Open Access Article
Open Access ArticleRecent developments in detoxication techniques for aristolochic acid-containing traditional Chinese medicines
Yang Fan,
Zongming Li and
Jun Xi *
*
School of Chemical Engineering, Sichuan University, Chengdu 610065, China. E-mail: xijun@scu.edu.cn; Fax: +86 28 85405209; Tel: +86 28 85405209
First published on 8th January 2020
Abstract
Aristolochic acids (AAs) have attracted significant attention because they have been proven to be the culprits in the mass incidents of AA nephropathy that occurred in Belgium in 1993. From then on, the door to sales of medicines containing AAs has been closed. As aristolochic acid (AA)-containing traditional Chinese medicine (TCM) has a potent therapeutic effect on some diseases, research into detoxication techniques for AA-containing traditional Chinese medicines (TCMs) should be considered to be absolutely essential. Therefore, in this paper, the use of AA-containing TCMs has been investigated and detoxication techniques, such as, processing (Paozhi, Chinese name), compatibility (Peiwu, Chinese name), pressurized liquid extraction (PLE) and supercritical fluid extraction (SFE), have been reviewed in detail. A large number of relevant studies have been reviewed and it was found that processing with honey or alkaline salts is the most widely used method in practical production. As the AAs are a group of weak acids, relatively speaking, processing with alkaline salts can achieve a high rate of reduction of the AAs. Meanwhile, it is necessary to consider the compatibility of AA-containing TCMs and other herbal medicines. In addition, PLE and SFE can also achieve an excellent reducing rate for AAs in a much shorter processing time. Therefore, the promotion of alkaline salt processing technology should be strengthened in the future. At the same time, some advanced modern extraction technologies also have good potential and should be further studied.
1. Introduction
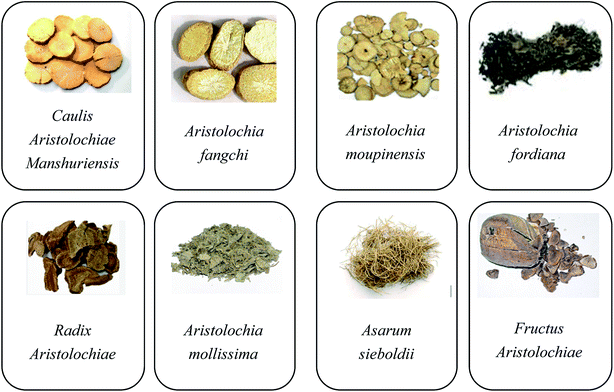
Aristolochic acids (AAs) are a family of carcinogenic and nephrotoxic phytochemicals commonly found in species of the genus Asarum and Aristolochia.1,2 Since AAs were finally proven to be the culprits in the mass incidents of aristolochic acid nephropathy that occurred in Belgium in 1993, many countries have imposed a ban on the therapeutic use of aristolochic acid (AA)-containing traditional Chinese medicines (TCMs). Nowadays, AAs are considered to be one of the most potent carcinogens, especially AA-I and AA-II. 3–5 Although the true carcinogenic mechanism has not been fully identified, it is generally acknowledged that AA-I and AA-II are both important carcinogens and have a relatively high content compared to other AA analogues in most AA-containing herbs. Meanwhile, the cytotoxic data suggests that the nitro and methoxy groups are determinants of the nephrotoxicological potency.6Over 600 species of Aristolochia have already been found, of which 99 species have already been used as medicines to help patients recover from sexually transmitted diseases, gastrointestinal complaints, poisoning due to snakebites, eczema, fungal skin diseases, and so forth.7 As shown in Fig. 1, the eight typical AA-containing TCMs (Caulis Aristolochiae Manshuriensis, Aristolochia fangchi, Aristolochia moupinensis, Aristolochia fordiana, Radix Aristolochiae, Aristolochia mollissima, Asarum sieboldii, and Fructus Aristolochiae) are commonly used. As AA-containing TCMs have such a high pharmaceutical value and they cannot be completely replaced by other AA-free herbal remedies, more and more researchers have begun to focus on the selective detoxication of AAs from the related herbs to allow their safe use in disease therapy.8,9 There are two kinds of detoxication methods, named processing (Paozhi, Chinese name) and compatibility (Peiwu, Chinese name) used in China for the pretreatment of TCMs. Nowadays these methods are increasingly being accepted and are becoming popular in western medicine.10 However, there are still several disadvantages to them, such as being time-consuming and labor-intensive.11 In addition, the chemical profiles of medicines are changed during the processing, which influences the pharmacological properties.12 In recent years, more efficient removal techniques such as pressurized liquid extraction (PLE)13 and supercritical fluid extraction (SFE)14 have also been proposed. These detoxication methods differ from the processing and compatibility methods, and use temperature and pressure for the detoxication of AAs.
The aim of this paper is to provide a detailed review of traditional and current methods used for potential detoxication of AA-containing TCMs, including processing (Paozhi), compatibility (Peiwu), PLE and SFE. Furthermore, the main problems and future development directions of these techniques will also be discussed.
2. Summary of AAs
2.1 AA-containing TCMs
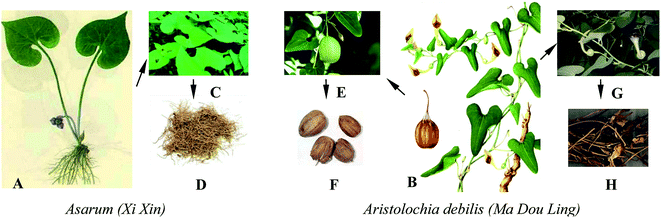
The AAs mainly occur in the genera Aristolochia and Asarum, of which only three kinds of herbs are still recorded in the China pharmacopoeia (2015). One is Asarum (Xi Xin, Chinese name), whose roots and rhizomes can be used as medicine, this is distributed in most parts of northern China. The other two herbs are Northern Dutchmanspipe Vine (Tian Xian Teng, Chinese name) and Fructus Aristolochiae (Ma Dou Ling, Chinese name), which have the same original plants of Aristolochia debilis Sieb. et Zucc. or Aristolochia contorta Bge and thus are termed Aristolochia debilis, the stem and fruit of which can be used as medicine (Table 1 and Fig. 2).| English name | Chinese name | Species (name given in original sources) | Genus | Family | Part used | Main chemical composition | Illness or function | Usage |
|---|---|---|---|---|---|---|---|---|
| Northern Dutchmanspipe Vine or Herba Aristolochiae or Aristolochia Mollisima Hance | Tian Xian Teng | Aristolochia debilis Sieb. et Zucc. or Aristolochia contorta Bge. | Aristolochia | Aristolochiaceae | Stem | Aristolochic acid D, magnoflorine, β-sitosterol | Abdominal pain, joint pain, edema in pregnancy | Oral: 4.5–9 g |
| Fructus Aristolochiae | Ma Dou Ling | Aristolochia | Aristolochiaceae | Fruit | β-Sitosterol, magnoflorine, aristolochic acid A (in seed), aristolochic acid C, aristolochic acid D, aristolactone | Antitussive, expectorant, hemorrhoids | Oral: decoction 3–9 g | |
| Manchurian Wildginger, Herb of Manchurian Wildginger, Herb of Seoul Wildginger, Herb of Siebold Wildginger | Xi Xin | Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag. or Asarum sieboldii Miq. var. seoulense Nakai or Asarum sieboldii Miq. | Asarum | Aristolochiaceae | Roots and rhizomes | Asarinin, volatile oil (methyl eugenol, croweacin, asaricin and so on), aristolochic acid | Cold, sore throat, headache | Oral: decoction 1.5–9 g, powder: 1–3 g. external |
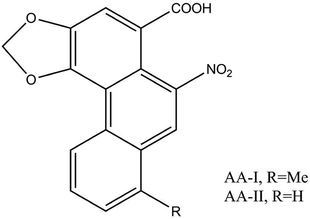
Aristolochic acids refer to a mixture of carboxylic acids. Among these a toxicological study on AA-I and AA-II (Fig. 3) revealed that the main constituents considered to be responsible for the nephrotoxic and carcinogenic effects are relatively abundant.11,15 Many studies have also found that not only AA-I and AA-II, but also AA-III, AA-IIIa, AA-IV, AA-IIIa-6-β-D-glucoside, aristolochin, aristolactam-I (AL-I) and so on, 178 aristolochic acid analogs (AAAs) in total, may have pathogenic potential.16
Generally speaking, most of the species of Asarum contain lower amounts of AA-I and AA-II than the Aristolochia species. Zang et al. determined the total content of AAs in Asarum bought from different places in China.17 They found that a sample from Liaoning Province had the highest content of AAs of 0.6633 mg g−1. In the genera Aristolochia, Xu et al. separated 12 kinds of AAAs from the dry fruit of Aristolochia contorta which included three kinds of AAs and three kinds of aristolactams (ALs).18 Tian et al. determined the average content of AL-I, AA-III, 7-hydroxy-aristolochic acid I and AA-I in Aristolochia debilis and found that the contents of AA-III and AA-I could reach 1.2 mg g−1 and 0.8 mg g−1, respectively.19 In contrast, the disparity of the content of AAs in Asarum and Aristolochia can be easily observed. Meanwhile it is clear that the contents of AAs in Asarum and Aristolochia are still relatively high and research into the detoxication of Asarum and Aristolochia is very important.
