Open Access Article
Open Access ArticleHumic acid as an efficient and reusable catalyst for one pot three-component green synthesis of 5-substituted 1H-tetrazoles in water†
Hongshe Wang *a,
Yichun Wangb,
Yinfeng Hana,
Weixing Zhaoa and
Xiaomei Wanga
*a,
Yichun Wangb,
Yinfeng Hana,
Weixing Zhaoa and
Xiaomei Wanga
aCollege of Chemistry and Chemical Engineering, Baoji University of Arts and Sciences, Baoji 721013, China. E-mail: baojizwx@126.com
bCollege of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang 712046, China
First published on 3rd January 2020
Abstract
Humic acid is a non toxic, inexpensive, easily available high-molecular weight polymer. A simple and facile one pot three-component synthesis of 5-substituted 1H-tetrazoles from aldehydes, hydroxyamine hydrochloride and sodium azide using humic acid as an efficient catalyst in water is described. The method reported has several advantages such as high to excellent yields, easy work-up, mild reaction conditions, use of water as a green solvent, and a commercially available, nontoxic and reusable catalyst.
Introduction
Tetrazoles are synthetic nitrogen-rich compounds with a wide range of applications that are receiving considerable attention among the stable heterocycles.1 They play important roles in energetic materials,2 pharmaceuticals3 and coordination chemistry.4 Because of their potent usefulness, various synthetic methods have been developed for the construction of tetrazole frameworks, such as the reactions of aryl halides with potassium ferrocyanide,5 primary amides with triazidochlorosilane,6 and oximes with sodium azide7 or diphenyl phosphorazidate.8 Traditional synthesis of 5-substituted 1H-tetrazole has been reported to proceed via [3 + 2] cycloaddition of azide ions with expensive and toxic nitrile.9 In view of the availability, lower toxicity and ease of handling of aldehydes as compared to nitriles, the application of aldehydes for the synthesis of tetrazole derivatives is a highly attractive and advantageous strategy. From the literature survey, we came to know that some methods were reported for the synthesis of 5-substituted 1H-tetrazole from aldehyde and the catalysts used were (NH4)4Ce(SO4)4·2H2O,10 TiCl3,11 Cu(OAc)2,12 PVA@Cu Schiff base complex,13 I2,14 Cu-MCM-41,15 [bmim]N3/Cu(OAc)2 (ref. 16) and P2O5.17 Although each of the above methods has its own merit, most of these methods are associated with certain disadvantages including the use of commercially unavailable catalysts, toxic organic solvents and high reaction temperatures. To avoid such drawbacks, development of more simple and efficient protocols is still in demand.Nowadays, one pot multicomponent reactions (MCRs) have received much attention for the synthesis of diverse compounds and contribute to sustainability by simplifying the synthetic route.18 MCRs combine three or more starting reagents at a time in the same pot to create the target molecule since there is no need of separating intermediate which help to reduce the energy consumption, solvent waste and reaction time and thus have the advantages of synthetic efficiency, simplicity, atom economy. Compared with the conventional multistep synthetic routes, MCRs are more powerful procedures in the diversity-oriented convergent synthesis of organic heterocycles from simple and easily available substrates.
Humic acid is a high-molecular weight polymer that is primarily derived from biodegradation of dead organic matter and appears mostly in peat, soil, coal, upland streams, dystrophic lakes and well water. Because humic acid is non toxic, inexpensive and easily available, the study of its catalytic activity may be very important. Humic acid is an acidic catalyst owing to the presence of abundant carboxyl and phenolic hydroxyl groups in the structure of it. There are only very few reports in which catalytic activity of humic acid has been described.19,20 In view of the efforts toward development of the potential of humic acid as a catalyst, herein, we report an efficient one pot three-component synthesis of 5-substituted 1H-tetrazoles from less expensive and easily available starting material aldehydes along with hydroxylamine hydrochloride and sodium azide (Scheme 1).
