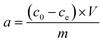Open Access Article
Open Access ArticleHighly-efficient removal of Pb(II), Cu(II) and Cd(II) from water by novel lithium, sodium and potassium titanate reusable microrods†
Monika Motlochová *ab,
Václav Slovákc,
Eva Pližingrováa,
Sven Lidinb and
Jan Šubrta
*ab,
Václav Slovákc,
Eva Pližingrováa,
Sven Lidinb and
Jan Šubrta
aInstitute of Inorganic Chemistry of the Czech Academy of Sciences, Husinec-Řež 1001, CZ-250 68 Řež, Czech Republic. E-mail: motlochova@iic.cas.cz
bCentre for Analysis and Synthesis, Lunds Universitet, Naturvetarvägen 14, Lund 222-61, Sweden
cFaculty of Science, University of Ostrava, 30. dubna 22, Ostrava, CZ-701 30, Czech Republic
First published on 22nd January 2020
Abstract
In this work, we report on the efficient removal of heavy metal ions with nanostructured lithium, sodium and potassium titanates from simulated wastewater. The titanates were obtained via a fast, easy and cost effective process based on extraction of sulfate ions from the crystals of titanyl sulfate and their replacement with hydroxyl groups of NaOH, LiOH and KOH solutions leaving the Ti–O framework intact. The as-prepared titanates were carefully examined by scanning and transmission electron microscopy. Furthermore, the effect of contact time, pH, annealing temperature, together with adsorption in real conditions including competitive adsorption and reusability were studied. It was found that the maximum adsorption capacity, as calculated from the Langmuir adsorption model, is up to 3.8 mmol Pb(II) per g, 3.6 mmol Cu(II) per g and 2.3 mmol Cd(II) per g. Based on the characterization results, a possible mechanism for heavy metal removal was proposed. This work provides a very efficient, fast and convenient approach for exploring promising materials for water treatment.
1. Introduction
All over the world, the pressure on industries for reduction of heavy metals in water is on the rise. Disposal of wastewater is one of the most pressing issues in the world, as such effluents usually contain considerable amounts of heavy metal ions such as Pb(II), Cd(II), Cr(VI), Hg(II), and Ni(II).1–8 One of the fields of interest in this area focuses on environmental clean-up,9–11 mainly the quality of public health and drinking water.There are various methods of removing metals from water, including chemical precipitation, electrochemical or adsorption methods.12 Nanoparticles could be another alternative option as a sorbent of metallic contaminants. Their larger surface area translates into larger sorption capacity, i.e. decrease in the required sorbent volume resulting in less waste in need of disposal. Furthermore, the smaller the particle size the larger the surface of an unsaturated system with more functional groups available, thus, leading to more reactivity and possibly a nanometric effect with particles smaller than 20 nm.12 Therefore, it has been proposed that they have high adsorption capacity.13
Titanium dioxide and different titanates are among the materials that are often mentioned in this context due to their outstanding properties.4,12,14–23 Recently, mesoporous TiO2 has attracted great attention due to its large surface area, which significantly increases its adsorption capacity potential. Its solubility is negligible and the zero charge point at neutral pH allows the adsorption of metal ions to TiO2 over a wide range of pH and ionic strengths.24 The titanium dioxide nanoparticles have shown to be effective as sorption agents,15 nevertheless, with the drawback of forming stable dispersion in the aqueous medium resulting in a very difficult, almost impossible separation from an aqueous medium.25 Layered titanate nanotubes prepared by the hydrothermal method possess flexible interlayer distances, high cation exchange capacity, high surface area and high density of functional hydroxyl group on the surface.4,20,21,23,26 The basic mechanism is expected to be the ion exchange3,4,6 even though other mechanisms are also possible (e.g. complexation).
Conventionally, anatase nanoparticles and their aggregates are prepared by sol–gel methods27 or by precipitation from an aqueous medium.25,27,28 Despite the clear benefits of these methods to the preparation of titanium dioxide nanoparticles with controlled properties, they also present a drawback in the form of high input requirements, therefore, rendering it expensive for large scale use and with product properties being often sub-optimal. Among other general limitations in use of nanoparticles, we can state e.g. longevity, stability, toxicity or recovery.