The AAs have a highly specific tissue distribution in the genera Asarum and Aristolochia. In the species Aristolochia, taking Caulis Aristolochiae Manshuriensis as an example, the contents of AA-I in the leaves and roots were much higher than that in the stem, especially in the growing period, which implied that AA-I might be a secondary metabolite of photosynthesis.20 In the species Asarum, Hus et al. found that the amount of AA-I was highest in the leaves, followed by the petioles, rhizomes and roots.21
2.2 Understanding Asarum and Aristolochia debilis toxicity
The toxicity of Asarum was first recorded in the earliest Chinese medicinal classic work Shennong Bencao Jing, “It can only be used in combination properly with other herbs.” In the Northern and Southern Dynasties, the record in Leigong Paozhi Lun (a famous book of Drug Processing, written by Lei Xiao, a famous pharmacologist) says that “Its leaves need to be removed because of their toxicity.”In Song Dynasty, according to the record in Bencao Bie Shuo, written by Chen Cheng, a famous physician, “Asarum cannot use more than 2.5 g for a single powder, or people will be in danger of life”. The famous Chinese medicinal classic work, Compendium of Materia Medica (Herbalism masterpiece, written by Li Shizhen in Ming Dynasty), says that “Asarum cannot use more than 5 g for a single powder, or people will be suffocated to death.” Up until the Qing Dynasty, there are other records stating that “If it can be used in the proper combination with other herbs, its usage limitation will not be so obvious.” It is clear that the ancient Chinese neglected to note the presence of AAs in Asarum. Nowadays, the China pharmacopoeia (2015) recommends the dosage of Asarum that should be used is 1–3 g and suggests the risk of nephrotoxicity should be noted.
Chinese people realized the toxicity of Aristolochia debilis a long time ago. According to many records, Aristolochia debilis is loaded with cold yin energy, which means that it is better to use in moderation. It was first recorded in Leigong Paozhi Lun, “The peels and diaphragm need to be completely shucked before using”. In the Compendium of Materia Medica, it is noted that “they are potent in expelling pathogenic heat but will cause vomiting if over consumed”. Similarly, almost no ancient records note its nephrotoxicity. Later, the China pharmacopoeia (2015) stipulated that the amount of AA-I contained in herbal medicine, no matter the genera Aristolochia or Asarum, should not be more than 0.001%.
Therefore, we can see that in ancient times, people did not have a clear understanding of the toxicity of AA-containing TCMs, and their opinions were different in these different times. Moreover, people did not try to explore the detoxification methods of the related medicines, but avoided possible harm by controlling the dosage or eliminating usage.
2.3 Preparation and determination
In order to further research the AAs, some researchers have tried to find and explore various qualitative and quantitative methods for the preparation and detection of the AA content.22–24 To enrich AA pre-treatment compounds, the earliest inventors used CHCl3 or CH2Cl2 for extraction and enrichment to obtain AA-I with a higher purity.22 This method is unfavorable with regards to environmental protection and recycling. Later, some researchers invented a method utilizing a macroporous absorption resin extraction column, the recovery rate of which is higher and the stability is better than that of the traditional liquid–liquid extraction.23 There is also a method of making molecularly imprinted polymers using silica gel to enrich the content for further detection, this method has a better selectivity. Therefore, it is more conducive to the quantitative analysis of AAs.24At present, there are many detection methods for AA content, some of which are very simple and convenient. Some specific methods are given in some patents, for example, the reduction method is used to reduce the nitro group, which inhibits fluorescence on the AAs, to an amino group to obtain a fluorescence enhanced reduction product. The excitation and emission spectra of the product can be measured to determine the AA content.25 Another researcher invented a method using fluorescent test paper, which is even more convenient and accurate. Only a single handheld ultraviolet lamp is needed to carry out the real-time quick detection of AAs.26 Rapid detection methods usually use fluorescent detection. In addition, the AA content in Chinese herbal medicines can also be determined by using the method of near infrared spectroscopy. This is a more accurate quantitative method with a higher resolution.27 It can be seen that detection methods for AAs are becoming more and more mature.
2.4 Metabolism and pathogenesis
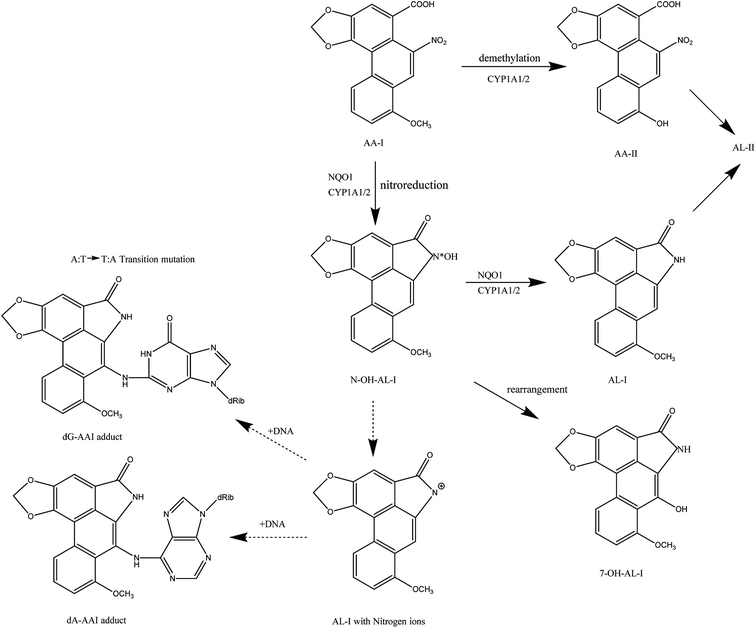
Aristolochic acids are etiologic agents in clinical syndromes known as Balkan endemic nephropathy, and cause a global burden of chronic kidney disease and urothelial cancer.28 Currently, research on the metabolism of AA-I is relatively comprehensive and definite.29 It mainly has two kinds of metabolites in vivo (Fig. 4), one is the harmful DNA adducts and the other is harmless and easily excreted. Under anoxic conditions, the carcinogens of 7-(deoxyadenosin-N6-yl) aristolactam I (dA-AL-I) and 7-(deoxyguanosin-N2-yl) aristolactam I (dG-AL-I), a kind of aristolactam-DNA adduct, will be produced.30 Numerous in vitro and in vivo animal models have proved that aristolactam-DNA adducts in human tissues serve as biomarkers for AA-I exposure and play a critical role in the development of upper tract urothelial cancers.28 These aristolactam-DNA (AL-DNA) adducts can lead to mutations of adenine (A) and thymine (T) in the genes reports of transgenic mice after the injection of AAs.31,32 By studying the transitional cell carcinomas of patients with endemic nephropathy (EN), researchers have found that almost all patients have at least one A:T → T:A mutation, and it is heavily concentrated in the p53 gene. This is totally different from transitional cell carcinomas with a common nephropathy.33,34 Under aerobic conditions, NQO1 and CYP1A1/A2, the main metabolic enzymes that can activate and detoxify poly cyclic aromatic hydrocarbons carcinogens, can convert AA-I into AA-II, AL-I or 7-OH-AL-I by O-demethylation or rearrangement. AA-II and AL-I can be further converted into AL-II, which can be rapidly excreted by the digestive system. Some researches indicated that the affinity of AA-I with NQO1 and CYP1A1/A2, and the reduction and activation of CYP1A1/A2 to AA-I will affect the degree of nitroreduction or O-demethylation of AA-I.35,36 Therefore, it provides a good solution to prevent the formation of aristolactam-DNA adducts by detecting the activity of AA-I metabolism-related enzymes in patients and selecting inhibitors of the corresponding enzymes or CYP1A1/A2 inducers.In addition to AA-I, some other compounds such as AA-II are also found to be harmful and can also lead to the induction of apoptosis through experiments on HK-2 cells. To further distinguish the possible different renal toxicity of AA-I and AA-II, some mice experiments were carried out and showed that both AA-I and AA-II will cause acute tubular necrosis and extensive cortical interstitial fibrosis, which are typical symptoms of cancer, while AA-II-treated mice exhibit comparatively mild symptoms. The body weight of AA-I-treated mice decreased by more than 30% after the experiment and those treated with AA-II decreased by about 12%.11 If we consider the entire digestive system, with time and dose accumulation of AAs, the toxicity might be greatly undervalued in some ways. Chang et al., designed a “microphysiological system” (MPS) which could simulate organ and tissue function, and responded to injury.28 To evaluate the role of hepatic metabolism in AA-induced kidney injuries, they linked a faux human kidney on a chip (MPS) to an MPS populated with human hepatocytes. Cytotoxicity assays demonstrated that the amount of renal cell apoptosis increased after AA-I treatment following prehepatic metabolism (liver → kidney) compared to that of infusing AA-I into the kidney MPS directly. The result indicated that the overall effect of hepatic metabolism was to bioactivate, rather than to detoxify.