Results and discussion
In order to determine the best reaction conditions, the effect of solvent (Table 1), catalyst loading (Table 2) and temperature (Table 3) were examined in the reaction of benzaldehyde, hydroxylamine hydrochloride and sodium azide. This reaction was very dependent on the nature of the solvent utilized. It was observed that the use of high boiling point polar solvents such as DMF and DMSO resulted in moderate conversion to the cycloaddition product (Table 1, entries 2 and 3). But no product was formed with weak polar solvent toluene (Table 1, entry 5), it may be due to the poor solubility of hydroxylamine hydrochloride and sodium azide in toluene. An additional disadvantage of DMF and DMSO is their solubility in both organic solvents and water, thus removing DMF and DMSO from tetrazole is difficult. Water was the best solvent for this protocol in terms of yield (Table 1, entry 6). However, no reaction was observed under solvent-free conditions (Table 1, entry 7). The reaction did not proceed with no catalyst (Table 2, entry 1). Decreasing the catalyst concentration resulted in lower yields under the same conditions (Table 2, entries 2 and 3). The best result was achieved by carrying out the reaction with 0.1 g of humic acid (Table 2, entry 4). Further increase in catalyst concentration did not show any significant effect on yields (Table 2, entries 5 and 6). At room temperature a useful conversion was not observed (Table 3, entry 1). However, the productivity increased on raising the temperature. The increase of reaction temperature will accelerate the thermal movement of molecules and increase the probability of intermolecular collision, thus, the rate of the reaction is improved. Different research groups established that using high temperature is necessary for cycloaddition reactions,10–12 so it seems reasonable to think that increasing the reaction temperature would be a good choice for improvement in the yield. The best result for this reaction was obtained at 100 °C (Table 3, entry 5).| Entry | Solvent | Yieldc (%) |
|---|---|---|
| a Reaction condition: benzaldehyde (1 mmol), hydroxylamine hydrochloride (1.2 mmol), sodium azide (1.5 mmol), solvent (2 mL), humic acid (0.1 g) at 100 °C for 4 h.b Reaction was performed under reflux condition.c Isolated yield. | ||
| 1b | CH3CN | 43 |
| 2 | DMF | 81 |
| 3 | DMSO | 64 |
| 4b | EtOH | 31 |
| 5 | Toluene | 0 |
| 6 | H2O | 92 |
| 7 | Solvent-free | 0 |
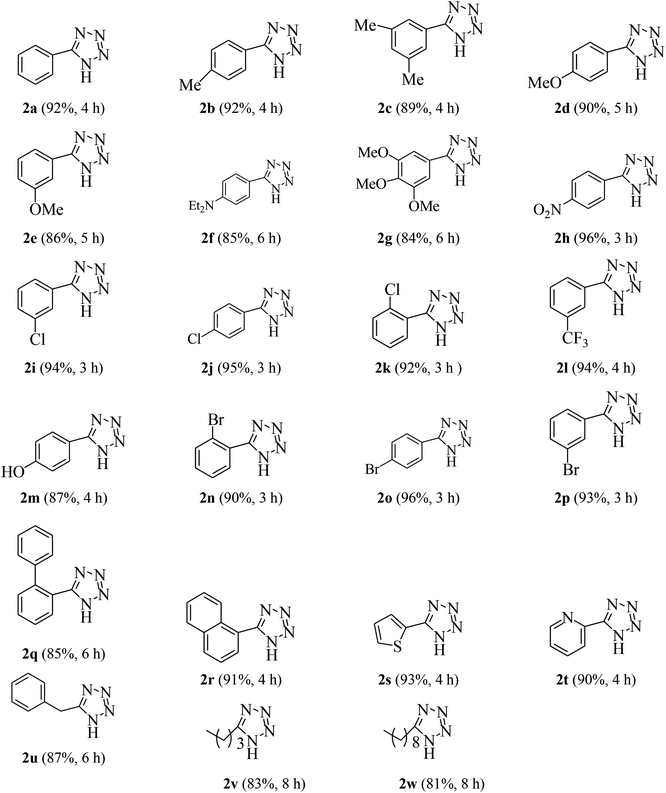
After optimization of the reaction conditions, to expand the efficiency and generality of this methodology, aldehydes were treated with hydroxylamine hydrochloride and sodium azide in the presence of 0.1 g of humic acid in water at 100 °C and a large variety of tetrazole derivatives were formed. The results are summarized in Table 4. This strategy applies to all types of aldehydes (aromatic, heterocyclic and aliphatic). Aromatic aldehydes containing both electron-donating and electron-withdrawing groups reacted successfully to give the corresponding tetrazoles in good to excellent yields irrespective of the electronic nature and substituent position on the aromatic ring (Table 4, 2a–2r). Heterocyclic aldehydes also reacted well to give corresponding products in high yields (Table 4, 2s and 2t). In addition, good results were also obtained with aliphatic aldehydes (Table 4, 2u–2w). From the above results, it is concluded that humic acid is an excellent catalyst for the synthesis of 5-substituted 1H-tetrazoles which tolerate a wide range of substituents.