Hydrous titanium oxide usually prepared by mixing titanyl oxalate or TiCl4 solutions with sodium hydroxide was suggested as effective sorbent for heavy metals or radionuclides many years ago.29 Inorganic ion-exchange materials, particularly, titanium(IV) hydroxides have been widely studied for purification of liquid radioactive waste and outflows, which contain heavy nonferrous metals.30 Ion-exchange properties of these materials depend on their morphology and surface properties. The analysis of literature sources concerning the inorganic titanium ion exchanger showed that many of the synthetic ways are based on deposition of amorphous precipitates using the sol–gel method. The synthetic process includes several stages like formation of titanium(IV) hydro(oxo) complexes, the formation of multinuclear complexes, the growth of polymeric particles with the sol formation, and coagulation of initial colloid particles with gel formation. In such unstable systems it is complicated to control the system and to maintain stable properties of products. An uncontrolled synthesis, proceeding without the supervision of the solid phase formation rate, leads to the irregularity of phase and dispersive composition and has a negative influence on the characteristics of the final products. The regulation of the titanium containing ion exchanger synthesis is even more difficult when concentrated acidic titanium solutions, particularly, the solutions of titanium(IV) sulphates, are used. In addition, it is known that the titanium oxide compounds form stable, practically unfilterable colloids in the aqueous medium that are difficult to separate.31 This also complicates the use of titanium oxides as sorbents for both radionuclides and heavy metals. Thus, the development of an inexpensive titanium hydroxide-based sorbent, characterized by high sorption capacity, aqueous stability and easy separation from the aqueous environment, is still a significant problem.
Our group has previously prepared rod-shaped titania material precipitated by aqueous ammonia and tested it as an adsorbent for radionuclides.32 Following the general demand for preparation of heavy metal adsorbents, the group efforts focused on tailoring suitable modifications of this material.
In the present work, various titanate nanostructures with different precipitation agents were synthesized in a low-cost and input efficient manner and their application as adsorbents for the removal of heavy metal ions was studied.
2. Materials and methods
2.1. Synthesis of metatitanates
Based on alkaline controlled hydrolysis, three types of titanium dioxide materials with different ratio of Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti, Na
Ti, Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti and K
Ti and K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti were prepared according to the following procedure:32,33 100 mL of cooled distilled water was mixed with 50 g of ice and appropriate amounts of hydroxides (see Table 1; Penta Czech Republic), afterwards 4.80 g of titanyl sulfate dihydrate (Sigma Aldrich) was added to the cold alkaline solution. The resulting suspension was stirred for 120 min. Then, the suspension was decanted twice and filtered off. Finally, the solid product was dried at room temperature (RT).
Ti were prepared according to the following procedure:32,33 100 mL of cooled distilled water was mixed with 50 g of ice and appropriate amounts of hydroxides (see Table 1; Penta Czech Republic), afterwards 4.80 g of titanyl sulfate dihydrate (Sigma Aldrich) was added to the cold alkaline solution. The resulting suspension was stirred for 120 min. Then, the suspension was decanted twice and filtered off. Finally, the solid product was dried at room temperature (RT).
| Alkaline | Amount of alkaline (mol) | pH of resulting suspensions | Sample name |
|---|---|---|---|
| LiOH | 0.07 | 8 | TIG-5 mL-LiOH |
| 0.21 | 10 | TIG-15 mL-LiOH | |
| NaOH | 0.07 | 9 | TIG-5 mL-NaOH |
| 0.21 | 11 | TIG-15 mL-NaOH | |
| KOH | 0.07 | 9 | TIG-5 mL-KOH |
| 0.21 | 12 | TIG-15 mL-KOH |
2.2. Characterization of the products
The following methods were used for morphological, structural, and chemical characterization of the product: scanning electron microscopy (SEM/EDS), transmission electron microscopy (TEM/EDS), X-ray diffraction (XRD) and atomic absorption spectroscopy (AAS). Details including experimental conditions are described in ESI† – Characterization methods.2.3. Adsorption experiments
To investigate the removal capacity of the metatitanates, toxic metal ions such as Pb(II), Cu(II) and Cd(II) ions in water solution were used. A series of systematic experiments has been performed to examine the removal of heavy metals.For kinetic measurements, approximately 0.1 g of sample was mixed with 50 mL of 10 mmol L−1 solution of Pb(NO3)2, Cu(NO3)2 or Cd(NO3)2 and put on a shaker at RT while the time was measured. Nine samples (0.5 mL) were taken from each mixture during the experiment (10–1440 min) and the concentration of heavy metal was determined by AAS.
Equilibrium experiments for the determination of the heavy metal ions adsorption and alkali metal ions desorption isotherms were performed by adding 0.1 g of the adsorbent into 50 mL of Pb(NO3)2, Cu(NO3)2 or Cd(NO3)2 aqueous solutions with concentration range of 0.5–10 mmol L−1 and shaken for 24 hours. The solid residue was filtered off and the concentration of metal ions (heavy metal and appropriate alkali metal) in solution was determined by AAS.
The pH values of solutions after adsorption were determined between 6.3–7.1 in case of Pb(II), 7.1–7.9 in case of Cu(II) and 6.9–7.6 in case of Cd(II).