3 Detoxication techniques of AA-containing TCMs
Traditional Chinese medicine (TCM) has a very wide range of applications in the field of medicine owing to the advantages of being a more holistic treatment. It usually has more than one effect, which is different from Western medicine. However, because TCMs generally have a complex chemical composition, some of them will inevitably also be harmful to the human body.37 The presence of AAs in the Aristolochia and Asarum species is a very typical example. Some cancer-genomic researchers believe that AAs have a direct link to mutation, although they still cannot completely identify the dose response relationship between the rate of prevalence of cancer and the exposure to AAs.38 A controversial policy was introduced in 2018, which stipulated that TCMs no longer needed to pass safety and efficacy trials in humans in China. Therefore, it is conceivable that the research studying detoxication techniques for TCMs will play a more important role in the safe use of TCMs.As for the detoxication techniques, these can be roughly divided into traditional methods, that is, processing and compatibility, which originated in ancient China and modern methods, such as, PLE and SFE. The majority of methods used to determine the optimal conditions for processing and compatibility are based on experience and extensive attempts. The modern methods, PLE and SFE, have a relatively definite scientific basis, therefore they have a clearer mechanism and research direction. So far, related research into detoxication of AAs is insufficient and further research is still needed. We will highlight each method and discuss the features respectively in the following sections. The rates of reduction of AA-I of AA-containing TCMs are comprehensively summarized in Table 2.
| Herb | Species | Excipient | Proportion | Processing method | Time | Reducing rate of AA-I in decoction | Ref. |
|---|---|---|---|---|---|---|---|
| a CAM: Caulis Aristolochiae Manshuriensis; FA: Fructus Aristolochiae; RA: Radix Aristolochiae. | |||||||
| Xi Xin | Asarum | Yellow rice wine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
Soaking | Until dry | 17.4% | Liu42 |
| Vinegar | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
Soaking | 2.89% | ||||
| Ginger juice | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
Soaking | 5.53% | ||||
| Honey | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.24 0.24 |
Soaking | 13.06% | ||||
| 0.1 mol L−1 NaHCO3 | Abundant excipient | Soaking | 3 d | 57.12% | |||
| Ma Dou Ling | Aristolochia | Honey | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
Stirring | 15 min | 26.4–29.2% | Liang et al.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
Ultrasonic treatment | 1 h | 16.9–51.7% (AAAs), 32.7% (AA-I) | Li et al.45 | |||
| Abundant excipient | Stirring | 25 min | 40–50% | Zhang et al.43 | |||
| CAM | 0.1 mol L−1 NaHCO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Soaking | 5 d | 93.60% | Wang and Zhang45 | |
| Coptis | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Plenty | 74.10% | Wu et al.40 | |||
| Poria cocos | 23.39% | ||||||
| Cortex Moutan | Boiling | 19.20% | Wang and Deng65 | ||||
| Ultrasonic treatment | 50 min | 75.19% | Zhu66 | ||||
| Glycyrrhiza juice | Boiling | 2 h | 36.10% | Lu and Pan49 | |||
| NaHCO3 | 46.40% | ||||||
| Daochi powder with Coptis chinensis | 85.52% | ||||||
| Frutus Gardeniae | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
57.49% | |||||
| Poria cocos | 23.39% | ||||||
| Coptis chinensis | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Ultrasonic treatment | 30 min | 75.85% | Ma et al.67 | ||
| 0.1 mol L−1 NaHCO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Soaking | 3 d | 84.70% | Lu and Pan49 | ||
| Longdanxiegan decoction | Boiling | 50 min | 48.47% | Xu and Xie60 | |||
| Ethanol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
SFE | 12 h | 81.30%, 81.20% (AA-II) | Liang et al.20 | ||
| FA | 65.20%, 59.10% (AA-II) | ||||||
| RA | Methanol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
PLE | Rapid | 1080 mg kg−1 | Ong13 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
Ultrasonic | 30 min | 1018 mg kg−1 | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
Soxhlet extraction | 7–8 h | 937 mg kg−1 | ||||
3.1 Processing
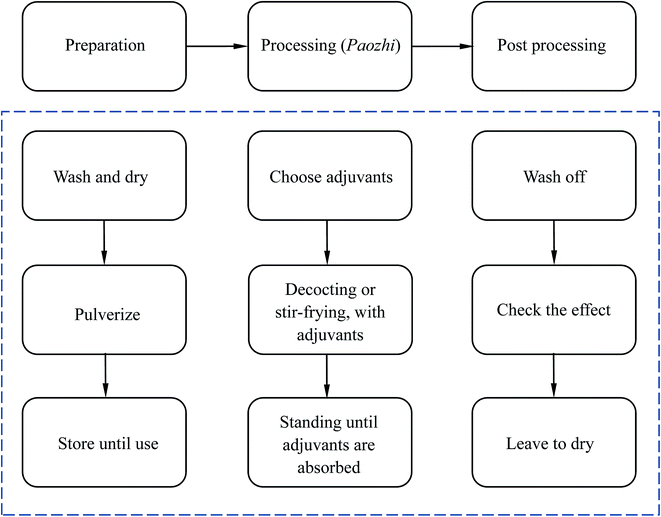
The processing methods for Chinese herbal medicines, called Paozhi in Chinese, play an important role in detoxifying toxic herbs. These methods have a wide range of applications in TCM, because some of the herbs not only include the effective constituent, but also include toxicants, which can lead to side effects.39 These methods are usually used to pretreat special herbs which are smelly or have significant side effects on the human body.40 Paozhi can be used to retain the beneficial chemical components for health and reduce the harmful ones.40 As for the detoxication of AAs, proper processing such as processing with NaHCO3 can achieve a relatively high reducing rate, but requires a relatively longer processing time and a greater amount of solvent, because it is usually a simple neutral reaction in the absence of an external force essentially. Nevertheless, some studies indicate that some other compounds will appear after processing by determination of high performance liquid chromatography (HPLC), therefore further research is required.The principle of this method is relatively easy to understand. Herbs are made of plant cells which have a dynamic, complex membrane and a rigid cell wall, therefore the entry of solvents faces some obstacles. These barriers can be disrupted by the processing. In general, the processing method is simple. The sample matrix is usually mixed with other supplements, and then the mixture is soaked, ground, or even fried. This acceleration process is to raise the temperature or increase the contact surface of the sample and supplements. Therefore the entire process can help the harmful compounds react with other chemicals in the supplements. With regards to the effective processing methods of AA-containing TCMs, the physical methods mainly involve processing with honey, salt and wine and the chemical methods of processing use alkaline salts or vinegar.41,42 The basic procedure for these methods is shown in Fig. 5. Among these, honey and alkaline salts are the most widely used in for the practical processing of AA-containing TCMs.40
Mostly, processing with honey can not only remove the bitter taste, but also has a certain effect on the removal of AAs. Liang et al. studied the removal rate of AA-I from the seeds of Fructus Aristolochiae after processing with honey in a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 g g−1. The sample was first soaked in honey for 2 h, and then fried for 5–20 min over a gentle heat. Later, the content change of the AA-I from the seeds was determined. The results showed the removal rate of AA-I could reach about 40% after frying for 20 min. Li et al. detected a change in the content of AA-I, AA-II, 7-OH AA-I, AA C and AA D in the Fructus Aristolochiae after processing with honey.45 They found that the reducing rates of these compounds in the decoction were between 16.9% and 28.7%, and the reducing rate of AA-I was 25.8%. Zhang et al. researched the change in the composition after processing with honey.43 They utilized the HPLC fingerprint to determine the chemical ingredients of Fructus Aristolochiae from different areas of China before and after processing with honey. The results showed that the reducing rate of AA-I could exceed 36%. Nevertheless, the content of AA-I was still above 0.2 mg g−1, which was the minimum toxic dose in the human body.19
0.25 g g−1. The sample was first soaked in honey for 2 h, and then fried for 5–20 min over a gentle heat. Later, the content change of the AA-I from the seeds was determined. The results showed the removal rate of AA-I could reach about 40% after frying for 20 min. Li et al. detected a change in the content of AA-I, AA-II, 7-OH AA-I, AA C and AA D in the Fructus Aristolochiae after processing with honey.45 They found that the reducing rates of these compounds in the decoction were between 16.9% and 28.7%, and the reducing rate of AA-I was 25.8%. Zhang et al. researched the change in the composition after processing with honey.43 They utilized the HPLC fingerprint to determine the chemical ingredients of Fructus Aristolochiae from different areas of China before and after processing with honey. The results showed that the reducing rate of AA-I could exceed 36%. Nevertheless, the content of AA-I was still above 0.2 mg g−1, which was the minimum toxic dose in the human body.19
As the decrease of AAs cannot represent the effect of detoxication completely. Some researchers have demonstrated in mice that Fructus Aristolochiae processed using honey is definitely safer. Yang et al. researched the LD50 of Fructus Aristolochiae before and after processing with honey in a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 g g−1, and they found that the LD50 values were 34.0 and 62.6 g kg−1 d−1 respectively.46 The 95% confidence limits were 27.7–42.0 g kg−1 d−1 and 55.1–71.1 g kg−1 d−1 respectively. In conclusion, there is a certain effect on the detoxication of Fructus Aristolochiae from the processing method using honey. However, the reducing rate of AA-I is lower, and the mechanism is still ambiguous. Therefore, the processing technique is still in need of improvement.
0.3 g g−1, and they found that the LD50 values were 34.0 and 62.6 g kg−1 d−1 respectively.46 The 95% confidence limits were 27.7–42.0 g kg−1 d−1 and 55.1–71.1 g kg−1 d−1 respectively. In conclusion, there is a certain effect on the detoxication of Fructus Aristolochiae from the processing method using honey. However, the reducing rate of AA-I is lower, and the mechanism is still ambiguous. Therefore, the processing technique is still in need of improvement.
