, 2H, Ar-H), 6.47 (s, 1H, Ar-H), 3.90 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 1.74 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 158.7 (s, Ar-C), 158.4 (s, Ar-C), 147.9 (s, Ar-C), 147.7 (s, Ar-C), 146.4 (s, Ar-C), 132.5 (d, 2 × Ar-CH), 129.9 (d, 2 × Ar-CH), 129.6 (s, Ar-C), 128.9 (s, Ar-C), 128.7 (s, Ar-C), 125.9 (s, Ar-C), 114.0 (d, 2 × Ar-CH), 113.9 (s, Ar-C), 112.8 (d, 2 × Ar-CH), 107.5 (d, Ar-CH), 106.3 (d, Ar-CH), 77.1 (s, Ar-C), 56.2 (q, OCH3), 55.8 (q, OCH3), 55.1 (q, OCH3), 55.0 (q, OCH3), 27.0 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C27H29O5]+ = [M + H]+: 433.2010; found 433.2018.
5,6,7-Trimethoxy-1,1-dimethyl-3,4-diphenyl-1H-isochromene (3fa)
GP was carried out with 2-(2-bromo-3,4,5-trimethoxyphenyl)propan-2-ol 1f (76 mg, 0.25 mmol), (phenylethynyl)benzene 2a (111.2 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 to 80
15 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain isochromene 3fa (19 mg, 19%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 80
20) to obtain isochromene 3fa (19 mg, 19%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), Rf (2a) = 0.6, Rf (3fa) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2936, 2364, 1742, 1695, 1649, 1515, 1461, 1258, 1099, 1025, 758, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.24–7.19 (m, 4H, Ar-H), 7.18–7.12 (m, 3H, Ar-H), 7.11–7.08 (m, 3H, Ar-H), 6.57 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.09 (s, 3H, OCH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 152.6 (s, Ar-C), 150.4 (s, Ar-C), 148.1 (s, Ar-C), 142.3 (s, Ar-C), 139.6 (s, Ar-C), 136.6 (s, Ar-C), 134.6 (s, Ar-C), 130.9 (d, 2 × Ar-CH), 128.6 (d, 2 × Ar-CH), 127.4 (d, 2 × Ar-CH), 127.3 (d, Ar-CH), 127.3 (d, 2 × Ar-CH), 125.9 (d, Ar-CH), 119.2 (s, Ar-C), 115.3 (s, Ar-C), 102.1 (d, Ar-CH), 77.6 (s, Ar-C), 60.6 (q, OCH3), 60.1 (q, OCH3), 56.2 (q, OCH3), 26.6 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O4]+ = [M + H]+: 403.1904; found 403.1907.
20), Rf (2a) = 0.6, Rf (3fa) = 0.5, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2936, 2364, 1742, 1695, 1649, 1515, 1461, 1258, 1099, 1025, 758, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.24–7.19 (m, 4H, Ar-H), 7.18–7.12 (m, 3H, Ar-H), 7.11–7.08 (m, 3H, Ar-H), 6.57 (s, 1H, Ar-H), 3.91 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.09 (s, 3H, OCH3), 1.75 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 152.6 (s, Ar-C), 150.4 (s, Ar-C), 148.1 (s, Ar-C), 142.3 (s, Ar-C), 139.6 (s, Ar-C), 136.6 (s, Ar-C), 134.6 (s, Ar-C), 130.9 (d, 2 × Ar-CH), 128.6 (d, 2 × Ar-CH), 127.4 (d, 2 × Ar-CH), 127.3 (d, Ar-CH), 127.3 (d, 2 × Ar-CH), 125.9 (d, Ar-CH), 119.2 (s, Ar-C), 115.3 (s, Ar-C), 102.1 (d, Ar-CH), 77.6 (s, Ar-C), 60.6 (q, OCH3), 60.1 (q, OCH3), 56.2 (q, OCH3), 26.6 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C26H27O4]+ = [M + H]+: 403.1904; found 403.1907.