One of the most significant features of a catalyst is its ability for reusability. Thus, we studied the reusability of the catalyst humic acid for the synthesis of 5-phenyl 1H-tetrazole (2a) under the optimized reaction conditions. The catalyst was separated from the reaction mixture by convenient filtration. According to the data presented in Table 5, the catalyst was reusable for five consecutive runs without any significant reactivity loss.
A comparison among humic acid and other catalysts reported in the literature for the preparation of 5-phenyl 1H-tetrazole reveals the advantages of humic acid over most of the other catalysts in terms of higher yield and shorter reaction time (Table 6).
| Entry | Conditions | Yield (%) | Ref. |
|---|---|---|---|
| a Commercially available catalyst.b Commercially unavailable catalyst. | |||
| 1a | (NH4)4Ce(SO4)4·2H2O (20 mol%), DMF, reflux, 5 h | 72 | 10 |
| 2a | TiCl3 (20 mol%), DMF, reflux, 4 h | 86 | 11 |
| 3a | Cu(OAc)2 (20 mol%), choline chloride–urea, 100 °C, 12 h | 90 | 12 |
| 4b | PVA@Cu Schiff base complex (0.43 mol%), H2O, r.t., 7 min | 98 | 13 |
| 5b | Cu-MCM-41 (30 mg mmol−1), DMF, 140 °C, 12 h | 90 | 15 |
| 6a | P2O5 (200 mol%), DMF, reflux, 9 h | 90 | 17 |
| 7a | Humic acid (0.1 g mmol−1), H2O, 100 °C, 4 h | 92 | This work |
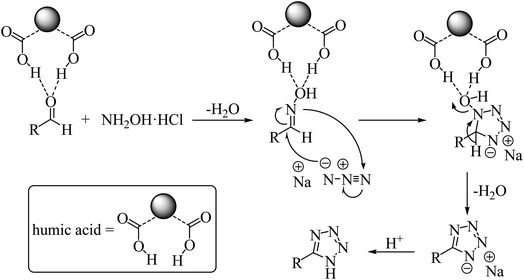
A plausible mechanism for the synthesis of 5-substituted 1H-tetrazole is represented in Scheme 2. After the activation of the carbonyl group of aldehyde catalyzed by protic acids on the humic acid surface and the nucleophilic attack of the nitrogen atom of hydroxylamine to the active carbonyl group, aldehyde oxime is obtained. The coordination of the protic acids with oxygen atom of aldehyde oxime assists to activate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond to form intermediate for nucleophilic addition of NaN3. The reaction proceeds via [3 + 2] cycloaddition pattern. Finally, the elimination of water and acidic hydrolysis give the corresponding 5-substituted 1H-tetrazole. The active site on the humic acid represented as –COOH is considered in the mechanism.