Compared to honey, the detoxication effect is better by processing with NaHCO3. Liu soaked 50 g Asarum in 0.1 mol L−1 NaHCO3 for 24 h, washed the herb materials, repeated this process four times and then dried them in an oven.42 When the ratio of material to solvent was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 g mL−1, it was found that the reducing rate of the AAs could reach more than 85%. He et al. soaked Caulis Aristolochiae Manshuriensis in 0.05 mol L−1 NaHCO3 for 24 hours, then washed it out with water and repeated this process three times.17 After that, the content of AA-I was determined by HPLC and the reducing rate of AA-I was 83.74%. Lu and Pan soaked Caulis Aristolochiae Manshuriensis in 0.1 mol L−1 NaHCO3 and boiled the solution until the water was all evaporated. Later they washed the herb material, dried it out and determined the residual AA-I.50 When the ratio of material to solvent was 1
40 g mL−1, it was found that the reducing rate of the AAs could reach more than 85%. He et al. soaked Caulis Aristolochiae Manshuriensis in 0.05 mol L−1 NaHCO3 for 24 hours, then washed it out with water and repeated this process three times.17 After that, the content of AA-I was determined by HPLC and the reducing rate of AA-I was 83.74%. Lu and Pan soaked Caulis Aristolochiae Manshuriensis in 0.1 mol L−1 NaHCO3 and boiled the solution until the water was all evaporated. Later they washed the herb material, dried it out and determined the residual AA-I.50 When the ratio of material to solvent was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g mL−1, the reducing rate of AA-I was 46.4%. It could be found that the content change of AAs mainly depended on the processing time, temperature and washing-leaching times. The rinsing process could wash off the sodium salt of AAs, a higher temperature could accelerate the evaporation of water and the repetitive process could increase the concentration difference of AAs for diffusion and produce a better detoxication effect.
10 g mL−1, the reducing rate of AA-I was 46.4%. It could be found that the content change of AAs mainly depended on the processing time, temperature and washing-leaching times. The rinsing process could wash off the sodium salt of AAs, a higher temperature could accelerate the evaporation of water and the repetitive process could increase the concentration difference of AAs for diffusion and produce a better detoxication effect.
In order to obtain a more comprehensive understanding of the actual detoxication effect, Wang et al. studied the pharmacodynamics and toxicology of Asarum in mice before and after processing with NaHCO3.50 The analgesic and anti-inflammatory effect of the processed Asarum was tested in a hot plate test, writhing test and xylene induced ear swelling test. The results showed that the number of times the body twisted in the high dose group of Asarum was 11.01 ± 2.83 times, which was more than that of the processed Asarum which was 8.71 ± 2.04 times (P < 0.05), and the degree of foot swelling 2 h after administration of the crude decoction was 14.00 ± 2.30 mg, which was higher than that in the processed Asarum decoction of 10.00 ± 2.30 mg (P < 0.05). The degree of ear swelling in the low dose group for the crude Asarum decoction was 13.30 ± 1.35 mg, which was higher than that of the processed Asarum decoction of 9.70 ± 1.41 mg (P < 0.05). The rate of inflammation inhibition in a xylene induced swollen mouse ear was 17.30% for the crude Asarum decoction, which was significantly lower than that of processed Asarum decoction of 22.80% (P < 0.05).
In the actual production, rice vinegar is usually used to neutralize the excess alkaline salt. In order to determine the detoxication effect in practice, Wang et al. studied the methods of processing with NaHCO3 and rice vinegar. Firstly, they mixed 10 times the amount of 0.1 mol L−1 NaHCO3 solvent with the crude Caulis Aristolochiae Manshuriensis, soaked for 24 h and rinsed with water three times after the process of soaking, repeated six times, then fried the products until the water evaporated.51 Secondly, they diluted rice vinegar by 2.5-fold with water, soaked the processed Caulis Aristolochiae Manshuriensis with diluted rice vinegar in the proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 g g−1 for a sufficient time and fried them until they were dry. Lastly, they compared the dissolution rate of the treated sample and the crude one in the decoction and found that the treated one decreased by about 80%. In the entire whole process, the carboxyl on the AAs can react with NaHCO3 to form soluble sodium salts and most of them can be washed out. Residues of the salts will be reduced to the free acid and will be hard to dissolve. Therefore, this process method can also provide a good detoxication effect.
1 g g−1 for a sufficient time and fried them until they were dry. Lastly, they compared the dissolution rate of the treated sample and the crude one in the decoction and found that the treated one decreased by about 80%. In the entire whole process, the carboxyl on the AAs can react with NaHCO3 to form soluble sodium salts and most of them can be washed out. Residues of the salts will be reduced to the free acid and will be hard to dissolve. Therefore, this process method can also provide a good detoxication effect.
3.2 Compatibility
The compatibility (Peiwu, Chinese name) of AAs with other medicinal materials has been used by generations of physicians to achieve a detoxication and synergistic effects.37 In brief, the aim of this method is to select the optimum combination of two or more Chinese herbs which have a similar effect to improve the overall effectiveness. Meanwhile, the mixture could result in a decreased in the toxicity of the herbs. Therefore, it could achieve the result of one plus one being larger than two.51 However, there remains a lack of systemic analyses of the compatibility rationale of TCM. The formula for TCM is mainly based on extensive attempts and experience.10,12,52Compared to processing, the compatibility of different herbal medicines has the same principle in essence. TCM theory is aimed toward mixing different materials together to maximize the efficacy and minimize the adverse effects of the main herbs.53 The main difference between the processing and compatibility is the selection of the supplement. Compatibility is the combining of different herbs together, therefore it is more difficult to determine the complex compounds contained in herbs.54 As for the detoxication of AAs, the following section will give a comprehensive review of the different compatibilities by taking Caulis Aristolochiae Manshuriensis as an example.10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be considered to be the standard recipe and sometimes bamboo leaves will also be added to improve the heat-clearing function.56 The major active components of licorice and Radix Rehmanniae are glycyrrhizin and iridoid glycosides, respectively.
1 can be considered to be the standard recipe and sometimes bamboo leaves will also be added to improve the heat-clearing function.56 The major active components of licorice and Radix Rehmanniae are glycyrrhizin and iridoid glycosides, respectively.Some researches indicated that the compatibility of licorice and Radix Rehmanniae had a good inhibition for the dissolution of AAs and obtained a good effect in the alleviation of renal tissue injury. Ding et al. found that the content of AAs in a decoction of Daochi powder was 40–60%, which was less than that of the single decoction of Caulis Aristolochiae Manshuriensis.57 Li et al. found that the content of AAs was 0.1605 mg mL−1 in Daochi powder, which was less than half of the content of AAs in the single decoction of Caulis Aristolochiae Manshuriensis.58 Later, in a mouse model, they compared the renal function and pathological changes in renal tissue between the mice by injecting them with 6 g kg−1 d−1 of the decoction of Daochi powder and a single decoction of Caulis Aristolochiae Manshuriensis. In contrast, the group injected with Daochi powder exhibited reduced kidney damage and the renal tissue morphology was almost the same as that of the control group.
A comparative research of the dissolution rate of AA-I among Longdanxiegan decoction, the decoction of single Caulis Aristolochiae Manshuriensis and the Longdanxiegan decoction without Radix Angelicae Sinensis, Radix Rehmanniae, and liquorice was carried out by Xu and Xie.60 The result showed that the amount of dissolution of AAs was 0.0326, 0.0627 and 0.0454 mg mL−1, respectively, which indicated that the compatibility of Longdanxiegan decoction could reduce the dissolution of AA-I and that the Radix Angelicae Sinensis, Radix Rehmanniae, and liquorice contained within it played a more important role. This might be due to the complexation reaction between AA-I and the dissolved metal ions in the decoction which comes from Radix Angelicae Sinensis and the Radix Rehmanniae in the prescription. Meanwhile, the glucuronic acid formed by glycyrrhizin also combines with a carboxyl on AA-I to reduce its dissolution.60 Another study investigated the nephrotoxicity and pharmacokinetic characteristics of the Longdanxiegan decoction in mice, and found that the level of Scr, BUN, UPRO and tubular interstitial lesions were significantly high in the serum of the group treated with the Longdanxiegan decoction and the single decoction of Caulis Aristolochiae Manshuriensis, while the level of damage to the former was less than that of the latter. The results indicated that the Longdanxiegan decoction had some effect on detoxication, but its function was not ideal.61
The famous prescriptions, Daochi Powder, Longdanxiegan decoction, Xiaoji Yinzi, and so forth are all compatible with Rehmannia Root while using Caulis Aristolochiae Manshuriensis. It was recorded that the combination of these two herbs can promote diuresis, clear dampness, pathogenic heat and restore the balance of yin and yang.61 To study the effect of changes in the compatibility ratio on the efficacy, some researchers compared the content of AAs with different compatibility ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and found that the proportion of 1
6) and found that the proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 g g−1 of Caulis Aristolochiae Manshuriensis and Rehmannia Root could obtain the highest reducing rate of more than 50%.62,63 The result also showed that the content of AA-I did not decrease gradually with the increased usage of Rehmannia Root, which excluded the possibility that the decrease in AA-I was due to the adsorption effect of the Rehmannia Root.