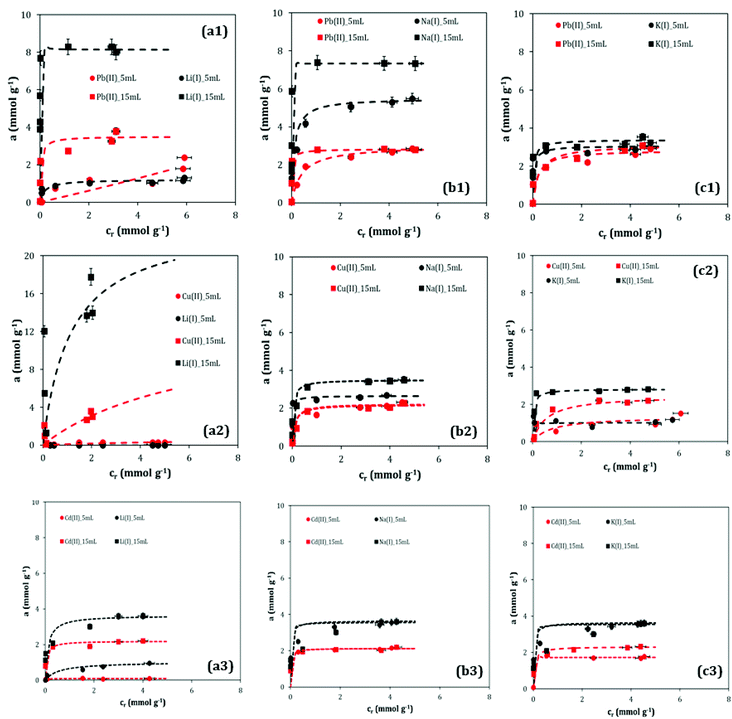
The adsorbed amount of heavy metals was calculated by using the following equation:
The equivalent desorbed amount of alkali metals ions was calculated by using:
The equilibrium adsorption isotherms were analysed by using the Langmuir model:
The influence of pH on sorption was studied by preparing solutions of metal ions at 10 mM wherein the pH levels were adjusted to fixed values with 5% HNO3 and 5% NaOH. 0.1 g of adsorbent was added into the individual solutions and equilibrated for 24 h before analysis of adsorbed amounts in aqueous phase. Afterwards, the pH was measured and, if necessary, adjusted again to the fixed value and the procedure was repeated.
In order to determine the influence of annealing temperature on the prepared samples and the resulting sorption capacity of heavy metal ions, appropriate portions of each sample were annealed at 300 °C, 450 °C, 600 °C and 1000 °C, in argon, heating rate 10 °C min−1.
A batch of experiments to determine the effect of real sample conditions was performed to compare the adsorption behaviour in distilled and tap water. Approximately 0.1 g of the sample was mixed with 50 mL of 10 mmol L−1 solutions of Pb(NO3)2, Cu(NO3)2 or Cd(NO3)2 in distilled, resp. tap water and put on a shaker at RT, equilibrated for 24 h and adsorbed amounts were determined by AAS.
Measurement of competitive adsorption was performed in solution containing Pb(NO3)2, Cu(NO3)2 and Cd(NO3)2 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with total concentration 10 mmol L−1. Approximately 0.1 g of the sample was mixed with 50 mL of this solution, put on a shaker at RT, equilibrated for 24 h and adsorbed amounts were determined by AAS.
1 ratio with total concentration 10 mmol L−1. Approximately 0.1 g of the sample was mixed with 50 mL of this solution, put on a shaker at RT, equilibrated for 24 h and adsorbed amounts were determined by AAS.
The reusability of materials was tested by washing the already used in adsorption material in solution of NaOH for 2 hours and then, the adsorption experiment was performed again. Approximately 0.1 g of the sample was mixed with 50 mL of 10 mmol L−1 solutions of Cu(NO3)2 in distilled water and put on a shaker at RT, equilibrated for 24 h and adsorbed amounts were determined by AAS.
All the adsorption experiments were repeated twice and the average values are presented.
3. Results and discussion
3.1. Characterization of the rod-shaped nanostructures
A typical morphology as obtained by SEM is shown in Fig. 1. The starting material is composed of rod-shaped crystals with a length of about 10–15 μm and diameter of 2 μm. The basic shape of the particles of titanyl sulfate remained unchanged throughout the controlled hydrolysis process which is in good agreement with the data for rods immersed into ice-cold concentrated aqueous ammonia published earlier.32,33 The XRD patterns of the metatitanates (not shown) were identical to those reported in previous literature,34 the diffraction patterns obtained in this study did not exhibit any peaks assignable to impurity phases and showed that the prepared materials are amorphous (Fig. S1†) as described in detail in the thermoanalytical study.34 | ||
| Fig. 1 SEM micrographs of adsorbents obtained by the controlled hydrolysis, TIG-5 mL-LiOH (a1), TIG-5 mL-NaOH (b1), TIG-5 mL-KOH (c1), TIG-15 mL-LiOH (a2), TIG-15 mL-NaOH (b2), TIG-15 mL-KOH (c2). | ||
The total content of alkali metals in prepared materials was determined by dissolving 0.1 g of samples in 50 mL of concentrated HNO3 under heating and analysing the obtained solution by AAS. The results are summarized in Table 2 and show that the higher the amount of hydroxide used for the synthesis, the higher amount of alkali metal in the prepared sample.
| Sample | Alkali metal | Content of alkali metal | |
|---|---|---|---|
| mg g−1 | mmol g−1 | ||
| TIG-5 mL-LiOH | Li | 58.2 | 8.36 |
| TIG-15 mL-NaOH | 127.5 | 18.35 | |
| TIG-5 mL-NaOH | Na | 134.1 | 5.61 |
| TIG-15 mL-NaOH | 179.3 | 7.54 | |
| TIG-5 mL-KOH | K | 136.6 | 3.42 |
| TIG-15 mL-KOH | 151.8 | 3.89 | |
TEM investigation (Fig. 2) of materials used for the adsorption revealed that the morphology of titanyl sulfate rods used in the syntheses is well preserved in the transformed amorphous samples including, e.g. laminar structure and other details.32 The amorphous character of the material is demonstrated by the corresponding electron diffraction pattern with only very broad diffraction rings. The TEM/EDS spectra (20 measurements were taken for each material) of materials before sorption experiments show that Na and K (Li can't be detected by EDS) are homogeneously distributed in samples and the amounts are in good agreement with the results determined by the extraction in nitric acid (Table 2).
 | ||
| Fig. 2 TEM/ED/EDS observations of TIG-5 mL-LiOH (a1 and a2), TIG-5 mL-NaOH (b1 and b2) and TIG-5 mL-KOH (c1 and c2) before adsorption tests of lead. | ||
Decreasing trend of alkali metal content (in mmol g−1) in series Li > Na > K is in agreement with increasing ionic radii. Small Li+ cation can be more easily accommodated in the cavities of titanate structure and its amount corresponds to stoichiometry Li1.25TiO2.625. On the contrary, the molar portion of heavier alkali metals is much smaller corresponding to Na0.47TiO2.235 and K0.26TiO2.13, respectively. Thus, the increasing mass and size of alkali metal ion used for preparation shifts the composition of final material closer to pure TiO2 while the use of LiOH lead to almost stoichiometric lithium titanate.
3.2. Adsorption experiments
The dynamics of adsorption process is shown in Fig. 3. Results show that the adsorption rate of materials towards all the tested heavy metals is comparable. The time necessary to reach the equilibrium is in range of 6–8 hours from the initial contact of adsorbents with the solution containing heavy metal ions which is faster than for titania nanoflowers,4 zeolite iron composites37 or zeolites38,39 but also much slower than for titania nanotubes40 where only 120 min were necessary to reach the equilibrium. Based on the kinetic experiments the contact time of 24 h was used for further equilibrium adsorption tests.
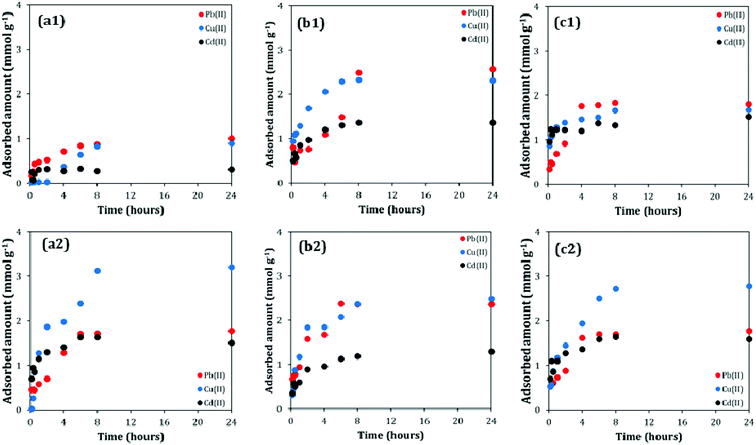
Materials precipitated with LiOH (Fig. 4(a1)) and NaOH (Fig. 4(b1)) present themselves as ion exchangers, i.e. the amount of adsorbed Pb(II) is nearly equivalent to the amount of desorbed alkali metal. This exchange is characterized by the fact that only a portion of the alkali metal is utilized while the remainder of the alkali metal is firmly incorporated into the titanate structure and the exchange capacity has, therefore, significant limitations due to this characteristic. The results achieved with the TIG-KOH (Fig. 4(c1)) show a behaviour quite different to the previously mentioned sorbents. Unlike the aforementioned partial alkali metal exchange, the TIG-KOH material desorbed and substituted almost all of the present K(I) ions by their Pb(II) equivalent during the adsorption process (compared with Table 2). In addition, compared to a standard ion exchange, the TIG-KOH material adsorbs more Pb(II) ions, therefore, a different way of binding has to be considered, e.g. complexation. Niu et al. have attributed this behaviour to the formation of anionic negatively charged surface complexes.22
Comparison of the experimental data with the theoretical Langmuir isotherm enables a determination of adsorption capacities of tested materials for Pb(II) and maximal desorbed amount for alkali metals (Fig. 5(a)). The Langmuir adsorption capacities of prepared materials have increased in series TIG-NaOH < TIG-KOH < TIG-LiOH. The maximum adsorbed amounts for Pb(II) as calculated from the Langmuir adsorption model are 3.8 mmol g−1, 2.8 mmol g−1, and 3.1 mmol g−1, respectively. It is apparent that the adsorption capacities are larger than those of titanate nanoflowers (1.47, 0.71 and 0.51 mmol g−1),4 amorphous titanate (0.71, 0.51 and 0.54 mmol g−1)20 or zeolites (0.08 and 1.6 mmol g−1).19,38