N bond to form intermediate for nucleophilic addition of NaN3. The reaction proceeds via [3 + 2] cycloaddition pattern. Finally, the elimination of water and acidic hydrolysis give the corresponding 5-substituted 1H-tetrazole. The active site on the humic acid represented as –COOH is considered in the mechanism.
Micellar catalysis provides a means for synthesizing novel and conventional materials in aqueous media, resulting in improved reaction rates and eliminating the need for organic solvents. Humic acid generally forms micelle-like structure in aqueous environment, which is an effective way to realize [3 + 2] cycloaddition of aldehydes, hydroxyamine hydrochloride and sodium azide, and the addition of inorganic electrolyte (hydroxyamine hydrochloride and sodium azide) into the aqueous phase can markedly promote the catalysis effect.21 The critical micelle concentration (CMC) value of the humic acid used in this study was 5.11 ± 0.10 g L−1.
Conclusions
In conclusion, we have disclosed that humic acid works as a non toxic, inexpensive and efficient catalyst for one pot three-component synthesis of 5-substituted 1H-tetrazoles from aldehydes, hydroxylamine hydrochloride and sodium azide in water. Advantages of this methodology are use of commercially available and reusable catalyst and water as a green solvent, simple experimental and work-up procedure, high yields and environment friendly. This environmentally friendly method to synthesizing 5-substituted 1H-tetrazoles holds promising potential for future applications in both academic and industrial research.Experimental
Materials and instrumentation
Humic acid and other chemicals were all purchased from Aladdin Reagent Company and used as received unless mentioned otherwise. Melting points were determined on an XT4A electrothermal apparatus equipped with a microscope and are uncorrected. NMR spectra were recorded on a Bruker Avance 400 spectrometer in DMSO-d6. Elemental analyses were performed on a PerkinElmer 240-C instrument.General procedure for the synthesis of 5-substituted 1H-tetrazoles
Aldehyde (1 mmol), hydroxylamine hydrochloride (1.2 mmol), sodium azide (1.5 mmol) and humic acid (0.1 g) were added to water (2 mL) and the mixture was heated at 100 °C for requisite time. The progress of reaction was monitored by TLC. After completion of reaction, the mixture was filtered to separate out the catalyst. The filtrate was treated with HCl (4 N, 10 mL), extracted with ethylacetate, and washed by water a few times. The extract was dried over anhydrous Na2SO4, purified by columnchromatography on silica-gel (60–120 mesh) using petroleum ether/ethyl acetate (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) as eluent to give the corresponding pure product. All the products were characterized by 1H NMR and 13C NMR spectroscopy.
25) as eluent to give the corresponding pure product. All the products were characterized by 1H NMR and 13C NMR spectroscopy.
A gram scale synthesis of 5-phenyl 1H-tetrazole (2a)
To a 200 mL round-bottomed flask equipped with a magnetic stirrer was added benzaldehyde (10.6 g, 0.1 mol), hydroxylamine hydrochloride (4.2 g, 0.1 mol), NaN3 (0.5 g, 0.11 mol), humic acid (10.0 g) and water (100 mL). Then the reaction mixture was heated to 100 °C with vigorous stirring. After completion of reaction (4 h), as detected by TLC, the mixture was cooled to room temperature and filtered to separate out the catalyst. The filtrate was adjusted pH to 1.0 with concentrated HCl and extracted with ethylacetate (3 × 50 mL). The combined organic phases were washed with water (2 × 100 mL), dried over anhydrous Na2SO4 and evaporated under reduced pressure to give the pure product 5-phenyl 1H-tetrazole as a white solid (13.2 g, 90% yield).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support provided by Shaanxi Key Laboratory for Phytochemistry (17JS008) and Baoji University of Arts and Sciences (No. ZK14008).Notes and references
- (a) L. Lang, B. Li, W. Liu, L. Jiang, Z. Xu and G. Yin, Chem. Commun., 2010, 46, 448 RSC; (b) F. Ling, Z. Xie, J. Chen, C. Ai, H. Shen, Z. Wang, X. Yi and W. Zhong, Adv. Synth. Catal., 2019, 361, 3094 CrossRef CAS; (c) F. Verma, A. Sahu, P. K. Singh, A. Rai, M. Singh and V. K. Rai, Green Chem., 2018, 20, 3783 RSC; (d) B. Gutmann, J. P. Roduit, D. Roberge and C. O. Kappe, Angew. Chem., Int. Ed., 2010, 49, 7101 CrossRef CAS PubMed; (e) W. Luo, B. Shao, J. Li, D. Song, X. Yi, F. Ling and W. Zhong, Tetrahedron Lett., 2019, 60, 151206 CrossRef.