0.5 g g−1 of Caulis Aristolochiae Manshuriensis and Rehmannia Root could obtain the highest reducing rate of more than 50%.62,63 The result also showed that the content of AA-I did not decrease gradually with the increased usage of Rehmannia Root, which excluded the possibility that the decrease in AA-I was due to the adsorption effect of the Rehmannia Root.
Several studies combined Caulis Aristolochiae Manshuriensis with Tree Peony Bark at different ratios, including 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and obtained the same optimal ratio of 1
6 and obtained the same optimal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.63,65,66 Specifically, Zhang et al. treated the compatibility group and the single medicine group with ultrasound for 50 min and found that the reducing rate of the AAs was reduced by 73.5% in the former.63 Wang and Deng conducted a more detailed study.65 They compared not only the AA-I content in the compatibility decoction with single Caulis Aristolochiae Manshuriensis, but also its residual content in drug sediments, respectively. In addition, they analyzed the components of the decoction and residue before and after the compatibility using HPLC, and found that the amounts of AA-I remaining in the decoction and the drug sediments of the compatibility group were both reduced significantly. Meanwhile another obvious chromatographic peak appeared logically beside that of the AA-I, it could be inferred that AA-derived analogs were produced during decocting. Interestingly, a similar but smaller adjacent peak also exists in the single decoction group, but it does not appear after the non-heat treatment of ultrasound. Therefore, the results illustrated that the structure of AA-I was unstable at the relatively higher temperature of poaching, and paeonol in Tree Peony Bark could not reduce the dissolution of AA but would promote its reaction and turn it into the new compounds. Whether the compatibility is effective was better illustrated by another mouse experiment.66 It was found that the related indexes of renal function such as β2 microglobulin, Scr and BUN were significantly lower than those in the single decoction group after 6 weeks of gavage in rats, and the differences were statistically significant. Therefore, the result proved the detoxication effect of the compatibility.
1.63,65,66 Specifically, Zhang et al. treated the compatibility group and the single medicine group with ultrasound for 50 min and found that the reducing rate of the AAs was reduced by 73.5% in the former.63 Wang and Deng conducted a more detailed study.65 They compared not only the AA-I content in the compatibility decoction with single Caulis Aristolochiae Manshuriensis, but also its residual content in drug sediments, respectively. In addition, they analyzed the components of the decoction and residue before and after the compatibility using HPLC, and found that the amounts of AA-I remaining in the decoction and the drug sediments of the compatibility group were both reduced significantly. Meanwhile another obvious chromatographic peak appeared logically beside that of the AA-I, it could be inferred that AA-derived analogs were produced during decocting. Interestingly, a similar but smaller adjacent peak also exists in the single decoction group, but it does not appear after the non-heat treatment of ultrasound. Therefore, the results illustrated that the structure of AA-I was unstable at the relatively higher temperature of poaching, and paeonol in Tree Peony Bark could not reduce the dissolution of AA but would promote its reaction and turn it into the new compounds. Whether the compatibility is effective was better illustrated by another mouse experiment.66 It was found that the related indexes of renal function such as β2 microglobulin, Scr and BUN were significantly lower than those in the single decoction group after 6 weeks of gavage in rats, and the differences were statistically significant. Therefore, the result proved the detoxication effect of the compatibility.
Wu et al. investigated the compatibility of Caulis Aristolochiae Manshuriensis and Rhizoma Coptidis with different proportions.40 The content of AA-I in each compatibility group was significantly lower than the single Caulis Aristolochiae Manshuriensis decoction. In the compatibility group with the proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 g g−1, the reducing rate reached a maximum of about 75.85%. Furthermore, the research also indicated that the results were not influenced despite other herbal medicines being added during the decocting process. Based on experiments with mice, Ma et al. observed that the combination could significantly improve the appetite, body weight, coat color, mental state, renal morphology and the biochemical items of renal function compared to the single Caulis Aristolochiae Manshuriensis decoction-treated rats.67,68
1 g g−1, the reducing rate reached a maximum of about 75.85%. Furthermore, the research also indicated that the results were not influenced despite other herbal medicines being added during the decocting process. Based on experiments with mice, Ma et al. observed that the combination could significantly improve the appetite, body weight, coat color, mental state, renal morphology and the biochemical items of renal function compared to the single Caulis Aristolochiae Manshuriensis decoction-treated rats.67,68
To summarize, according to the experiments in vivo and in vitro, most of compatibility and different processing methods could reduce the acute nephrotoxicity and improve the metabolism of AA-containing TCMs such us Caulis Aristolochiae Manshuriensis, Fructus Aristolochiae, and Radix Aristolochiae (Table 3). However, there are still some difficulties with these traditional extraction methods. Firstly, observation of the pharmacological effects of other compounds produced by these methods are still scarce and further research is needed. Secondly, these methods also have the problem of having a long treatment time, large solvent usage, and so forth, and the efficiency still needs to be improved. Thirdly, the detoxification mechanism of these traditional extraction methods is still not completely clear and further determination is needed.
| Herb | Material | Dosage | Duration | Serum creatinine (Scr, μmol L−1) | Blood urea nitrogen (BUN, mmol L−1) | Urine protein (uPro, mg) | Urine volume (UV, mL) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a The decoction was given orally to healthy SD rats, female, 160–200 g. CAM: Caulis Aristolochiae Manshuriensis; FA: Fructus Aristolochiae; RA: Radix Aristolochiae. | ||||||||
| CAM | Crude CAM | 6 g kg−1 d−1 | 8 weeks | 57.91 ± 11.81 | 7.58 ± 2.61 | 9.10 ± 1.70 | Xie and Zhang81 | |
| Longdanxiegan | 57.91 ± 11.81 | 6.07 ± 0.55 | 6.15 ± 2.07 | |||||
| Blank | 7 d | 30.85 ± 4.05 | 6.25 ± 1.25 (30d), 6.18 ± 1.10 (90d) | — | 10–15 | Liu et al.82 | ||
| Crude CAM | 3 g kg−1 d−1 | 65.06 ± 4.50 | — | 5–6 | ||||
| 0.5 g kg−1 d−1 | 31.20 ± 3.08 | 6.80 ± 1.56 (30d), 7.80 ± 1.60 (90d) | — | 10–15 | ||||
| Blank | 56.90 ± 1.70 | 5.86 ± 1.13 | 0.91 ± 0.34 | 6.01 ± 3.52 | Zhu et al.83 | |||
| Crude CAM | 6 g kg−1 d−1 | 3 d | 65.34 ± 0.25 | 10.21 ± 0.89 | 2.05 ± 0.51 | 8.28 ± 1.21 | ||
| 7 d | 78.35 ± 1.43 | 18.92 ± 1.13 | 5.28 ± 2.41 | 12.11 ± 2.42 | ||||
| 15 d | 94.56 ± 1.51 | 26.42 ± 1.49 | 6.92 ± 1.92 | 10.65 ± 2.52 | ||||
| CAM and liquorice (LE) | 6 g kg−1 d−1 (CAM) + 2 g kg−1 d−1 (LE) | 3 d | 69.42 ± 2.35 | 13.14 ± 1.32 | 3.29 ± 0.21 | 11.31 ± 1.02 | ||
| 7 d | 82.49 ± 2.31 | 21.92 ± 0.98 | 6.31 ± 1.99 | 14.11 ± 1.92 | ||||
| 15 d | 99.66 ± 2.32 | 29.32 ± 1.03 | 9.14 ± 2.02 | 13.64 ± 2.02 | ||||
| Blank | 7 d | 181.11 ± 23.88 | 16.33 ± 2.34 | 6.01 ± 1.29 | 11.50 ± 1.31 | Li et al.84 | ||
| Crude CAM | 12 g kg−1 d−1 | 244.38 ± 49.50 | 23.83 ± 3.71 | 9.68 ± 1.49 | 18.73 ± 4.82 | |||
| Daochi powder | 6 g kg−1 d−1 | 182.13 ± 14.11 | 16.75 ± 1.16 | 6.13 ± 1.21 | 10.56 ± 1.71 | |||
| 12 g kg−1 d−1 | 192.10 ± 15.80 | 19.38 ± 1.59 | 7.32 ± 1.74 | 13.96 ± 4.92 | ||||
| 24 g kg−1 d−1 | 232.63 ± 12.22 | 24.13 ± 4.25 | 9.29 ± 1.95 | 27.85 ± 7.32 | ||||
| FA | Crude FA | (34.1 ± 7.2) g kg−1 d−1 (LD50) | Yang et al.85 | |||||
| FA processed with honey | (62.6 ± 8.0) g kg−1 d−1 (LD50) | |||||||
| RA | Crude RA | 146.45 g kg−1 d−1 (LD50) | Jiang et al.86 | |||||
| Processed RA | 846.06 g kg−1 d−1 (LD50) | |||||||
3.3 Pressurized liquid extraction
Pressurized liquid extraction is a relatively new extraction method compared to the processing and compatibility methods.13,69 It can also be called accelerated solvent extraction, pressurized solvent extraction or enhanced solvent extraction. PLE is an environmental-friendly method which has emerged as an efficient way of increasing the automation, shortening the extraction time and reducing the amount of organic solvent.70In principal, the efficiency of PLE depends on the nature of the sample matrix, the target compounds to be extracted and their location within the matrix. The control step of PLE is diffusion, which can be subdivided into six steps, specifically: (1) rapid solvent entry; (2) desorption from the matrix active sites; (3) diffusion through swollen organic material; (4) solvation in the solvent at the matrix–solvent interface; (5) diffusion through the static solvent in the porous matrix; and (6) diffusion through the organic layer. Diffusion depends on the properties of the target compounds and the choice of solvent.71,72 PLE works at high temperature and high pressure to extract quickly with a few milliliters of water or organic solvent. The temperature applied for PLE should be higher than the boiling point and a sufficient pressure needs to be applied to maintain the solvent in the liquid state.13 Owing to the characteristics of dissolution, the analytes can be dissolved in the solvents which have a similar polarity. The dielectric constant is a measure of the polarity, and it can be changed markedly at higher pressure and temperature. Take water for example, the hydrogen-bonded lattice will be disrupted when the thermal motion increases apparently.72,73 The temperature needed to extract AAs is usually controlled between 100 to 120 °C and the corresponding pressure has no limitations. In this range, 25–40 mL methanol is selected as the solvent owing to its low polarity. It is worth noting that the raw herb should be ground by a microfine grinder before extraction, and the size of the particles selected must be less than 0.5 mm for a greater contact area with the solvent.13
As for the apparatus of PLE, the scheme for a typical unit and the basic extraction procedure are given in Fig. 6. The entire process contains several successive steps. Firstly, the sample is entered into the cell which is placed on the carousel and mixed with inert material. Secondly, the cell is rotated into position in the oven chamber by the carousel, transferred into it and automatically sealed at room temperature. Thirdly, the extraction process in the oven chamber will start after preheating and continue for a user-set static time. Finally, the solvent, which contains the extracted analytes, will be collected in a vial. After the whole process, the cell needs to be flushed and purged with an inert gas such as N2. Normally the total extraction time is 15–45 min and this can sometimes be extended if necessary.69,70 Ong et al. applied this extraction method to reduce the content of AA-I and AA-II in Radix Aristolochiae.13 The result showed it could achieve a higher amount of removal of AA-I compared to that of Soxhlet and ultrasonic assisted extraction,74 which was recorded as the standard method in the China pharmacopoeia (2015), on the premise of reduced solvent and time consumption. This technology still faces some challenges, because the study only reflected the removal of AA but did not mention the effective components, therefore preservation of the effective components under the extraction conditions of a high temperature and pressure requires further research and comprehensive consideration. Meanwhile, the extraction solvent used in the study was methanol. Considering its biological toxicity and environmental hazards, the extraction effect of other solvents such as water should be further studied.