 | ||
| Fig. 5 Comparison of adsorption capacity for Pb(II) (a), Cu(II) (b) and Cd(II) (c), (expressed in equivalents ½) with desorbed and total amount of alkali metals for all prepared metatitanates. | ||
By the experiments with copper and cadmium, it was proved that all three types of materials behaved as ion-exchangers, the amount of adsorbed copper (Fig. 4(a2, b2 and c2)) and cadmium (Fig. 4(a3, b3 and c3)) corresponded or was little higher than the amount of desorbed alkali metal. When comparing the influence of concentrations of alkali hydroxide during the synthesis, resp. the amount of alkali metals incorporated in the material, with the adsorbed amount of heavy metal, it seems that the amount of available alkali metal does not have a significant influence on the amount of adsorbed copper or cadmium with the exception of the TIG-5 mL-LiOH which is an unsuitable sorbent, the adsorbed amount is negligible.
The maximum adsorbed amounts for Cu(II) as calculated have increased in series TIG-NaOH < TIG-KOH < TIG-LiOH, in good agreement with data observed in adsorption experiments with lead. The maximum adsorbed amounts are 3.6 mmol g−1, 2.3 mmol g−1 and 2.7 mmol g−1 for materials precipitated with LiOH, NaOH and KOH, respectively. It is apparent that the adsorption capacities are larger than those of titanate nanotubes (2.0 mmol g−1 (ref. 23)) or hydroxotitanate (0.9 mmol g−1 (ref. 36)). These results indicate that prepared adsorbents can be considered as promising adsorbents for removal of copper from water.
The maximum adsorbed amounts for Cd(II) as calculated from the Langmuir adsorption model are 2.2 mmol g−1, 2.2 mmol g−1 and 2.3 mmol g−1 (NaOH < TIG-KOH < TIG-LiOH). It is apparent that the adsorption capacities are larger than those of titanate nanotubes (2.1 mmol g−1 (ref. 40)). These results indicate that prepared adsorbents can be considered as promising adsorbents for removal of cadmium from water.
The adsorption capacity for heavy metals was compared to desorbed amount of alkali metal and the total amount of alkali metal available (Fig. 5). Based on the results it can be concluded that the mechanism of sorption is ion-exchange. These promising results are an opening to possible applications of these sorbents compounds into remove heavy metals from contaminated water.
In real-life applications, the reusability of prepared samples is crucial. Therefore, the wash-out of copper adsorbed in the materials was repeatedly tested (by cycling). It was concluded that the materials are recyclable, the copper adsorbed in the sample can be easily removed from the material by washing out in sodium hydroxide with a slight decrease (up to 5%) in uptake in the consequent cycles.
By mapping of samples by SEM/EDS analysis after adsorption tests it was proved that the heavy metals are homogenously distributed in the sample in all rods as can be seen in Fig. 6 for lead sorption experiment for TIG-5 mL-LiOH, TIG-5 mL-NaOH and TIG-5 mL-KOH (other materials – see ESI, Fig. S2 and S3†).
 | ||
| Fig. 6 SEM/EDS mapping of oxygen, titanium and lead in TIG-5 mL-LiOH (a), TIG-5 mL-NaOH (b) and TIG-5 mL-KOH (c) after adsorption tests of lead. | ||
The influence of the equilibrium pH on the uptake of heavy metals was studied in range between 2–8 as described in detail in ESI.† As shown in Fig. S4,† the sorption capacity increased with increasing pH.
The thermal decomposition of prepared materials has been already described.41 It was discovered that the materials are amorphous up to crystallization into mixtures of anatase and titanates. The influence on annealing temperature on sorption of radionuclides on titania materials showed that the higher the annealing temperature the lower the adsorbed amount.42
In this study, the observation revealed very similar results as can be seen in ESI.† An increase in the annealing temperature led to a decrease in the adsorbed amount of heavy metal ions (Fig. S5†), therefore, the materials are not suitable for adsorption experiments when crystalline.
Real samples have an inherent presence of several inorganic ions which can interfere with heavy metal ion adsorption. For this reason, the influence of common cations and anions present in tap water (for composition see ESI, Table S1†) on heavy metal uptake was investigated and summarized in Table 3. The decrease of adsorption capacity of all three samples towards Pb(II), Cu(II) and Cd(II) ions was observed.
| Sample | Adsorbed amount of Pb(II) in water | Adsorbed amount of Cu(II) in water | Adsorbed amount of Cd(II) in water | |||
|---|---|---|---|---|---|---|
| Distilled | Tap | Distilled | Tap | Distilled | Tap | |
| TIG-5 mL-LiOH | 2.4 | 2.2 | 0.3 | 0.2 | 0.1 | 0.0 |
| TIG-15 mL-NaOH | 3.8 | 3.7 | 3.6 | 3.1 | 2.1 | 1.8 |
| TIG-5 mL-NaOH | 2.9 | 2.8 | 2.3 | 1.8 | 2.1 | 1.9 |
| TIG-15 mL-NaOH | 2.8 | 2.7 | 2.3 | 1.9 | 2.2 | 2.1 |
| TIG-5 mL-KOH | 2.9 | 2.9 | 1.5 | 1.3 | 1.8 | 1.4 |
| TIG-15 mL-KOH | 3.1 | 3.0 | 2.2 | 2.0 | 2.3 | 2.1 |
Based on the presented results, it can be concluded that the effect of interfering ions on the heavy metal uptake is not very significant, therefore, the prepared samples are meant to be suitable sorbents for sorption of Pb(II), Cu(II) and Cd(II) from drinking water.
The measurement of competitive adsorption showed that the lead ions are adsorbed preferably in all tested sorbents. The amount of adsorbed amounts decreases in series Pb(II) > Cd(II) > Cu(II) as can be seen in Fig. 7. The competitive behaviour can be explained by decreasing hydrated ion radii43 which decreases in the same series 1.20 > 0.96 > 0.62 Å. To confirm these results mapping of samples was conducted after the aforementioned procedures. Results confirmed that the adsorbed amount of heavy metals towards prepared rods decreases in series Pb(II) > Cd(II) > Cu(II), while being homogenously distributed among rods (Fig. 8).
 | ||
Fig. 7 Comparison of competitive behaviour of TIG-LiOH, TIG-NaOH and TIG-KOH towards solution containing Pb(II), Cu(II) and Cd(II) in ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
4. Conclusions
Amorphous lithium, sodium and potassium metatitanates were successfully synthetized, characterized and used as adsorbents for the removal of heavy metals from natural water.The adsorption rate of each heavy metal ion was quite low, the equilibrium was reached in about 8 hours. The maximum amounts of Pb(II), Cu(II) and Cd(II) were detected in pH range of 5–8. The study on equilibrium showed that the Langmuir model was the most appropriate and the adsorption capacities were in range of 2.4–3.8 mmol Pb(II) per g, 0.3–3.6 mmol Cu(II) per g and 0.1–2.3 mmol Cd(II) per g. It was discussed that the interfering ions from drinking water do not have any significant impact on the adsorbed amount and the competitive behaviour was also described. The influence of annealing temperature of prepared materials on Pb(II), Cu(II) and Cd(II) showed that the higher the annealing temperature the lower the adsorbed amount.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank for financial support provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2015073 and to the Technological Agency of Czech Republic under Project No. TJ01000154. The authors gratefully acknowledge Mariana Klementova for the TEM measurements.References
- J. S. Hu, L. S. Zhong, W. G. Song and L. J. Wan, Synthesis of hierarchically structured metal oxides and their application in heavy metal ion removal, Adv. Mater., 2008, 20, 2977–2982 CrossRef CAS.
- A. Dabrowski, Z. Hubicki, P. Podkoscielny and E. Robens, Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method, Chemosphere, 2004, 56, 91–106 CrossRef CAS PubMed.
- M. N. Akieh, M. Lahtinen, A. Vaisanen and M. Sillanpaa, Preparation and characterization of sodium iron titanate ion exchanger and its application in heavy metal removal from waste waters, J. Hazard. Mater., 2008, 152, 640–647 CrossRef CAS PubMed.
- J. Huang, Y. Cao, Z. Liu, Z. Deng, F. Tang and W. Wang, Efficient removal of heavy metal ions from water system by titanate nanoflowers, Chem. Eng. J., 2012, 180, 75–80 CrossRef CAS.
- H. Leinonen, J. Lehto and A. Makela, Purification of nickel and zinc from waste-waters of metal-plating plants by ion-exchange, React. Polym., 1994, 23, 221–228 CrossRef CAS.
- L. di Bitonto, A. Volpe, M. Pagano, G. Bagnuolo, G. Mascolo, V. La Parola, P. Di Leo and C. Pastore, Amorphous boron-doped sodium titanates hydrates: efficient and reusable adsorbents for the removal of Pb2+ from water, J. Hazard. Mater., 2017, 324, 168–177 CrossRef CAS.
- W. Liu, T. Wang, A. G. L. Borthwick, Y. Q. Wang, X. C. Yin, X. Z. Li and J. R. Ni, Adsorption of Pb2+, Cd2+, Cu2+ and Cr3+ onto titanate nanotubes: competition and effect of inorganic ions, Sci. Total Environ., 2013, 456, 171–180 CrossRef PubMed.
- T. Wang, W. Liu, L. Xiong, N. Xu and J. R. Ni, Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes, Chem. Eng. J., 2013, 215, 366–374 CrossRef.
- W. T. Liu, Nanoparticles and their biological and environmental applications, J. Biosci. Bioeng., 2006, 102, 1–7 CrossRef CAS PubMed.
- H. J. Shipley, S. Yean, A. T. Kan and M. B. Tomson, Adsorption of arsenic to magnetite nanoparticles: effect of particle concentration, pH, ionic strength, and temperature, Environ. Toxicol. Chem., 2009, 28, 509–515 CrossRef CAS PubMed.
- P. G. Tratnyek and R. L. Johnson, Nanotechnologies for environmental cleanup, Nano Today, 2006, 1, 44–48 CrossRef.
- K. E. Engates and H. J. Shipley, Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: effect of particle size, solid concentration, and exhaustion, Environ. Sci. Pollut. Res., 2011, 18, 386–395 CrossRef CAS PubMed.
- P. Liang, T. Q. Shi and J. Li, Nanometer-size titanium dioxide separation/preconcentration and FAAS determination of trace Zn and Cd in water sample, Int. J. Environ. Anal. Chem., 2004, 84, 315–321 CrossRef CAS.
- T. Ohnuki and N. Kozai, Adsorption behavior of radioactive cesium by non-mica minerals, J. Nucl. Sci. Technol., 2013, 50, 369–375 CrossRef CAS.
- R. Chakravarty and A. Dash, Role of Nanoporous Materials in Radiochemical Separations for Biomedical Applications, J. Nanosci. Nanotechnol., 2013, 13, 2431–2450 CrossRef CAS PubMed.
- B. K. Singh, R. Tomar, S. Kumar, A. S. Kar, B. S. Tomar, S. Ramanathan and V. K. Manchanda, Role of the humic acid for sorption of radionuclides by synthesized titania, Radiochim. Acta, 2014, 102, 255–261 CAS.
- W. F. Yang, L. D. Guo, C. Y. Chuang, P. H. Santschi, D. Schumann and M. Ayranov, Influence of organic matter on the adsorption of Pb-210, Po-210, Be-7 and their fractionation on nanoparticles in seawater, Earth Planet. Sci. Lett., 2015, 423, 193–201 CrossRef CAS.
- C. Santhosh, V. Velmurugan, G. Jacob, S. K. Jeong, A. N. Grace and A. Bhatnagar, Role of nanomaterials in water treatment applications: a review, Chem. Eng. J., 2016, 306, 1116–1137 CrossRef CAS.
- D. J. Yang, Z. F. Zheng, H. W. Liu, H. Y. Zhu, X. B. Ke, Y. Xu, D. Wu and Y. Sun, Layered Titanate Nanofibers as Efficient Adsorbents for Removal of Toxic Radioactive and Heavy Metal Ions from Water, J. Phys. Chem. C, 2008, 112, 16275–16280 CrossRef CAS.
- J. Huang, Y. Cao, Z. Deng and H. Tong, Formation of titanate nanostructures under different NaOH concentration and their application in wastewater treatment, J. Solid State Chem., 2011, 184, 712–719 CrossRef CAS.
- H. Kochkar, A. Turki, L. Bergaoui, G. Berhault and A. Ghorbel, Study of Pd(II) adsorption over titanate nanotubes of different diameters, J. Colloid Interface Sci., 2009, 331, 27–31 CrossRef CAS PubMed.
- H. Y. Niu, J. M. Wang, Y. L. Shi, Y. Q. Cai and F. S. Wei, Adsorption behavior of arsenic onto protonated titanate nanotubes prepared via hydrothermal method, Microporous Mesoporous Mater., 2009, 122, 28–35 CrossRef CAS.
- S. S. Liu, C. K. Lee, H. C. Chen, C. C. Wang and L. C. Juang, Application of titanate nanotubes for Cu(II) ions adsorptive removal from aqueous solution, Chem. Eng. J., 2009, 147, 188–193 CrossRef CAS.
- S. Wang, L. Q. Tan, J. L. Jiang, J. Chen and L. D. Feng, Preparation and characterization of nanosized TiO2 powder as an inorganic adsorbent for aqueous radionuclide Co(II) ions, J. Radioanal. Nucl. Chem., 2013, 295, 1305–1312 CrossRef CAS.
- M. Kolar, H. Mest'ankova, J. Jirkovsky, M. Heyrovsky and J. Subrt, Some aspects of physico-chemical properties of TiO2 nanocolloids with respect to their age, size, and structure, Langmuir, 2006, 22, 598–604 CrossRef CAS PubMed.
- R. Z. Ma, T. Sasaki and Y. Bando, Alkali metal cation intercalation properties of titanate nanotubes, Chem. Commun., 2005, 948–950 RSC.
- D. H. Chen and R. A. Caruso, Recent Progress in the Synthesis of Spherical Titania Nanostructures and Their Applications, Adv. Funct. Mater., 2013, 23, 1356–1374 CrossRef CAS.
- V. Privman, D. V. Goia, J. Park and E. Matijevic, Mechanism of formation of monodispersed colloids by aggregation of nanosize precursors, J. Colloid Interface Sci., 1999, 213, 36–45 CrossRef CAS PubMed.
- V. Vesely and V. Pekarek, Synthetic Inorganic Ion-Exchangers. 1. Hydrous Oxides and Acidic Salts of Multivalent Metals, Talanta, 1972, 19, 219–262 CrossRef CAS PubMed.
- L. G. Gerasimova, M. V. Maslova and A. I. Nikolaev, Application of Colloid Titanium-Containing Precursors in the Inorganic Ion-Exchange Technology, Glass Phys. Chem., 2011, 37, 401–405 CrossRef CAS.
- P. Fernandez-Ibanez, J. Blanco, S. Malato and F. J. de las Nieves, Application of the colloidal stability of TiO2 particles for recovery and reuse in solar photocatalysis, Water Res., 2003, 37, 3180–3188 CrossRef CAS.
- M. Klementová, M. Motlochová, J. Boháček, J. Kupčík, L. Palatinus, E. Pližingrová, L. Szatmáry and J. Šubrt, Metatitanic acid pseudomorphs after titanyl sulfates: nanostructured sorbents and precursors for crystalline titania with desired particle size and shape, Cryst. Growth Des., 2017, 17, 6762–6769 CrossRef.
- M. Palkovska, V. Slovak, J. Subrt, J. Bohacek, Z. Barbierikova, V. Brezova and R. Fajgar, Investigation of the thermal decomposition of a new titanium dioxide material, J. Therm. Anal. Calorim., 2016, 125, 1071–1078 CrossRef CAS.
- M. Motlochová, V. Slovák, E. Pližingrová, M. Klementová, P. Bezdička and J. Šubrt, Thermal Decomposition Study of Nanostructured Amorphous Lithium, Sodium and Potassium Metatitanates, Thermochim. Acta, 2018, 670, 148–154 CrossRef.
- W. Liu, H. Chen, A. G. L. Borthwick, Y. F. Han and J. R. Ni, Mutual promotion mechanism for adsorption of coexisting Cr(III) and Cr(VI) onto titanate nanotubes, Chem. Eng. J., 2013, 232, 228–236 CrossRef CAS.
- A. Volpe, M. Pagano, C. Pastore, C. Cuocci and A. Milella, Sorption properties of an amorphous hydroxo titanate towards Pb2+, Ni2+, and Cu2+ ions in aqueous solution, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2016, 51, 1121–1130 CrossRef CAS PubMed.
- S. A. Kim, S. Kamala-Kannan, K. J. Lee, Y. J. Park, P. J. Shea, W. H. Lee, H. M. Kim and B. T. Oh, Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zero-valent iron composite, Chem. Eng. J., 2013, 217, 54–60 CrossRef CAS.
- S. Wang and E. Ariyanto, Competitive adsorption of malachite green and Pb ions on natural zeolite, J. Colloid Interface Sci., 2007, 314, 25–31 CrossRef CAS PubMed.
- X. Y. Wang, J. H. Cai, Y. J. Zhang, L. H. Li, L. Jiang and C. R. Wang, Heavy metal sorption properties of magnesium titanate mesoporous nanorods, J. Mater. Chem. A, 2015, 3, 11796–11800 RSC.
- L. Xiong, C. Chen, Q. Chen and J. R. Ni, Adsorption of Pb(II) and Cd(II) from aqueous solutions using titanate nanotubes prepared via hydrothermal method, J. Hazard. Mater., 2011, 189, 741–748 CrossRef CAS.
- M. Motlochova, V. Slovak, E. Plizingrova, M. Klementova, P. Bezdicka and J. Subrt, Thermal decomposition study of nanostructured amorphous lithium, sodium and potassium metatitanates, Thermochim. Acta, 2018, 670, 148–154 CrossRef CAS.
- M. Motlochova, V. Slovak, E. Plizingrova, L. Szatmary, P. Bezdicka and J. Subrt, The influence of annealing temperature on properties of TiO2 based materials as adsorbents of radionuclides, Thermochim. Acta, 2019, 673, 34–39 CrossRef CAS.
- I. Persson, Hydrated metal ions in aqueous solution: how regular are their structures?, Pure Appl. Chem., 2010, 82, 1901–1917 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08737k |
| This journal is © The Royal Society of Chemistry 2020 |