- (a) G. Steinhauser and T. M. Klapotke, Angew. Chem., Int. Ed., 2008, 47, 3330 CrossRef CAS PubMed; (b) R. P. Singh, R. D. Verma, D. T. Meshri and J. M. Shreeve, Angew. Chem., Int. Ed., 2006, 45, 3584 CrossRef CAS PubMed.
- P. Srihari, P. Dutta, R. S. Rao, J. S. Yadav, S. Chandrasekhar, P. Thombare, J. Mohapatra, A. Chatterjee and M. R. Jain, Bioorg. Med. Chem. Lett., 2009, 19, 5569 CrossRef CAS PubMed.
- V. Pimenta, J. Lhoste, A. Hemon-Ribaud, M. Leblanc, J. M. Greneche, L. Jouffret, A. Martel, G. Dujardin and V. Maisonneuve, Cryst. Growth Des., 2015, 15, 4248 CrossRef CAS.
- M. Nasrollahzadeh, M. Maryami, M. Sajjadi and E. Mehdipour, Appl. Organomet. Chem., 2019, 33, 4730 CrossRef.
- A. A. S. Elahl, S. S. Elmorsy, A. H. Elbeheery and F. A. Amer, Tetrahedron Lett., 1997, 38, 1257 CrossRef CAS.
- S. D. Guggilapu, S. K. Prajapti, A. Nagarsenkar, K. K. Gupta and B. N. Babu, Synlett, 2016, 27, 1241 CrossRef CAS.
- M. Halder, M. M. Islam, P. Singh, A. S. Roy, S. M. Islam and K. Sen, ACS Omega, 2018, 3, 8169 CrossRef CAS.
- (a) N. Kaur, Synth. Commun., 2019, 49, 1633 CrossRef CAS; (b) R. Mittal and S. K. Awasthi, Synthesis, 2019, 51, 3765 CrossRef CAS; (c) H. Fatahi, M. Jafarzadeh and Z. Pourmanouchehri, J. Heterocycl. Chem., 2019, 56, 2090 CrossRef CAS; (d) M. A. E. A. A. A. El-Remaily and O. A. Elhady, Appl. Organomet. Chem., 2019, 33, 4989 Search PubMed; (e) S. Molaei and M. Ghadermazi, Appl. Organomet. Chem., 2019, 33, 4854 Search PubMed; (f) S. B. Bhagat and V. N. Telvekar, Synlett, 2018, 29, 874 CrossRef CAS; (g) B. Yakambram, A. J. Shree, L. S. Reddy, T. Satyanarayana, P. Naveen and R. Bandichhor, Tetrahedron Lett., 2018, 59, 445 CrossRef CAS; (h) J. M. Chretien, G. Kerric, F. Zammattio, N. Galland, M. Paris, J. P. Quintard and E. L. Grognec, Adv. Synth. Catal., 2019, 361, 747 CrossRef CAS; (i) D. Cantillo, B. Gutmann and C. O. Kappe, J. Am. Chem. Soc., 2011, 133, 4465 CrossRef CAS PubMed; (j) Z. P. Demko and K. B. Sharpless, J. Org. Chem., 2001, 66, 7945 CrossRef CAS.