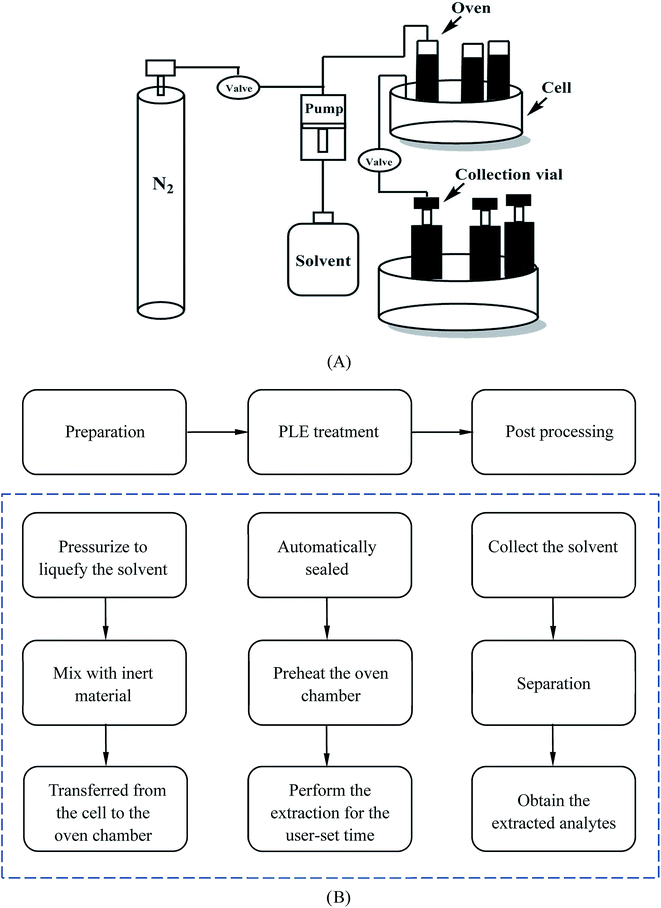 | ||
| Fig. 6 The structure and procedure of a PLE system (A), and a schematic description of the procedure (B). | ||
3.4 Supercritical fluid extraction
Supercritical fluid extraction has been widely used since the 1990s. As the name implies, this method uses supercritical fluid as the solvent. Low viscosity samples appear as a gas, high density as a liquid, and intermediate as diffusion between the gas and liquid phases, varying with the density. Many compounds can be used as supercritical solvents, such as water, benzene, toluene, ethane, ethanol, and so forth. The high density of these fluids gives them a high solvation power, whereas its high diffusion and low viscosity values provide the desired penetration power in the solid matrix.75 Among them, CO2 is the most commonly used owing to the fact that it is economical, eco-friendly, easily available and easy to remove after extraction. Additionally, CO2 is a non-oxidizing medium and its critical point (304 K, 7.4 Mpa) requires low temperature extraction conditions.75,76 Therefore, supercritical CO2 is an excellent alternative to chemical solvents and can be widely applied to extract the thermally unstable or easily oxidized compounds. In this aspect, SFE can be effective in protecting the active ingredients contained in herbal material such as AA-containing TCMs.76However, CO2 has a low polarity and thus cannot be effectively utilized for all tasks, because sometimes the target compounds may have a high polarity. According to the theory that liquids of a similar polarity can be easily solved in each other, raising the polarity to adapt to different kinds of target compounds is important.77 To receive a better extraction efficiency, sometimes some modifiers need to be added and these modifiers play a crucial role in SFE. To raise the polarity of CO2, at least 17 modifiers, such as water, ethanol, methanol, acetic acid, ethylene glycol, and so forth, have been studied. It was found that methanol, the most commonly used solvent, is an effective polar modifier when used in conjunction with CO2. By taking the environmental pollution into consideration, we can also use ethanol as a substitute even though its effect is not as good as methanol for plant materials. As for the AAs, methanol can achieve a good detoxication efficiency.77 In addition, as demonstrated in another study,78 Freon-22 (chlorodifluoromethane) has been proved to be useful for the extraction of carboxylic acids, which means it could be appropriate for the AA-containing TCMs owing to the structure containing a carboxylic acid group.
Supercritical fluid extraction is an efficient extraction method with good selectivity. However, there are many variables that need to be controlled in the extraction process. Mostly, parameters such as the temperature, pressure, modifiers, flow rate, sample moisture content and sample particle size can influence the extraction effects of SFE. Specifically, an appropriate temperature and pressure are critical because, to a great extent, they will determine the extraction yield and extract composition. As mentioned above, the modifiers usually play an important role in the extraction of polar compounds. Increasing the flow rate within a permissive extent can obtain a more homogeneous mixture of the solvent and material and a shorter extraction time. Meanwhile, sample preparation also needs to be seriously considered. A smaller particle size can increase the surface area of the sample exposed to the solvent and improve the extraction efficiency. However, a high moisture content will make the sample agglomerate and reduce the amount of contact between the solvent and sample. Moreover, moisture can be a barrier to the diffusion of the solvent in the sample, as well as the diffusion of the extractant outside the sample, therefore it is important to dry the raw material before extraction.79
The basic SFE instrumentation and its basic extraction procedure are shown in Fig. 7. A high pressure pump is used to deliver the extraction fluid to a high-pressure extraction cell. Before this, the modifier, which can be pumped in by an additional pump for the majority of the time, may be added to the fluid to change its polarity. The cell, usually constructed from stainless steel, can withstand a high pressure. By placing it into an oven, the cell can be maintained at a temperature above the critical temperature of the fluid. A restrictor is then used to maintain the desired extraction pressure. When the fluid has passed through the restrictor it can be simply depressurized and the analyte will be trapped in a collection device. The collection device can be a vial containing a solvent or a cartridge containing chromatographic packing material. For the whole system, a control center will be used to adjust the operation.69,80 Liang et al. ground 1 g of sample into 100–120 mesh size and pretreated it with 2 mL ethanol for 12 h.14 They found that the removal rate of AA-I and AA-II were respectively 65.2% and 59.1% for Fructus Aristolochiae and 81.3% and 81.2% for Caulis Aristolochiae Manshuriensis under the optimum conditions of a pressure of 194 bar, a temperature of 50 °C, an ethanol concentration of 0.2 mole fraction and a processing time of 4 h. However, the results did not mention the amount of remaining active ingredients and no toxicity studies were performed on the processed material. Therefore we cannot make a comprehensive assessment based on the one-sided data and analysis. At present, the application of the SFE method in TCM extraction is mainly limited to the extraction of effective components from single TCMs. However, for research on the detoxification of AA-containing TCMs, the goal is to remove AAs while preserving as many effective components as possible, therefore this was also a crucial problem for this technology. Furthermore, it also faced the challenges of the expensive process equipment, difficulties in cleaning and realizing industrial production, which need to be further studied and overcome.