5,6,7-Trimethoxy-1,1-dimethyl-3,4-bis(3-methylphenyl)-1H-isochromene (3fb)
GP was carried out with 2-(2-bromo-3,4,5-trimethoxyphenyl)propan-2-ol 1f (76 mg, 0.25 mmol),1-methyl-3-[(3-methylphenyl)ethynyl]benzene 2b (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 90
7 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain isochromene 3fb (21.5 mg, 20%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 90
10) to obtain isochromene 3fb (21.5 mg, 20%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), Rf (2b) = 0.5, Rf (3fb) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2926, 2856, 2365, 1696, 1650, 1514, 1463, 1097, 753 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.08 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 7.05–7.01 (m, 3H, Ar-H), 7.00–6.86 (m, 4H, Ar-H), 6.54 (s, 1H, Ar-H), 3.89 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.09 (s, 3H, OCH3), 2.26 (s, 3H, CH3), 2.19 (s, 3H, CH3) 1.72 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 152.5 (s, Ar-C), 150.4 (s, Ar-C), 147.9 (s, Ar-C), 142.3 (s, Ar-C), 139.5 (s, Ar-C), 136.7 (s, Ar-C), 136.5 (s, Ar-C), 136.4 (s, Ar-C), 134.8 (s, Ar-C), 131.3 (d, Ar-CH), 129.2 (d, Ar-CH), 128.1 (d, Ar-CH), 128.0 (d, Ar-CH), 127.1 (d, 2 × Ar-CH), 126.5 (d, Ar-CH), 125.7 (d, Ar-CH), 119.5 (s, Ar-C), 115.4 (s, Ar-C), 102.0 (d, Ar-CH), 77.5 (s, Ar-C), 60.6 (s, Ar-C), 60.1 (q, OCH3), 56.1 (q, OCH3), 26.6 (q, 2 × CH3), 21.4 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C28H31O4]+ = [M + H]+: 431.2217; found 431.2214.
10), Rf (2b) = 0.5, Rf (3fb) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2926, 2856, 2365, 1696, 1650, 1514, 1463, 1097, 753 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.08 (ddd, J = 7.8, 7.8 and 1.0 Hz, 1H, Ar-H), 7.05–7.01 (m, 3H, Ar-H), 7.00–6.86 (m, 4H, Ar-H), 6.54 (s, 1H, Ar-H), 3.89 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.09 (s, 3H, OCH3), 2.26 (s, 3H, CH3), 2.19 (s, 3H, CH3) 1.72 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 152.5 (s, Ar-C), 150.4 (s, Ar-C), 147.9 (s, Ar-C), 142.3 (s, Ar-C), 139.5 (s, Ar-C), 136.7 (s, Ar-C), 136.5 (s, Ar-C), 136.4 (s, Ar-C), 134.8 (s, Ar-C), 131.3 (d, Ar-CH), 129.2 (d, Ar-CH), 128.1 (d, Ar-CH), 128.0 (d, Ar-CH), 127.1 (d, 2 × Ar-CH), 126.5 (d, Ar-CH), 125.7 (d, Ar-CH), 119.5 (s, Ar-C), 115.4 (s, Ar-C), 102.0 (d, Ar-CH), 77.5 (s, Ar-C), 60.6 (s, Ar-C), 60.1 (q, OCH3), 56.1 (q, OCH3), 26.6 (q, 2 × CH3), 21.4 (q, 2 × CH3) ppm. HR-MS (APCI+) m/z calculated for [C28H31O4]+ = [M + H]+: 431.2217; found 431.2214.
5,6,7-Trimethoxy-1,1-dimethyl-3,4-bis(4-methylphenyl)-1H-isochromene (3fc)
GP was carried out with 2-(2-bromo-3,4,5-trimethoxyphenyl)propan-2-ol 1f (76 mg, 0.25 mmol),1-methyl-4-[(4-methylphenyl)ethynyl]benzene 2c (128.8 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 85
10 to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) to obtain isochromene 3fc (21.5 mg, 20%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 85
15) to obtain isochromene 3fc (21.5 mg, 20%) as a brown jelly compound, [TLC control (petroleum ether/ethyl acetate 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15), Rf (2c) = 0.5, Rf (3fc) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2925, 2855, 2365, 1742, 1697, 1649, 1512, 1462, 1232, 1158, 1099, 828, 758, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.15–7.06 (m, 4H, Ar-H), 7.01 (d, J = 7.3 Hz, 2H, Ar-H), 6.92 (d, J = 7.8 Hz, 2H, Ar-H), 6.53 (s, 1H, Ar-H), 3.88 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.07 (s, 3H, OCH3), 2.30 (s, 3H, CH3), 2.23 (s, 3H, CH3), 1.70 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 152.4 (s, Ar-C), 150.4 (s, Ar-C), 147.9 (s, Ar-C), 142.3 (s, Ar-C), 137.0 (s, Ar-C), 136.5 (s, Ar-C), 135.2 (s, Ar-C), 134.7 (s, Ar-C), 133.8 (s, Ar-C), 130.7 (d, 2 × Ar-CH), 128.5 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 128.0 (d, 2 × Ar-CH), 119.6 (s, Ar-C), 114.9 (s, Ar-C), 102.0 (d, Ar-CH), 77.2 (s, Ar-C), 60.6 (q, OCH3), 60.2 (q, OCH3), 56.1 (q, OCH3), 26.6 (q, 2 × CH3), 21.3 (q, CH3), 21.2 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C28H31O4]+ = [M + H]+: 431.2217; found 431.2220.
15), Rf (2c) = 0.5, Rf (3fc) = 0.4, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2925, 2855, 2365, 1742, 1697, 1649, 1512, 1462, 1232, 1158, 1099, 828, 758, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.15–7.06 (m, 4H, Ar-H), 7.01 (d, J = 7.3 Hz, 2H, Ar-H), 6.92 (d, J = 7.8 Hz, 2H, Ar-H), 6.53 (s, 1H, Ar-H), 3.88 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.07 (s, 3H, OCH3), 2.30 (s, 3H, CH3), 2.23 (s, 3H, CH3), 1.70 (s, 6H, 2 × CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 152.4 (s, Ar-C), 150.4 (s, Ar-C), 147.9 (s, Ar-C), 142.3 (s, Ar-C), 137.0 (s, Ar-C), 136.5 (s, Ar-C), 135.2 (s, Ar-C), 134.7 (s, Ar-C), 133.8 (s, Ar-C), 130.7 (d, 2 × Ar-CH), 128.5 (d, 2 × Ar-CH), 128.1 (d, 2 × Ar-CH), 128.0 (d, 2 × Ar-CH), 119.6 (s, Ar-C), 114.9 (s, Ar-C), 102.0 (d, Ar-CH), 77.2 (s, Ar-C), 60.6 (q, OCH3), 60.2 (q, OCH3), 56.1 (q, OCH3), 26.6 (q, 2 × CH3), 21.3 (q, CH3), 21.2 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C28H31O4]+ = [M + H]+: 431.2217; found 431.2220.
7-Chloro-1,1-dimethyl-3,4-diphenyl-1H-isochromene (3ga)
GP was carried out with 2-(2-bromo-5-chlorophenyl)propan-2-ol 1g (61.9 mg, 0.25 mmol), (phenylethynyl)benzene 2a (111.2 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3ga (51.8 mg, 60%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3ga (51.8 mg, 60%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2a) = 0.7, Rf (3ga) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3405, 3042, 1692, 1602, 1453, 1320, 1260, 1088, 1025, 793, 744, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.36–7.28 (3H, m), 7.25–7.18 (4H, m), 7.18–7.11 (4H, m), 7.07 (1H, dd, J = 8.4, 2.1 Hz), 6.80 (1H, d, J = 8.4 Hz), 1.76 (6H, s) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.3 (s, Ar-C), 137.9 (s, Ar-C), 136.6 (s, Ar-C), 135.6 (s, Ar-C), 132.1 (s, Ar-C), 131.5 (d, 2 × Ar-CH), 128.8 (d, 2 × Ar-CH), 128.7 (d, 2 × Ar-CH), 127.9 (d, Ar-CH), 127.5 (d, 2 × Ar-CH), 127.2 (d, Ar-CH), 127.1 (d, Ar-CH), 125.9 (s, Ar-C), 125.0 (d, Ar-CH), 122.5 (d, Ar-CH), 114.9 (s, Ar-C), 77.4 (s, Ar-C), 26.9 (q, 2 × CH3) ppm.
2), Rf (2a) = 0.7, Rf (3ga) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3405, 3042, 1692, 1602, 1453, 1320, 1260, 1088, 1025, 793, 744, 700 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.36–7.28 (3H, m), 7.25–7.18 (4H, m), 7.18–7.11 (4H, m), 7.07 (1H, dd, J = 8.4, 2.1 Hz), 6.80 (1H, d, J = 8.4 Hz), 1.76 (6H, s) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.3 (s, Ar-C), 137.9 (s, Ar-C), 136.6 (s, Ar-C), 135.6 (s, Ar-C), 132.1 (s, Ar-C), 131.5 (d, 2 × Ar-CH), 128.8 (d, 2 × Ar-CH), 128.7 (d, 2 × Ar-CH), 127.9 (d, Ar-CH), 127.5 (d, 2 × Ar-CH), 127.2 (d, Ar-CH), 127.1 (d, Ar-CH), 125.9 (s, Ar-C), 125.0 (d, Ar-CH), 122.5 (d, Ar-CH), 114.9 (s, Ar-C), 77.4 (s, Ar-C), 26.9 (q, 2 × CH3) ppm.
3,4-Diphenyl-1-propyl-1H-isochromene (3ha)
GP was carried out with 1-(2-bromophenyl)butan-1-ol 1h (57 mg, 0.25 mmol), (phenylethynyl)benzene 2a (111.2 mg, 0.62 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 98
1 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain isochromene 3ha (33.4 mg, 41%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98
2) to obtain isochromene 3ha (33.4 mg, 41%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Rf (2a) = 0.8, Rf (3ha) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3085, 2973, 2839, 1607, 1486, 1424, 1295, 1211, 1040, 831, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.40–7.28 (m, 3H, Ar-H), 7.28–7.20 (m, 4H, Ar-H), 7.20–7.03 (m, 6H, Ar-H), 6.86 (dd, J = 7.3 and 1.0 Hz, 1H, Ar-H), 5.28 (dd, J = 8.3 and 4.9 Hz, 1H, Ar-H), 2.29–2.11 (m, 1H, CH2), 2.02–1.84 (m, 1H, CH2), 1.77–1.61 (m, 1H, CH2) 1.60–1.46 (m, 1H, CH2), 1.01 (t, J = 7.34 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.9 (s, Ar-C), 137.0 (s, Ar-C), 135.7 (s, Ar-C), 132.8 (s, Ar-C), 131.6 (d, 2 × Ar-CH), 128.7 (d, Ar-CH), 128.7 (s, Ar-C), 128.6 (d, 2 × Ar-CH), 127.7 (s, Ar-C), 127.1 (d, Ar-CH), 127.5 (d, Ar-CH), 127.4 (d, 2 × Ar-CH), 126.9 (d, Ar-CH), 126.5 (d, Ar-CH), 123.6 (d, Ar-CH), 123.2 (d, Ar-CH), 115.9 (s, Ar-C), 77.4 (s, Ar-C), 36.0 (t, CH2), 18.7 (t, CH2), 14.0 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H23O]+ = [M + H]+: 327.1743; found 327.1746.
2), Rf (2a) = 0.8, Rf (3ha) = 0.7, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3085, 2973, 2839, 1607, 1486, 1424, 1295, 1211, 1040, 831, 698 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.40–7.28 (m, 3H, Ar-H), 7.28–7.20 (m, 4H, Ar-H), 7.20–7.03 (m, 6H, Ar-H), 6.86 (dd, J = 7.3 and 1.0 Hz, 1H, Ar-H), 5.28 (dd, J = 8.3 and 4.9 Hz, 1H, Ar-H), 2.29–2.11 (m, 1H, CH2), 2.02–1.84 (m, 1H, CH2), 1.77–1.61 (m, 1H, CH2) 1.60–1.46 (m, 1H, CH2), 1.01 (t, J = 7.34 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 148.9 (s, Ar-C), 137.0 (s, Ar-C), 135.7 (s, Ar-C), 132.8 (s, Ar-C), 131.6 (d, 2 × Ar-CH), 128.7 (d, Ar-CH), 128.7 (s, Ar-C), 128.6 (d, 2 × Ar-CH), 127.7 (s, Ar-C), 127.1 (d, Ar-CH), 127.5 (d, Ar-CH), 127.4 (d, 2 × Ar-CH), 126.9 (d, Ar-CH), 126.5 (d, Ar-CH), 123.6 (d, Ar-CH), 123.2 (d, Ar-CH), 115.9 (s, Ar-C), 77.4 (s, Ar-C), 36.0 (t, CH2), 18.7 (t, CH2), 14.0 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H23O]+ = [M + H]+: 327.1743; found 327.1746.
3,4-Bis(4-fluorophenyl)-1-propyl-1H-isochromene (3hh)
GP was carried out with 1-(2-bromophenyl)butan-1-ol 1h (57 mg, 0.25 mmol), 1-fluoro-4-[(4-fluorophenyl)ethynyl]benzene 2h (133.7 mg, 0.625 mmol), palladium acetate (2.8 mg, 5 mol%), L-proline (5.6 mg, 20 mol%), TBAI (92.3 mg, 0.25 mmol), K2CO3 (138.6 mg, 1 mmol) and water (0.5 mL). The crude material was then refined by silica gel column chromatography (petroleum ether/ethyl acetate, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 99
0 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain isochromene 3hh (39.8 mg, 44%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 100
1) to obtain isochromene 3hh (39.8 mg, 44%) as a light yellow jelly compound, [TLC control (petroleum ether/ethyl acetate 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), Rf (2h) = 0.7, Rf (3hh) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3096, 2974, 2930, 2842, 1610, 1491, 1423, 1297, 1220, 1044, 837, 701 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.24–7.13 (m, 6H, Ar-H), 7.13–6.99 (m, 3H, Ar-H), 6.91–6.77 (m, 3H, Ar-H), 5.26 (dd, J = 8.6 and 5.1 Hz, 1H, Ar-H), 2.24–2.07 (m, 1H, CH2), 1.99–1.83 (m, 1H, CH2), 1.73–1.46 (m, 2H, CH2), 1.01 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 162.1 (d, J = 248.0 Hz, Ar-C), 161.9 (s, J = 246.5 Hz, Ar-C), 148.3 (s, Ar-C), 133.2 (d, Ar-CH), 133.1 (d, Ar-CH), 132.1 (d, J = 103.4 Hz, Ar-C), 131.9 (d, J = 103.4 Hz, Ar-C), 131.5 (d, J = 103.4 Hz, Ar-C), 130.6 (d, Ar-CH), 130.5 (d, Ar-CH), 127.6 (d, 2 × Ar-CH), 126.7 (d, 2 × Ar-CH), 123.7 (d, 2 × Ar-CH), 123.0 (d, 2 × Ar-CH), 115.7 (d, J = 21.3 Hz, Ar-CH), 114.6 (d, J = 21.3 Hz, Ar-CH), 77.5 (d, Ar-CH) 35.9 (t, CH2) 18.70 (t, CH2) 14.03 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H21F2O]+ = [M + H]+: 363.1555; found 363.1563.
0), Rf (2h) = 0.7, Rf (3hh) = 0.6, UV detection]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3096, 2974, 2930, 2842, 1610, 1491, 1423, 1297, 1220, 1044, 837, 701 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.24–7.13 (m, 6H, Ar-H), 7.13–6.99 (m, 3H, Ar-H), 6.91–6.77 (m, 3H, Ar-H), 5.26 (dd, J = 8.6 and 5.1 Hz, 1H, Ar-H), 2.24–2.07 (m, 1H, CH2), 1.99–1.83 (m, 1H, CH2), 1.73–1.46 (m, 2H, CH2), 1.01 (t, J = 7.3 Hz, 3H, CH3) ppm. 13C{1H} NMR (CDCl3, 100 MHz): δ = 162.1 (d, J = 248.0 Hz, Ar-C), 161.9 (s, J = 246.5 Hz, Ar-C), 148.3 (s, Ar-C), 133.2 (d, Ar-CH), 133.1 (d, Ar-CH), 132.1 (d, J = 103.4 Hz, Ar-C), 131.9 (d, J = 103.4 Hz, Ar-C), 131.5 (d, J = 103.4 Hz, Ar-C), 130.6 (d, Ar-CH), 130.5 (d, Ar-CH), 127.6 (d, 2 × Ar-CH), 126.7 (d, 2 × Ar-CH), 123.7 (d, 2 × Ar-CH), 123.0 (d, 2 × Ar-CH), 115.7 (d, J = 21.3 Hz, Ar-CH), 114.6 (d, J = 21.3 Hz, Ar-CH), 77.5 (d, Ar-CH) 35.9 (t, CH2) 18.70 (t, CH2) 14.03 (q, CH3) ppm. HR-MS (APCI+) m/z calculated for [C24H21F2O]+ = [M + H]+: 363.1555; found 363.1563.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Department of Science and Technology-Science and Engineering Research Board (DST-SERB) [No. EMR/2017/005312], New Delhi, for financial support. K. R. thanks MHRD, New Delhi, for the award of a research fellowship.Notes and references
- (a) L. F. Tietze and N. Rackelmann, Pure Appl. Chem., 2004, 76, 1967–1983 CAS; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed; (c) G. Satyanarayana, C. Maichle-Mössmer and M. E. Maier, Chem. Commun., 2009, 1571–1573 RSC.
- (a) J. Lee and J. S. Panek, Org. Lett., 2014, 16, 3320–3323 CrossRef CAS PubMed; (b) J. M. Gao, S. X. Yang and J. C. Qin, Chem. Rev., 2013, 113, 4755–4811 CrossRef CAS PubMed.
- M. A. Brimble, L. J. Duncalf and M. R. Nairn, Nat. Prod. Rep., 1999, 16, 267–281 RSC.
- W. Wang, T. Li, R. Milburn, J. Yates, E. Hinnant, M. J. Luzzio, S. A. Noble and G. Attardo, Bioorg. Med. Chem. Lett., 1998, 8, 1579–1584 CrossRef CAS PubMed.
- R. Mutter and M. Wills, Bioorg. Med. Chem., 2000, 8, 1841–1860 CrossRef CAS PubMed.
- M. Masubuchi, K. Kawasaki, H. Ebiike, Y. Ikeda, S. Tsujii, S. Sogabe, T. Fujii, K. Sakata, Y. Shiratori, Y. Aoki, T. Ohtsuka and N. Shimma, Bioorg. Med. Chem. Lett., 2001, 11, 1833–1837 CrossRef CAS PubMed.
- A. Shimbashi and S. Nishiyama, Tetrahedron Lett., 2007, 48, 1545–1548 CrossRef CAS.
- (a) T. Oja, P. San Martin Galindo, T. Taguchi, S. Manner, M. Vuorela, K. Ichinose, M. Metsä-Ketelä and A. Fallarero, Antimicrob. Agents Chemother., 2015, 59, 6046–6052 CrossRef CAS PubMed; (b) Y. S. Nanayakkaraa, R. M. Woodsb, Z. S. Breitbachb, S. Handac, L. M. Slaughterc and D. W. Armstrong, J. Chromatogr. A, 2013, 1305, 94–101 CrossRef PubMed.
- H. S. Kang, E. M. Jun, S. H. Park, S. J. Heo, T. S. Lee, I. D. Yoo and J. P. Kim, J. Nat. Prod., 2007, 70, 1043–1045 CrossRef CAS PubMed.
- H. He, H. Y. Yang, S. W. Luckman, D. M. Roll and G. T. Carter, J. Antibiot., 2002, 55, 1072–1075 CrossRef CAS PubMed.
- I. S. Chen, I. W. Tsai, C. M. Teng, J. J. Chen, Y. L. Chang, F. N. Ko, M. C. Lu and J. M. Pezzuto, Phytochemistry, 1997, 46, 525–529 CrossRef CAS.
- H. Hussain, J. Hussain, S. Alib, A. Al-Harrasi, M. Saleem, G. A. Miana, M. Riaz, S. Anwar, S. Hussain and L. Alia, J. Asian Nat. Prod. Res., 2012, 14, 297–300 CrossRef CAS PubMed.
- (a) J. Kawai, P. K. Chikkade, Y. Shimizu and M. Kanai, Angew. Chem., Int. Ed., 2013, 52, 7177–7180 CrossRef CAS PubMed; (b) C. Chan, Y. Chan and M. Chang, Tetrahedron, 2016, 72, 1–8 CrossRef; (c) R. Mancuso, S. Mehta, B. Gabriele, G. Salerno, W. S. Jenks and R. C. Larock, J. Org. Chem., 2010, 75, 897–901 CrossRef CAS PubMed.
- (a) T. Uto, M. Shimizu, K. Ueura, H. Tsurugi, T. Satoh and M. Miura, J. Org. Chem., 2008, 73, 298–300 CrossRef CAS PubMed; (b) K. Morimoto, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 9548–9551 CrossRef CAS PubMed; (c) M. Fukui, Y. Hoshino, T. Satoh, M. Miura and K. Tanakaa, Adv. Synth. Catal., 2014, 356, 1638–1644 CrossRef CAS; (d) S. Nakanowatari and L. Ackermann, Chem.–Eur. J., 2014, 20, 5409–5413 CrossRef CAS PubMed; (e) D. Yue, N. D. Ca and R. C. Larock, J. Org. Chem., 2006, 71, 3381–3388 CrossRef CAS PubMed; (f) R. Mancuso, S. Mehta, B. Gabriele, G. Salerno, W. S. Jenks and R. C. Larock, J. Org. Chem., 2010, 75, 897–901 CrossRef CAS PubMed; (g) A. Varela-Fernández, C. González-Rodríguez, J. A. Varela, L. Castedo and C. Saá, Org. Lett., 2009, 11, 5350–5353 CrossRef PubMed; (h) M. Dell'Acqua, B. Castano, C. Cecchini, T. Pedrazzini, V. Pirovano, E. Rossi, A. Caselli and G. Abbiati, J. Org. Chem., 2014, 79, 3494–3505 CrossRef PubMed.
- E. K. Yum, M. J. Doty, K. K. C. Sham and R. C. Larock, J. Org. Chem., 1995, 60, 3270–3271 CrossRef.
- W. J. Ang, C. Tai, L. Lo and Y. Lam, RSC Adv., 2014, 4, 4921–4929 RSC.
- (a) A. Gopi Krishna Reddy and G. Satyanarayana, J. Org. Chem., 2016, 81, 12212–12222 CrossRef CAS PubMed; (b) C. Sreenivasulu and G. Satyanarayana, Eur. J. Org. Chem., 2018, 2846–2857 CrossRef CAS; (c) K. Ramesh and G. Satyanarayana, Eur. J. Org. Chem., 2018, 4135–4146 CrossRef CAS; (d) K. Ramesh and G. Satyanarayana, Green Chem., 2018, 20, 369–374 RSC; (e) K. Ramesh, S. Basuli and G. Satyanarayana, Eur. J. Org. Chem., 2018, 2171–2177 CrossRef CAS; (f) L. Mahendar and G. Satyanarayana, J. Org. Chem., 2014, 79, 2059–2074 CrossRef CAS PubMed; (g) J. Krishna, A. G. K. Reddy and G. Satyanarayana, Synlett, 2013, 24, 967–972 CrossRef CAS.
- (a) N. Ohtani, T. Ohta, Y. Hosoda and T. Yamashita, Langmuir, 2004, 20, 409–415 CrossRef CAS PubMed; (b) A. W. Herriott and D. Picker, J. Am. Chem. Soc., 1975, 97, 2345–2349 CrossRef CAS; (c) N. Ohtani and Y. Hosoda, Bull. Chem. Soc. Jpn., 2000, 73, 2263–2268 CrossRef CAS; (d) C. M. Starks and M. Halpern, Phase-Transfer Catalysis: Fundamentals, Applications, and Industrial Perspectives, Springer Science & Business Media, 2012 Search PubMed; (e) P. William, W. George and W. Gokel, Phase Transfer Catalysis in Organic Synthesis, Springer Science & Business Media, 2012 Search PubMed; (f) A. Bharat Kumar and S. Krishna Nand, Synthesis, 2011, 7, 1125 Search PubMed; (g) A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed., 1994, 33, 2379 CrossRef.
- (a) F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 3850 CrossRef CAS; (b) T. Konno, J. Chae, T. Ishihara and H. Yamanaka, Tetrahedron, 2004, 60, 11695–11700 CrossRef CAS; (c) A. Casnati, R. Maggi, G. Maestri, N. Della Ca and E. Motti, J. Org. Chem., 2017, 82, 8296–8303 CrossRef CAS PubMed; (d) Q. Tian, A. A. Pletnev and R. C. Larock, J. Org. Chem., 2003, 68, 339–347 CrossRef CAS PubMed; (e) F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 3850 CrossRef CAS; (f) R. C. Larock, M. J. Doty and X. Han, J. Org. Chem., 1999, 64, 8770–8779 CrossRef CAS PubMed; (g) H. Miao and Z. Yang, Org. Lett., 2000, 2, 1765–1768 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08792c |
| This journal is © The Royal Society of Chemistry 2020 |