- B. Mitra, S. Mukherjee, G. C. Pariyar and P. Ghosh, Tetrahedron Lett., 2018, 59, 1385 CrossRef CAS.
- R. R. Chakraborty and P. Ghosh, Tetrahedron Lett., 2018, 59, 3616 CrossRef.
- X. Xiong, C. Yi, X. Liao and S. Lai, Tetrahedron Lett., 2019, 60, 402 CrossRef CAS.
- M. Kazemnejadi and A. R. Sardarian, RSC Adv., 2016, 6, 91999 RSC.
- M. B. M. Reddy and M. A. Pasha, Synth. Commun., 2011, 41, 2081 CrossRef.
- M. Abdollahi-Alibeik and A. Moaddeli, New J. Chem., 2015, 39, 2116 RSC.
- M. M. Hevari, A. Fazeli, H. A. Oskooie, Y. S. Beheshtiha and H. Valizadeh, Synlett, 2012, 23, 2927 CrossRef.
- K. M. Khan, I. Fatima, S. M. Saad, M. Taha and W. Voelter, Tetrahedron Lett., 2016, 57, 523 CrossRef CAS.
- (a) P. Bao, H. Yue, N. Meng, X. Zhao, J. Li and W. Wei, Org. Lett., 2019, 21, 7218 CrossRef CAS PubMed; (b) L. Y. Xie, Y. L. Chen, L. Qin, Y. Wen, J. W. Xie, J. X. Tan, Y. Huang, Z. Cao and W. M. He, Org. Chem. Front., 2019, 6, 3950 RSC; (c) X. Geng, C. Wang, C. Huang, P. Zhao, Y. Zhou, Y. D. Wu and A. X. Wu, Org. Lett., 2019, 21, 7504 CrossRef CAS; (d) Y. Xia, H. Huang, F. Zhang and G. J. Deng, Org. Lett., 2019, 21, 7489 CrossRef CAS PubMed; (e) X. Li, T. Feng, D. Li, H. Chang, W. Gao and W. Wei, J. Org. Chem., 2019, 84, 4402 CrossRef CAS PubMed; (f) S. W. Tao, J. Y. Zhou, R. Q. Liu and Y. M. Zhu, J. Org. Chem., 2019, 84, 8121 CrossRef CAS PubMed; (g) L. H. Lu, Z. Wang, W. Xia, P. Cheng, B. Zhang, Z. Cao and W. M. He, Chin. Chem. Lett., 2019, 30, 1237 CrossRef CAS; (h) F. Ling, J. Chen, Z. Xie, H. Hou, Z. Pan, C. Feng, H. Shen and W. Zhong, J. Heterocycl. Chem., 2019, 56, 3333 CrossRef CAS; (i) C. Wu, L. H. Lu, A. Z. Peng, G. K. Jia, C. Peng, Z. Cao, Z. Tang, W. M. He and X. Xu, Green Chem., 2018, 20, 3683 RSC; (j) Z. Cao, Q. Zhu, Y. W. Lin and W. M. He, Chin. Chem. Lett., 2019, 30, 2132 CrossRef CAS; (k) M. Sun, J. Jiang, J. Chen, Q. Yang and X. Yu, Tetrahedron, 2019, 75, 130456 CrossRef.
- Z. Wei, J. Li, Z. Wang, P. Li and Y. Wang, Chin. J. Org. Chem., 2017, 37, 1835 CrossRef CAS.
- M. Klavins, J. Dipane and K. Babre, Chemosphere, 2001, 44, 737 CrossRef CAS.
- (a) M. K. Skopic, K. Gotte, C. Gramse, M. Dieter, S. Pospich, S. Raunser, R. Weberskirch and A. Brunschweiger, J. Am. Chem. Soc., 2019, 141, 10546 CrossRef CAS PubMed; (b) Q. Liu, M. Lu and W. Wei, Sci. China, Ser. B, 2009, 39, 440 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08523h |
| This journal is © The Royal Society of Chemistry 2020 |