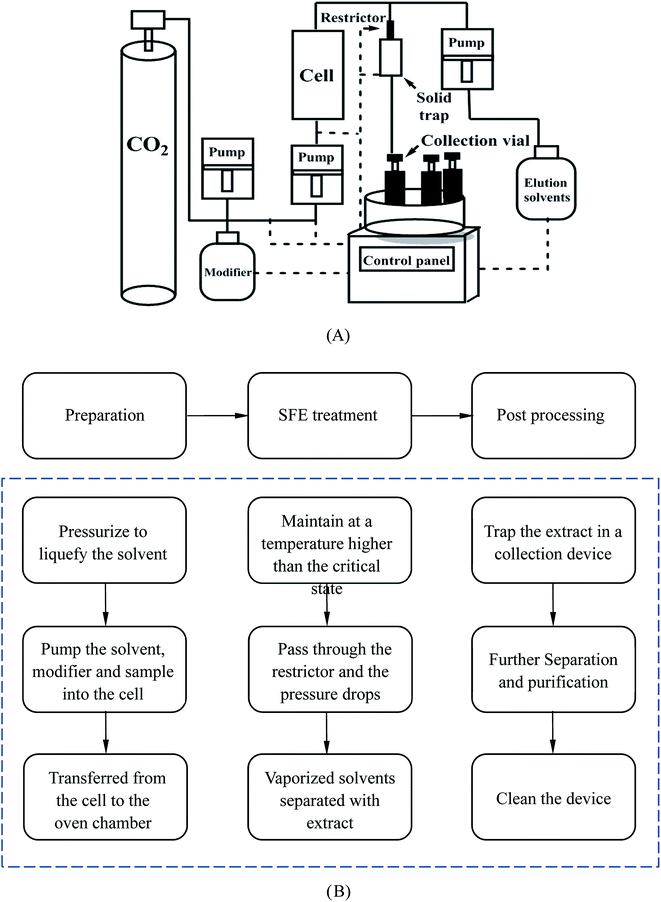 | ||
| Fig. 7 The structure and procedure of an SFE system (A), and a schematic description of the procedure (B). | ||
4 Conclusions and future trends
Recently, kidney disease from herbal medicines, also called aristolochic acid nephropathy, has resulted in AA-containing TCMs being largely boycotted by many countries. However, for thousands of years, AA-containing TCMs have been widely used to treat many diseases in clinical practice, such as sexually transmitted diseases, gastrointestinal complaints, eczema, and so forth. Many Chinese patent medicines are made from a variety of herbs, and toxic compounds such as AAs are still inevitably contained within. Therefore, it is important to study detoxication methods for AA-containing TCMs. This review has introduced and discussed different detoxication methods, including the processing, compatibility and new techniques of PLE and SFE, in detail. In fact, based on many experimental results, it has been found that the toxicity can actually be alleviated after the detoxication process. Thus, further improvements in detoxication techniques for AA-containing TCMs are realistic. However, there are still many challenges that need to be overcome in subsequent research.(1) In the current study, the available research on different detoxication techniques is not sufficient. Further attempts to find novel approaches are necessary, and they can help us to fully take into account all factors that may affect the extraction processing and finally find a method to give the best comprehensive evaluation.
(2) In the current research on the detoxification of AA-containing TCMs, many researchers only focus on the content of AA-I but overlook the other AAAs. Therefore, more bio-experiments are needed to fully understand the clinical effects of processed AA-containing TCMs.
(3) A comprehensive review of various detoxification methods has been provided, including the method of processing with NaHCO3 using non-toxic alkaline salts as an auxiliary agent and simultaneously utilizing the simple principle of acid–base neutralization, therefore it plays an important role in detoxification research for AAs. Future research should focus on improving the efficiency and further confirming the toxicity of the derivatives. As AAs have a strong accumulative effect in the human body, future research in the field of AA detoxification should further focus on improving the selectivity of detoxication. From this point of view, SFE is an ideal extraction method, and further optimization of the parameters, equipment structural design and clinical trials are needed to enable the products to be truly useful clinically and to allow industrial production.
Conflicts of interest
The authors affirm and confirm that there are no conflict of interest issues with regard to the content of this article.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21376150), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20100181120076) and the China Postdoctoral Science Foundation (No. 2013M530400, 2014T70871).References
- J. Michl, M. J. Ingrouille, M. S. Simmonds and M. Heinrich, Nat. Prod. Rep., 2014, 31, 676–693 RSC.
- A. W. T. Ng, S. L. Poon, M. N. Huang, J. Q. Lim, A. Boot, W. Yu, Y. Suzuki, S. Thangaraju, C. C. Y. Ng and P. Tan, et al., Sci. Transl. Med., 2017, 9, 1–12 Search PubMed.
- X. Wang, L. Zhang, Y. Gao and P. Zang, Chin. Tradit. Herb. Drugs, 2013, 4, 3242–3244 Search PubMed.
- J. Michl, G. C. Kite, S. Wanke, O. Zierau, G. Vollmer, C. Neinhuis, M. S. Simmonds and M. Heinrich, J. Nat. Prod., 2015, 79, 30–37 CrossRef PubMed.
- J. Holzbach, I. Nascimento and L. Lopes, J. Braz. Chem. Soc., 2017, 28, 2275–2279 CAS.
- P. Balachandran, F. Wei, R. C. Lin, I. A. Khan and D. S. Pasco, Kidney Int., 2005, 67, 1797–1805 CrossRef CAS PubMed.
- M. Heinrich, J. Chan, S. Wanke, C. Neihuis and S. J. M. Simmonds, J. Ethnopharmacol., 2009, 125, 108–144 CrossRef CAS PubMed.
- M. B. Al-Barham, H. I. Al-Jaber, M. A. Al-Qudah and Z. M. H. Abu, Nat. Prod. Res., 2017, 31, 245–252 CrossRef CAS PubMed.
- IARC Working Group, Plants containing aristolochic acid, Pharmaceuticals, France, 2012 Search PubMed.
- M. Zhou, Y. Hong, X. Lin, L. Shen and Y. Feng, J. Ethnopharmacol., 2017, 206, 363–375 CrossRef PubMed.
- S. Shibutani, H. Dong, N. Suzuki, S. Ueda, F. Miller and A. P. Grollman, Drug Metab. Dispos., 2007, 35, 1217–1222 CrossRef CAS PubMed.
- X. Wu, S. Wang, J. Lu, Y. Jing, M. Li, J. Cao, B. Bian and C. Hu, Chin. Med., 2018, 13, 2–13 CrossRef PubMed.
- E. S. Ong, S. O. Woo and Y. L. Yong, J. Chromatogr. A, 2000, 313, 57–64 CrossRef.
- Q. Liang, A. H. L. Chow, Y. Wang, H. H. Y. Tong and Y. Zheng, Sep. Purif. Technol., 2010, 72, 269–274 CrossRef CAS.
- L. V. Emma and M. Alistair, Aristolochic acid, Br. J. Pharmacol., 2016, 173, 1639–1652 CrossRef PubMed.
- J. Michl, O. Bello, G. C. Kite, M. S. J. Simmonds and M. Heinrich, Front. Pharmacol., 2017, 8, 1–9 Search PubMed.
- P. Zang, X. L. Wu, Y. G. Gao, H. Yang, Y. Zhao, L. Ma, X. H. Wang and L. X. Zhang, Chin. J. Appl. Chem., 2014, 31, 416–420 CAS.
- Y. Q. Xu, M. Y. Shang, Y. W. Ge, X. Wang and S. Q. Cai, China J. Chin. Mater. Med., 2010, 35, 2862–2865 CAS.
- H. Tian, X. Cheng and C. Wang, Chin. Tradit. Pat. Med., 2016, 38, 560–565 Search PubMed.
- Z. Liang, F. Xie, J. He, X. Chen and M. Wei, J. Guangdong Pharm. Univ., 2012, 6, 628–631 Search PubMed.
- Y. H. Hus, C. F. Lo, F. S. Liu and J. H. Lin, J. Food Drug Anal., 2009, 17, 274–281 Search PubMed.
- A. G. Hoechst, German patent, DE1186980-B, 1963.
- X. Zhang, H. Xiao, L. Wang, D. Liu, and Y. Qu, China Pat., CN106872247-A, 2015.
- Q. Fu, Y. Ge, P. Yu, H. Shu, C. Chang, and A. Zeng, China Pat., CN106770784-A, CN106770784-B, 2017.
- T. Zheng, and T. Yu, China Pat., CN106124461-A, CN106124461-B, 2016.
- G. Feng, and J. Liu, China Pat., CN109060754-A, 2018.
- W. Sun, T. Huang, X. Li, R. Zhao, and W. Xu, China Pat., CN108918460-A, 2018.
- S. Y. Chang, E. J. Weber, V. S. Sidorenko, A. Chapron, C. K. Yeung, C. Gao, Q. Mao, D. Shen, J. Wang and T. A. Rosenquist, et al., JCI Insight, 2017, 2, 1–14 CrossRef PubMed.
- E. L. Veale and A. Mathie, Aristolochic acid, Br. J. Pharmacol., 2016, 173, 1639–1652 CrossRef CAS PubMed.
- H. Zhou, J. Fu and Z. Cai, J. Chin. Mass Spectrom. Soc., 2017, 38, 30–36 CAS.
- L. P. McDaniel, E. R. Elander and X. Guo, Environ. Mol. Mutagen., 2012, 53, 358–368 CrossRef CAS PubMed.
- G. Z. Xing, X. M. Qi, M. Chen, Y. F. Wang, J. Yao, L. K. Gong, T. Nohmi, Y. Luan and J. Ren, Mutat. Res. Genet. Toxicol. Environ. Mutagen., 2012, 743, 52–58 CrossRef CAS PubMed.
- A. P. Grollman, S. Shibutani, M. Moriya, F. Miller, L. Wu, U. Moll, N. Suzuki, A. Fernandes, T. Rosenquist and Z. Medverec, et al., Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12129–12134 CrossRef CAS PubMed.
- M. Olivier, M. Hollstein and P. Hainaut, Cold Spring Harbor Perspect. Biol., 2010, 2, 1–17 Search PubMed.
- V. Martinek, F. Barta, P. Hodek, E. Frei, H. H. Schmeiser, V. M. Arit and M. Stiborova, Monatsh. Chem., 2017, 148, 1971–1981 CrossRef CAS PubMed.
- M. Stiborova, F. Barta, K. Levova, P. Hodek, H. H. Schmeiser, V. M. Arlt and V. Martinek, Int. J. Mol. Sci., 2015, 16, 27561–27575 CrossRef CAS PubMed.
- S. Liu, F. Li, Y. Li, W. Li, J. Xu and H. Du, J. Ethnopharmacol., 2017, 207, 237–250 CrossRef PubMed.
- S. L. Poon, M. N. Huang, Y. Choo, J. R. McPherson, W. Yu, H. L. Heng, A. Gan, S. S. Myint, E. Y. Siew and L. D. Ler, et al., Genome Med., 2015, 7, 1–10 CrossRef CAS PubMed.
- J. Singhuber, M. Zhu, S. Prinz and B. Kopp, J. Ethnopharmacol., 2009, 126, 18–30 CrossRef PubMed.
- J. Wu, Z. Zhang, Y. Lu, K. Sun and H. Li, Hubei Journal of Traditional Chinese Medicine, 2012, 14, 39–42 Search PubMed.
- R. K. Saini and Y. S. Keum, Food Chem., 2018, 240, 90–103 CrossRef CAS PubMed.
- Y. Liu, J. Jilin Agr. Univ., 2008, 1–50 CrossRef.
- Y. Zhang, R. Xiao, J. Huang, P. Wu, L. Lin and B. Xia, Chin. Pharm. J., 2017, 52, 1397–1402 CAS.
- H. Liu, M. Chen and K. Liu, Heilongjiang Anim. Sci. Vet. Med., 2015, 8, 92–93 Search PubMed.
- Z. Li, B. Yang, W. Yang, H. Chen, M. Yang, J. Yuan, Z. Yan and X. Luo, J. Chin. Med. Mater., 2013, 36, 538–540 CAS.
- B. Yang, M. Shi, Z. Li, W. Yang, H. Chen, Z. Yan, J. Yuan and X. Luo, Jiangxi Journal of Traditional Chinese Medicine, 2013, 44, 51–52 Search PubMed.
- C. Zhang, R. Chao and C. Zhang, J. Baotou Med. Colg., 2012, 28, 4–6 CAS.
- B. Yang, Z. Li, W. Yang, H. Chen and J. Yuan, Lishizhen Med. Mater. Med. Res., 2012, 23, 2553–2555 CAS.
- H. Lu and J. H. Pan, Chin. J. Inf. Tradit. Chin. Med., 2008, 15, 37–39 Search PubMed.
- X. Wang, L. K. Li, Y. F. Zhang, G. Z. Gong and Y. G. Zhao, China Med. Her., 2015, 12, 36–39 Search PubMed.
- H. Wang and J. Zhang, Chin. J. Integrated Tradit. West Med., 2005, 6, 200–203 Search PubMed.
- J. Chen and W. Xue, Guide of China Medicine, 2015, 13, 35–36 Search PubMed.
- X. Zhou, S. W. Seto, D. Chang, H. Kiat, V. Razmovski-Naumovski, K. Chan and A. Bensoussan, Front. Pharmacol., 2016, 7, 1–16 Search PubMed.
- Y. Fan, S. Man, H. Li, Y. Liu, Z. Liu and W. Gao, J. Pharm. Biomed. Anal., 2016, 120, 364–373 CrossRef CAS PubMed.
- L. L. Feng and A. P. Zhang, J. Chengde Med. College, 2007, 24, 389 Search PubMed.
- H. Y. Cui, J. N. Cui and Q. G. Wang, J. Liaoning Univ. Tradit. Chin. Med., 2009, 11, 41–42 Search PubMed.
- Y. J. Ding, C. X. Li, Y. L. Ding and Y. Y. Zhao, Chin. J. Clin. Pharmacol., 2004, 20, 4–6 Search PubMed.
- C. X. Li, Y. Li, W. Z. Wang and F. Ding, Chin. J. Basic Med. Tradit. Chin. Med., 2005, 11, 288–289 Search PubMed.
- Z. X. Zhang, Z. K. Huang, M. H. Zeng, Y. J. Zhang and W. F. Wu, Forum on Traditional Chinese Medicine, 2018, 33, 67–70 Search PubMed.
- G. Xu and M. Xie, Chin. J. Exp. Tradit. Med. Formulae, 2008, 14, 20–22 CAS.
- N. Zhang and M. Xie, China J. Chin. Mater. Med., 2007, 32, 619–622 Search PubMed.
- H. H. Zhao, Y. Q. Liu, N. Hou, X. R. Dou and P. Zhao, China J. Chin. Mater. Med., 2007, 32, 451–452 Search PubMed.
- Y. Zhang, Y. Liu and H. Zhao, Tianjin Pharmacy, 2005, 17, 3–6 Search PubMed.
- Y. K. Li, X. H. Hong and D. Zhang, Tradit. Chin. Drug Res. Clin. Pharmacol., 2010, 21, 254–256 CAS.
- Y. Wang and X. Deng, Chin. Tradit. Herb. Drugs, 2008, 39, 1805–1807 CAS.
- J. Zhu, China Practical Medicine, 2012, 7, 164 Search PubMed.
- Y. M. Ma, Y. H. Wang, M. Q. Song and B. W. Liu, Shanxi Journal of Traditional Chinese Medicine, 2015, 31, 57–58 Search PubMed.
- Y. M. Ma, R. H. Yan, Y. Y. Li, Y. H. Wang, Z. Y. Feng, Z. B. Nie and R. Zhou, Chin. Remedies Clin., 2010, 10, 248–250 Search PubMed.
- V. Camel, Analyst, 2001, 126, 1182–1193 RSC.
- A. M. Antonio and A. Trejo, Ind. Eng. Chem. Res., 2010, 49, 3342–3348 CrossRef.
- E. Bjorklund and T. Nilsson, Trends Anal. Chem., 2000, 19, 434–445 CrossRef CAS.
- A. Mustafa and C. Turner, Anal. Chim. Acta, 2011, 703, 8–18 CrossRef CAS PubMed.
- E. Puértolas, N. López, S. Condón, I. Álvarez and J. Raso, Trends Food Sci. Technol., 2010, 21, 247–255 CrossRef.
- S. R. Shirsath, S. H. Sonawane and P. R. Gogate, Chem. Eng. Process., 2012, 53, 10–23 CrossRef CAS.
- A. S. M. Bruna, G. P. Camila, B. N. Silmar, F. P. Francine and A. U. Marcelo, Sep. Sci. Technol., 2013, 48, 2741–2760 CrossRef.
- M. H. Zuknik, N. N. A. Nik and O. A. K. Mohd, J. Food Eng., 2012, 112, 253–262 CrossRef CAS.
- Q. Lang and C. Wai, Talanta, 2001, 53, 771–782 CrossRef CAS PubMed.
- M. Djas and M. Henczka, Sep. Purif. Technol., 2018, 201, 106–119 CrossRef CAS.
- F. Sahena, I. S. M. Zaidul, S. Jinap, A. A. Karim, K. A. Abbas, N. A. N. Norulaini and A. K. M. Omar, J. Food Eng., 2009, 95, 240–253 CrossRef CAS.
- I. J. Barnabas and J. R. Dean, Analyst, 1994, 119, 2381–2394 RSC.
- M. Xie and N. Zhang, J. Beijing Univ. Tradit. Chin. Med., 2007, 30, 674–677 Search PubMed.
- H. Liu, X. S. Chen, M. Z. Cui and C. Y. Li, Chin. J. Comp. Med., 2003, 13, 361–363 Search PubMed.
- S. Zhu, M. Liu, J. Liu, L. Chen, P. Wu and G. Wei, Journal of Hubei University of Medicine, 2002, 21, 270–272 Search PubMed.
- C. X. Li, Y. Li, W. Z. Wang and F. Ding, Chin. J. Basic Med. Tradit. Chin. Med., 2005, 11, 288–289 Search PubMed.
- B. Yang, M. Shi, Z. Li, W. Yang, H. Chen, Z. Yan, J. Yuan and X. Luo, Jiangxi Journal of Traditional Chinese Medicine, 2013, 44, 51–52 Search PubMed.
- X. Jiang, L. Li, W. H. Wang, J. H. Wang, H. M. Gao and Z. M. Wang, Chin. Remedies Clin., 2006, 6, 485–487 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |