Open Access Article
Open Access ArticleDegradation of a series of fluorinated acrylates and methacrylates initiated by OH radicals at different temperatures
P. Lugo Garcíaa,
C. B. Rivelaa,
R. G. Gibiliscob,
S. Salgadoc,
P. Wiesenb,
M. A. Teruel a and
M. B. Blanco
a and
M. B. Blanco *a
*a
aInstituto de Investigaciones en Fisicoquímica de Córdoba (INFIQC), Dpto. de Fisicoquímica, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, 5000 Córdoba, Argentina. E-mail: mblanco@fcq.unc.edu.ar
bInstitute for Atmospheric and Environmental Research, University of Wuppertal, DE-42097 Wuppertal, Germany
cDepartamento de Química Física, Facultad de Ciencias Químicas, Universidad de Castilla La Mancha, Avenida Camilo José Cela 10, 13071 Ciudad Real, Spain
First published on 27th January 2020
Abstract
Rate coefficients for the gas-phase reactions of OH radicals with a series of fluorinated acrylates and methacrylates: 2,2,2-trifluoroethylmethacrylate (k1), 1,1,1,3,3,3-hexafluoroisopropylacrylate (k2), 1,1,1,3,3,3-hexafluoroisopropylmethacrylate (k3), and 2,2,2-trifluoroethylacrylate (k4) have been measured for the first time as a function of temperature in the range 290–308 K. The kinetic data obtained were used to derive the following Arrhenius expressions (in units of cm3 per molecule per s): k1 = (2.13 ± 0.68) × 10−18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(4745 ± 206)/T], k2 = (8.72 ± 0.68) × 10−15
exp[(4745 ± 206)/T], k2 = (8.72 ± 0.68) × 10−15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(2166 ± 205)/T], k3 = (6.30 ± 0.51) × 10−17
exp[(2166 ± 205)/T], k3 = (6.30 ± 0.51) × 10−17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(3721 ± 153)/T] and k4 = (3.93 ± 0.43) × 10−16
exp[(3721 ± 153)/T] and k4 = (3.93 ± 0.43) × 10−16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(3140 ± 129)/T]. The experiments were performed at normal atmospheric pressure in synthetic air using a 1080 L photoreactor and coupled with FTIR analysis to monitor the decay of the substances of interest and the reference compounds. The obtained negative temperature dependencies are in agreement with a mechanism implying an initial addition of the OH radical to the double bond. Atmospheric implications are discussed with reference to the rate coefficients obtained as a function of the temperature.
exp[(3140 ± 129)/T]. The experiments were performed at normal atmospheric pressure in synthetic air using a 1080 L photoreactor and coupled with FTIR analysis to monitor the decay of the substances of interest and the reference compounds. The obtained negative temperature dependencies are in agreement with a mechanism implying an initial addition of the OH radical to the double bond. Atmospheric implications are discussed with reference to the rate coefficients obtained as a function of the temperature.
1 Introduction
Through international protocols, several countries have tried to join their efforts to combat air pollution. However, some alternative substances, despite their capability to reduce the man-made impact on the ozone layer, contribute to climate change because of their absorption properties acting as greenhouse gases.1–4 Because chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are important anthropogenic sources of active chlorine in the stratosphere, the Montreal Protocol and its subsequent amendments have banned the production of these compounds and many other substances decreasing stratospheric ozone. Accordingly, replacement compounds with similar physicochemical properties but less harmful to the environment have been developed and applied in industry.5–8These replacement compounds have a significantly greater reactivity in the troposphere due to the presence of hydrogen atoms or double bonds in the molecule which increased their oxidative photodegradation in the troposphere so that these species do not have the chance to reach in significant amounts the stratosphere, where shortwave UV radiation could lead fragments of the molecules that could react with stratospheric O3. Accordingly, to determinate the impact of these compounds on air quality, kinetic and mechanistic data are needed on their tropospheric degradation.9,10
Acrylates and methacrylates are unsaturated esters, which contain double bond in their molecule (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), a functional group (OH, COOH, etc.) and a highly reactive olefinic group and are used as intermediates in the manufacture of polymers and plastics.11 When these compounds are added to its structure fluorine atoms or CF3 substituents, some properties are improved, such as oil and water repellences, refractive index, dielectric constant and thermal resistance.12,13 Fluorinated acrylates and methacrylates are unsaturated esters that are used to develop a family of fluorinated compounds14–18 that have brought a greater interest in their application in various industries and research fields, such as 2,2,2-trifluoroethylmethacrylate (CH3CH2C(O)OCH2CF3) that has been used as a copolymer in stone monument coatings in Italy, since it prevents cyclization reactions.19 Another example is the use of these fluorinated unsaturated esters (UEF) in the area of medicine, with the application of relatively low doses of PFC emulsion (perfluorinated chemicals) to maintain tissue oxygenation, which means that the autologous blood (blood transfusion) can be preserved and reinfused in the patient towards the end of the surgery, or in the postoperative period, as necessary, allowing surgery to begin at a reduced cell volume, reducing cell loss during subsequent bleeding.20
C), a functional group (OH, COOH, etc.) and a highly reactive olefinic group and are used as intermediates in the manufacture of polymers and plastics.11 When these compounds are added to its structure fluorine atoms or CF3 substituents, some properties are improved, such as oil and water repellences, refractive index, dielectric constant and thermal resistance.12,13 Fluorinated acrylates and methacrylates are unsaturated esters that are used to develop a family of fluorinated compounds14–18 that have brought a greater interest in their application in various industries and research fields, such as 2,2,2-trifluoroethylmethacrylate (CH3CH2C(O)OCH2CF3) that has been used as a copolymer in stone monument coatings in Italy, since it prevents cyclization reactions.19 Another example is the use of these fluorinated unsaturated esters (UEF) in the area of medicine, with the application of relatively low doses of PFC emulsion (perfluorinated chemicals) to maintain tissue oxygenation, which means that the autologous blood (blood transfusion) can be preserved and reinfused in the patient towards the end of the surgery, or in the postoperative period, as necessary, allowing surgery to begin at a reduced cell volume, reducing cell loss during subsequent bleeding.20
The large-scale use of these unsaturated fluorinated esters could have a great environmental impact, among them we can mention climate change21 and although these have been used in several areas as mentioned earlier, only a limited number of studies have been carried out for their tropospheric degradation reactions when emitted into the atmosphere. Butt et al., 2009 (ref. 22) reported the rate coefficient of ambient temperature for OH-initiated oxidation of an unsaturated fluorinated acrylate (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)O(CH2)2C4F9) using a 140 L Pyrex reactor coupled to spectroscopy Fourier transformation infrared (FTIR) detection. In addition, Tovar and Teruel 2014,23 have studied the atmospheric degradation of the titled reactions at 298 K using the relative method by Gas Chromatography with Flame Ionization Detection (GC-FID).
CHC(O)O(CH2)2C4F9) using a 140 L Pyrex reactor coupled to spectroscopy Fourier transformation infrared (FTIR) detection. In addition, Tovar and Teruel 2014,23 have studied the atmospheric degradation of the titled reactions at 298 K using the relative method by Gas Chromatography with Flame Ionization Detection (GC-FID).
In addition, Rivela et al., 2018,24 reported on the degradation of 2,2,2-trifluoroethylmethacrylate, 1,1,1,3,3,3-hexafluoroisopropylacrylate, 1,1,1,3,3,3-hexafluoroisopropylmethacrylate and 2,2,2-trifluoroethylacrylate with Cl atoms at 298 K and 760 torr of air using an 80 L Teflon collapsible chamber trough the relative method by gas chromatography with flame ionization detection (GC-FID) and product identification studies were performed by these authors using the solid phase microextraction method (SPME), with derivatization of fiber products using o-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride, together with gas chromatography coupled by Mass Spectroscopy as detection system (GC-MS). Rivela et al., 2018 (ref. 24) have detected the formation of chloroacetone (CH3C(O)CH2Cl) and formaldehyde (HCOH) as products of the reaction of Cl atoms with 2,2,2-trifluoroethylmethacrylate and 1,1,1,3,3,3-hexafluoroisopropylmethacrylate, which could continue reacting with OH radicals or Cl atoms and contribute to the formation of ozone and secondary organic aerosols (SOA). Although these studies provide valuable information about the tropospheric degradation of these Unsaturated Fluorinated Esters (UFEs), there are limited studies of temperature dependences rate coefficients of fluorinated esters + OH reactions. In this sense, the objective of the present study was to extend the results obtained in our group and determine the temperature dependence of the reaction of OH radicals with 2,2,2-trifluoroethylmethacrylate (TFEM), 1,1,1,3,3,3-hexafluoroisopropylacrylate (HFIA), 1,1,1,3,3,3-hexafluoroisopropyl methacrylate (HFIM) and 2,2,2-trifluoroethylacrylate (TFEA) reactions in the temperature range 290–308 K and a pressure of 760 torr synthetic air in a 1080 L quartz reactor equipped with a long path FTIR system as follows:
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CF3 + OH → products, k1 C(CH3)C(O)OCH2CF3 + OH → products, k1
| (1) |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH(CF3)2 + OH → products, k2 CHC(O)OCH(CF3)2 + OH → products, k2
| (2) |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH(CF3)2 + OH → products, k3 C(CH3)C(O)OCH(CF3)2 + OH → products, k3
| (3) |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CF3 + OH → products, k4 CHC(O)OCH2CF3 + OH → products, k4
| (4) |
To the best of our knowledge, this work provides the first kinetic study of these reactions as a function of temperature. Furthermore, the lifetimes times of the esters studied have been calculated as a function of altitude in the troposphere.
Atmospheric lifetimes of the VOCs studied in this work, by their reactions with OH radicals, were calculated considering the experimental rate coefficients obtained.
2 Materials and methods
A detailed description of the reactor can be found elsewhere and only a brief general overview is given here.25All experiments were performed in a 1080 L quartz–glass reaction chamber in the temperature range (290–308) K and a total pressure of (760 ± 10) torr of synthetic air. The reactor chamber has two concentric tubes closed at the ends with aluminium flanges. The reactor is surrounded homogeneously distributed by two different types of fluorescent lamps. One lamp type is emitting light with a maximum at 360 nm (Philips TL 0.5 W) and the other type is emitting light with a maximum at 254 nm (Philips TUV 40 W). The reactor is equipped with a white type multiple reflection mirror system with an optical path length of 484.7 m. Due to the rather long absorption path length it is possible to perform experiments using concentrations of the species of interest which are comparable to the real atmosphere. The multiple reflection system is coupled with an FTIR spectrometer (Thermo Nicolet Nexus) equipped with an MCT detector (mercury–cadmium–telluride) cooled with liquid nitrogen (77 K). The FTIR spectrometer records infrared spectra from 700 to 4000 cm−1 with a resolution of 1 cm−1.
OH radicals were generated by the photolysis of hydrogen peroxide with the Philips TUV lamps:
| H2O2 + hν (λ = 254 nm) → 2OH | (5) |
Typical photolysis times ranged from 15 to 20 min. In the presence of OH radicals the Unsaturated Fluorinated Esters (UFEs) under investigation and the reference compounds are consumed by the following reactions:
| OH + UFE → products | (6) |
| OH + reference → products | (7) |
Provided that the reference compound and the UFE are lost only by reactions (6) and (7), it can be shown that:
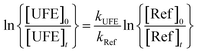 | (8) |
To verify this assumption, various tests were performed to assess the reaction of the reactants with hydrogen peroxide, photolysis, and wall losses. Reaction between hydrogen peroxide and the esters and reference compounds was found to be negligible over the time period of the experiments. Several tests were performed to evaluate the loss of reactants by reaction with hydrogen peroxide, photolysis and wall deposition, concluding that these processes were found to be insignificant for both the UFE and the reference compounds.
The following typical initial concentrations in ppmV (1 ppmV = 2.46 × 1013 molecule cm3 at 298 K and 760 torr total pressure) were used for the chemicals: (0.5–0.9) ppmV for TFEM; (0.3–0.6) ppmV for HFIA, (0.6–0.9) ppmV for HFIM; (0.4–0.7) ppmV for TFEA and (1.4–1.8) ppmV for propene. The concentration of H2O2 was typically around 13 ppm.
The following IR bands (in cm−1) were used for the analysis of the results: 1185 for TFEM; 1150 for HFIA, 1132.9 for HFIM; 1181.2 for TFEA and 911.8 for propene. The chemicals used in the experiments had the following purities as given by the manufacturer and were used as supplied: synthetic air (Messer, 99.999%), TFEM (Aldrich, 99%), HFIA (Aldrich, 99%), TFEA (Aldrich, 99%), HFIM (Aldrich, 99%), propene (Messer 99.95%) and H2O2 (Peroxid Chemie, 85% w/w).
3 Results and discussion
3.1 OH room temperature rate coefficient
Table 1 shows a comparison of the room temperature rate coefficients obtained in the present work with literature values obtained by our group at room temperature and atmospheric pressure using 80 L Teflon bag and GC-FID as detection system. The errors given for the kAlcohol/kReference ratios are the 2σ statistical errors from the linear regression fits to the plots. The measurements were made relative to the reaction of OH with isobutene and (E)-2-butene.| Unsaturated fluorinated ester | kOH (298 K) (cm3 per molecule per s) | ||
|---|---|---|---|
| Literature values | SARs calculation | This work | |
| a Ref. 24. | |||
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2(CF3) C(CH3)C(O)OCH2(CF3) |
(2.54 ± 0.12) × 10−11a | 1.82 × 10−11 | (1.70 ± 0.53) × 10−11 |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH(CF3)2 CHC(O)OCH(CF3)2 |
(1.41 ± 0.11) ×10−11a | 0.92 × 10−11 | (1.22 ± 0.26) × 10−11 |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH(CF3)2 C(CH3)C(O)OCH(CF3)2 |
(1.65 ± 0.14) ×10−11a | 1.81 × 10−11 | (1.67 ± 0.42) × 10−11 |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CF3 CHC(O)OCH2CF3 |
(1.25 ± 0.13) ×10−11a | 0.93 × 10−11 | (1.53 ± 0.37) × 10−11 |
The errors quoted for the individual kUFE values at each temperature are twice the standard deviation arising from the least-squares fit of the straight lines, to which a contribution has been added to take into account the recommended errors associated with the reference rate coefficient for reaction of OH with propene (reference reaction).
For comparison we have calculated the rate coefficients for these reactions using the SAR method of Kwok and Atkinson.26
Table 1 exhibits excellent agreement between the values from the present work and the previous experimental data for the reactions of 1,1,1,3,3,3-hexafluoroisopropylacrylate (k2), 1,1,1,3,3,3-hexafluoroisopropylmethacrylate and 2,2,2-trifluoroethylacrylate (k4) with OH radicals. For the reaction of 1 2,2,2-trifluoroethylmethacrylate with OH radicals, reaction (1), the rate coefficient of k1 = (1.70 ± 0.53) × 10−11 cm3 per molecule per s from the present study is in good agreement with the previously reported value of k1 = (2.54 ± 0.14) × 10−11 cm3 per molecule per s by Tovar and Teruel23 determined relative to the reactions of OH with 3-chloropropene, 2-methyl-3-buten-2-ol and diethyl ether in a 80 L Teflon chamber with GC-FID detection system. The small differences are within the experimental error of the used techniques.
The SAR method of Kwok and Atkinson26 gives values (in units of 10−11 cm3 per molecule per s) of 1.82, 0.92, 1.81 and 0.93 for the OH radical reactions with 2,2,2-trifluoroethylmethacrylate, 1,1,1,3,3,3-hexafluoroisopropylacrylate, 1,1,1,3,3,3-hexafluoroisopropylmethacrylate, and 2,2,2-trifluoroethylacrylate, respectively, which are in excellent agreement with the values from the present study (Table 1).
For methacrylate and acrylates esters it can be shown a slight decrease in the reaction rate coefficients with de degree of fluorination: kOH+CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CF3 = 1.70 × 10−11 cm3 per molecule per s > kOH+CH2
C(CH3)C(O)OCH2CF3 = 1.70 × 10−11 cm3 per molecule per s > kOH+CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2(CF3)2 = 1.67 × 10−11 cm3 per molecule per s and kOH+CH2
C(CH3)C(O)OCH2(CF3)2 = 1.67 × 10−11 cm3 per molecule per s and kOH+CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CF3 = 1.53 × 10−11 cm3 per molecule per s > kOH+CH2
CHC(O)OCH2CF3 = 1.53 × 10−11 cm3 per molecule per s > kOH+CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2(CF3)2 = 1.22 × 10−11 cm3 per molecule per s.
CHC(O)OCH2(CF3)2 = 1.22 × 10−11 cm3 per molecule per s.
Even though, this trend could be due to the electron withdrawing effect of F atoms, leading to a decrease in the rate coefficient value, the effect is not large because the CF3 group is distant from the double bond.
In addition, the rate coefficients presented in Table 1 for UEF + OH reactions can be compared with values reported by other authors for the OH rate coefficients with hydrogenated unsaturated esters, observing that rate coefficient values decrease when increase the number of F atoms in the molecule, such as: k(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CH3+OH) = (4.6 ± 0.6) × 10−11 cm3 per molecule per s27 > k(CH2
C(CH3)C(O)OCH2CH3+OH) = (4.6 ± 0.6) × 10−11 cm3 per molecule per s27 > k(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)O CH2CF3+OH) = (1.70 ± 0.53) × 10−11 cm3 per molecule per s (this work); k(CH2
C(CH3)C(O)O CH2CF3+OH) = (1.70 ± 0.53) × 10−11 cm3 per molecule per s (this work); k(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH(CH3)2+OH) = (2.3 ± 0.3) × 10−11(ref. 28) > k(CH2
C(CH3)C(O)OCH(CH3)2+OH) = (2.3 ± 0.3) × 10−11(ref. 28) > k(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH(CF3)2+OH) = (1.67 ± 0.42) × 10−11 cm3 per molecule per s (this work) and k(CH2
C(CH3)C(O)OCH(CF3)2+OH) = (1.67 ± 0.42) × 10−11 cm3 per molecule per s (this work) and k(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CH3+OH) = (1.7 ± 0.13) ×10−11 cm3 per molecule per s27 > k(CH2
CHC(O)OCH2CH3+OH) = (1.7 ± 0.13) ×10−11 cm3 per molecule per s27 > k(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CF3+OH) = (1.53 ± 0.37) × 10−11 cm3 per molecule per s−1 (this work). This can be explained due to the negative inductive effect of F in CF3 group reduces the partial negative charge, so the attack of OH radicals is less favored compared to hydrogenated unsaturated esters.
CHC(O)OCH2CF3+OH) = (1.53 ± 0.37) × 10−11 cm3 per molecule per s−1 (this work). This can be explained due to the negative inductive effect of F in CF3 group reduces the partial negative charge, so the attack of OH radicals is less favored compared to hydrogenated unsaturated esters.
3.2 Temperature dependence studies
The temperature dependence of the rate coefficients was determined in the temperature range 290–308 K and atmospheric pressure. Kinetic plots at 290, 298 and 308 K are presented in Fig. 1–4 for the reactions studied.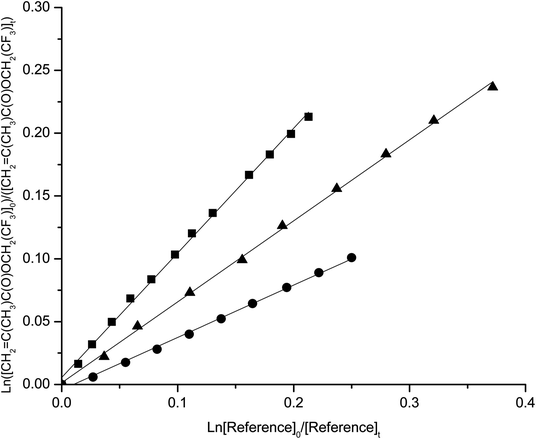 | ||
| Fig. 1 Plots of the kinetic data for the reaction of OH radicals with 2,2,2-trifluoroethyl methacrylate at 290 K (■) 298 K(▲) and 308 K (●) using propene as reference. | ||
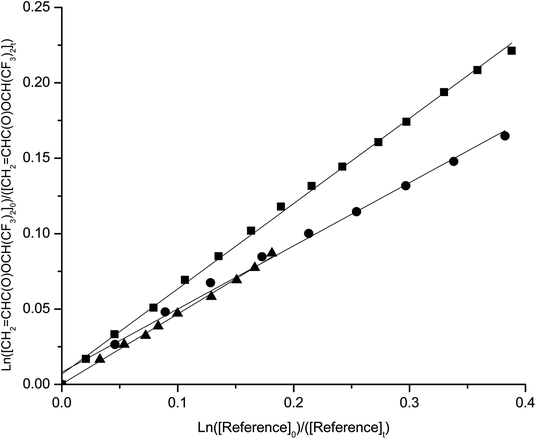 | ||
| Fig. 2 Plots of the kinetic data for the reaction of OH radicals with 1,1,1,3,3,3-hexafluoroisopropylacrylate at 290 K (■) 298 K(▲) and 308 K (●) using propene as reference. | ||
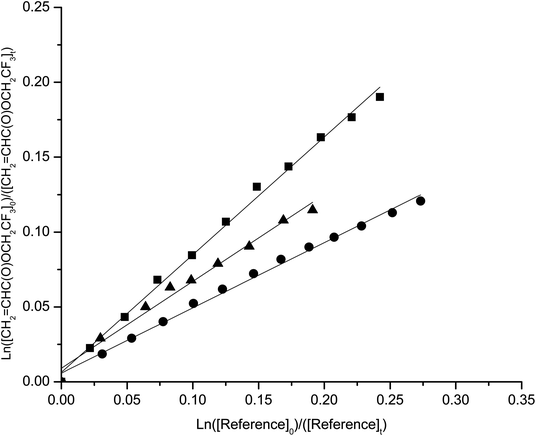 | ||
| Fig. 3 Plots of the kinetic data for the reaction of OH radicals with 2,2,2-trifluoroethylacrylate at 290 K (■) 298 K(▲) and 308 K (●) using propene as reference. | ||
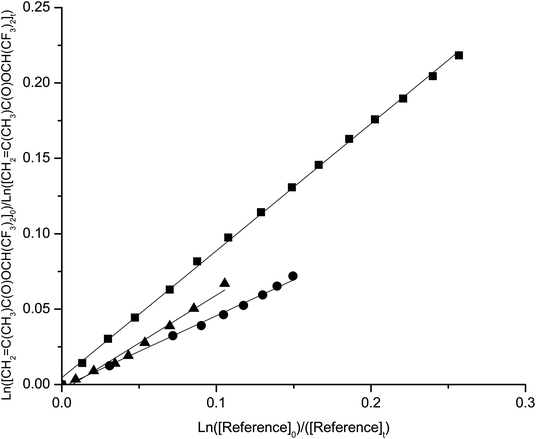 | ||
| Fig. 4 Plots of the kinetic data for the reaction of OH radicals with 1,1,1,3,3,3-hexafluoroisopropylmethacrylate at 290 K (■) 298 K(▲) and 308 K (●) using propene as reference. | ||
Table 2 lists the rate coefficient ratios obtained at each temperature from linear least-squares analyses of the kinetic data plotted according to eqn (8) using propene as reference compound using the following Arrhenius expression which has been derived between 290 and 467 K:29
| k = 4.85 × 10−12 [cm3 per molecule per s] e4.19 [±0.38 kJ mol−1]/RT |
| Unsaturated ester | T (K) | kOH × 10−11 (cm3 per molecule per s) | −Ea/R (K) | A (cm3 per molecule per s) |
|---|---|---|---|---|
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CF3 C(CH3)C(O)OCH2CF3 |
290 | (2.67 ± 0.57) | 4745 ± 206 | (2.13 ± 0.68) × 10−18 |
| 295 | (2.40 ± 0.49) | |||
| 298 | (1.70 ± 0.53) | |||
| 303 | (1.36 ± 0.29) | |||
| 308 | (1.04 ± 0.22) | |||
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH(CF3)2 CHC(O)OCH(CF3)2 |
290 | (1.56 ± 0.33) | 2166 ± 205 | (8.72 ± 0.68) × 10−15 |
| 295 | (1.34 ± 0.28) | |||
| 298 | (1.22 ± 0.26) | |||
| 303 | (1.07 ± 0.23) | |||
| 308 | (1.02 ± 0.23) | |||
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH(CF3)2 C(CH3)C(O)OCH(CF3)2 |
290 | (2.32 ± 0.48) | 3721 ± 153 | (6.30 ± 0.51) × 10−17 |
| 295 | (1.96 ± 0.39) | |||
| 298 | (1.67 ± 0.42) | |||
| 303 | (1.33 ± 0.30) | |||
| 308 | (1.12 ± 0.27) | |||
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CF3 CHC(O)OCH2CF3 |
290 | (1.99 ± 0.45) | 3140 ± 129 | (3.93 ± 0.43) × 10−16 |
| 295 | (1.63 ± 0.34) | |||
| 298 | (1.53 ± 0.37) | |||
| 303 | (1.27 ± 0.30) | |||
| 308 | (1.04 ± 0.23) |
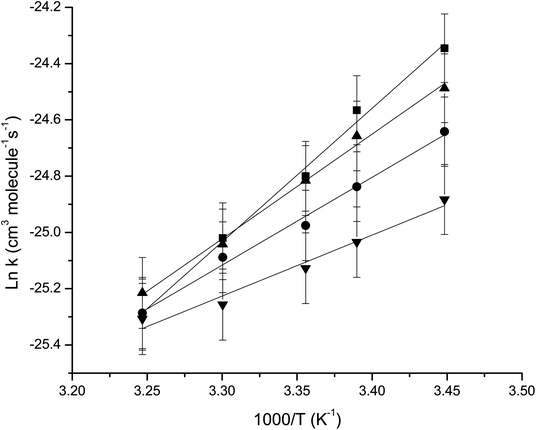
The absolute rate coefficients obtained for OH radical reactions with the UFEs derived from the rate ratios and the corresponding Arrhenius parameters obtained from the Arrhenius plots of the rate data are summarized in Fig. 5.
From Fig. 5 the following Arrhenius expressions were derived, which adequately describe the data (cm3 per molecule per s):
k1 = (2.13 ± 0.68) × 10−18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(4745 ± 206)/T] exp[(4745 ± 206)/T] |
k2 = (8.72 ± 0.68) × 10−15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(2166 ± 205)/T] exp[(2166 ± 205)/T] |
k3= (6.30 ± 0.51) × 10−17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(3721 ± 153)/T] exp[(3721 ± 153)/T] |
k4 = (3.93 ± 0.43) × 10−16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(3140 ± 129)/T] exp[(3140 ± 129)/T] |
The errors in the activation term and the pre-exponential factor are the 2σ random statistical errors from the least squares analyses of the data presented in Table 2 and plotted in Fig. 5.
At present, there are no previous kinetics studies of fluorinated acrylates and methacrylates at different reaction temperatures with OH radicals, accordingly this work represents the first determination of Arrhenius parameters for the titled reactions.
Table 3 shows the activation energies (Ea) for the OH radical reactions of different fluorinated and hydrogenated unsaturated esters. It can be seen that for the same temperature range around 287 and 314 K the values of Ea/R for fluorinated unsaturated esters are higher (values between 1029 and 4744 K−1) than the Ea/R for the reactions with unsaturated non fluorinated compounds (values between 413 and 1117 K−1). For example, Ea/R for CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CF3 is (4744 ± 206)K−1, which is higher than the Ea/R corresponding value for CH2
C(CH3)C(O)OCH2CF3 is (4744 ± 206)K−1, which is higher than the Ea/R corresponding value for CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH3, which is (921 ± 52) K−1. The only difference between the two molecules is that a hydrogen atom has been replaced by a –CF3 group.
C(CH3)C(O)OCH3, which is (921 ± 52) K−1. The only difference between the two molecules is that a hydrogen atom has been replaced by a –CF3 group.
| Unsaturated esters | T (K) | −Ea/R (K) |
|---|---|---|
| a This work.b Ref. 29.c Ref. 30.d Ref. 31. | ||
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CF3 C(CH3)C(O)OCH2CF3 |
290–308 | (4744 ± 206)a |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH(CF3)2 CHC(O)OCH(CF3)2 |
(2166 ± 205)a | |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH(CF3)2 C(CH3)C(O)OCH(CF3)2 |
(3721 ± 153)a | |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CF3 CHC(O)OCH2CF3 |
(3142 ± 129)a | |
CH3CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 |
287–313 | (1029 ± 82)b |
(E/Z)-CF3CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHF CHF |
(1765 ± 181)b | |
(CF3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 |
(3210 ± 159)b | |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH3 C(CH3)C(O)OCH3 |
287–313 | (921 ± 52)c |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)O(CH2)3CH3 C(CH3)C(O)O(CH2)3CH3 |
(413 ± 34)c | |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)O(CH2)3CH3 CHC(O)O(CH2)3CH3 |
(1117 ± 105)c | |
CH3C(O)OCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 |
(540 ± 49)c | |
CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH3 CHC(O)OCH3 |
(750 ± 159)d | |
(CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH3 CHC(O)OCH3 |
288–314 | (838 ± 182)d |
CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CH3 C(CH3)C(O)OCH2CH3 |
(522 ± 114)d | |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2C(O)OCH3 CHCH2C(O)OCH3 |
(834 ± 185)d | |
This can be explained due to the presence of F substituents in the molecule, which confers greater electronegativity than the C–H bond. This could make the molecule more stable against the attack of the OH radical and result in a greater amount of activation energy for the reaction to occur.
For all reactions studied the OH rate coefficients decrease with increasing temperature in the range 290–308 K. For bimolecular gas-phase reactions, negative activation energies can be explained if one assumes the formation of an intermediate complex. This allows the rate determining step to have a tight transition state with a small or negative potential energy relative to the reactants. As a result, at low temperature the average energy of the transition state will be less than that of the reactants and the activation energy will be negative.32,33
The presence of negative activation energy indicates also an electrophilic addition of OH to the double bond34 of the fluorinated unsaturated esters.
3.3 Tropospheric OH radical reaction loss rates for the fluorinated acrylates and methacrylates
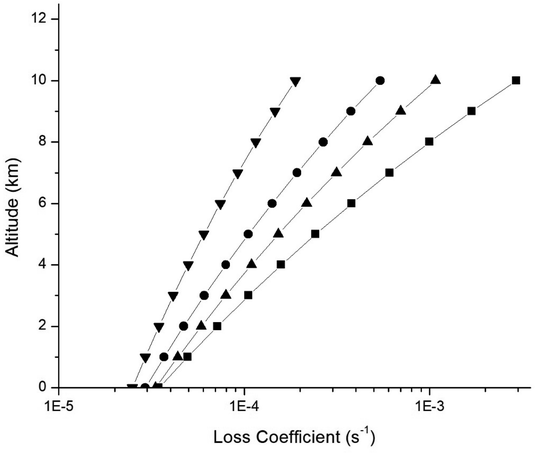
An estimation of the loss rate of the unsaturated fluorinated esters (UFE) by the reaction with OH radicals was performed using the temperature dependences of the reported rate coefficients from sea level to near the tropopause.Using the OH radical concentration of 2 × 106 molecule per cm3 (12 h daytime average)35 and the Arrhenius parameters reported in this work we have calculated the temperature profiles between 0 and 10 km, height of the troposphere, considering a lapse rate in the troposphere of −6.5 K km−1.36
Table 4 shows the OH removal rates for the four fluorinated compounds studied as a function of altitude in the troposphere assuming a temperature of 298.15 K at 0 km. The results shown in Table 4 are plotted in Fig. 6. The loss rates (in s−1) of the UFE at sea level (0 km) are 3.47 × 10−5, 2.49 × 10−5, 3.31 × 10−5 and 2.92 × 10−5 and near to the tropopause (∼10 km) around 2.93 × 10−3, 1.88 × 10−4, 1.08 × 10−3 and 5.41 × 10−4 for TFEM, HFIA, HFIM and TFEA, respectively.
| Altitude (km) | Temperature (K) | kUEE [OH] (s−1) | |||
|---|---|---|---|---|---|
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH2CF3 C(CH3)C(O)OCH2CF3 |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH(CF3)2 CHC(O)OCH(CF3)2 |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)C(O)OCH(CF3)2 C(CH3)C(O)OCH(CF3)2 |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)OCH2CF3 CHC(O)OCH2CF3 |
||
| 0 | 298.15 | 3.47 × 10−5 | 2.49 × 10−5 | 3.31 × 10−5 | 2.92 × 10−5 |
| 1 | 291.65 | 4.94 × 10−5 | 2.92 × 10−5 | 4.38 × 10−5 | 3.69 × 10−5 |
| 2 | 285.15 | 7.17 × 10−5 | 3.46 × 10−5 | 5.86 × 10−5 | 4.71 × 10−5 |
| 3 | 278.65 | 1.05 × 10−4 | 4.14 × 10−5 | 7.95 × 10−5 | 6.08 × 10−5 |
| 4 | 272.15 | 1.58 × 10−4 | 4.98 × 10−5 | 1.09 × 10−4 | 7.95 × 10−5 |
| 5 | 265.65 | 2.43 × 10−4 | 6.05 × 10−5 | 1.52 × 10−4 | 1.05 × 10−4 |
| 6 | 259.15 | 3.80 × 10−4 | 7.43 × 10−5 | 2.17 × 10−4 | 1.41 × 10−4 |
| 7 | 252.65 | 6.09 × 10−4 | 9.21 × 10−5 | 3.14 × 10−4 | 1.92 × 10−4 |
| 8 | 246.15 | 1.00 × 10−3 | 1.16 × 10−4 | 4.63 × 10−4 | 2.67 × 10−4 |
| 9 | 239.65 | 1.69 ×10−3 | 1.46 × 10−4 | 6.98 × 10−4 | 3.76 × 10−4 |
| 10 | 233.15 | 2.93 ×10−3 | 1.88 × 10−4 | 1.08 × 10−3 | 5.41 × 10−4 |
These values were calculated considering that at a given temperature corresponding to a given altitude in the troposphere, then the rate of loss of the unsaturated fluorinated esters is the product between the OH rate coefficient (at this temperature) and [OH] at this altitude.
The loss rate of 2,2,2-trifluoroethylmethacrylate is the largest and the loss rate of 1,1,1,3,3,3-hexafluoroisopropylacrylate is the smallest at all altitudes while the other two UFEs have intermediate values, as expected taking into account the larger and lower rate coefficients kOH (T) and Ea/R for 2,2,2-trifluoroethylmethacrylate and 1,1,1,3,3,3-hexafluoroisopropylacrylate, respectively.
3.4 Atmospheric implications
Tropospheric lifetimes at 298 K were calculated by Rivela et al. 2018 (ref. 37) for the reactions of the fluorinated unsaturated esters, studied here, using the following expression:| τx = 1/kx[X] | (9) |
They reported values were 5, 8, 10 and 11 hours for the OH degradation of 2,2,2-trifluoroethylmethacrylate, 1,1,1,3,3,3-hexafluoroisopropylmethacrylate, 1,1,1,3,3,3-hexafluoroisopropylacrylate and 2,2,2-trifluoroethylacrylate, respectively.23
For chlorine-initiated degradation the lifetimes are 8 days for 1,1,1,3,3,3-hexafluoroisopropylacrylate and 5 days for the other UFE studied.39
Thus, the main tropospheric chemical removal of these unsaturated fluorinated esters seems to be the reaction of OH radicals. The relatively short atmospheric lifetimes indicate that these unsaturated oxygenated compounds will be oxidized rapidly by reaction with OH radicals near their anthropogenic origin where the oxidation can contribute to ozone and photooxidant formation in these areas.
The main products formed in these reactions are expected to be esters, fluorinated pyruvates and glyoxylates27,40 that, after subsequent reactions with OH radicals, could form fluorinated carboxylic acids which in general are ubiquitous contaminants of the hydrosphere.41–43
4 Conclusions
The reaction of OH radicals with the unsaturated fluorinated esters (UFE) 2,2,2-trifluoroethyl methacrylate, 1,1,1,3,3,3-hexafluoroisopropylacrylate, 1,1,1,3,3,3-hexafluoroisopropylmethacrylate, and 2,2,2-trifluoroethylacrylate have been studied for the first time as a function of the temperature.The observed negative temperature dependence of these reactions can be explained by assuming that the lifetime of the excited bimolecular complex, formed between the OH radical and the UFE, with respect to decomposition back to the reactants decreases as the temperature increases. Therefore, the probability of the excited adduct being stabilized by collision with a third body decreases with increasing temperature.
It has been observed that the estimated lifetimes of these UFE studied with respect to reaction with OH decrease with altitude (from sea level to near the tropopause).
The temperature dependent rate coefficients determined in this study could help to improve master chemicals mechanism models if the temperature variation in the reactions are considered.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to acknowledge EUROCHAMP-2020, the Deutsche Forschungsgemeinschaft (DFG), Germany as well as FONCyT, CONICET and SECyT UNC, Argentina for financial support P. G. L wishes to acknowledge CONICET for a doctoral fellowship and financial support. R. G. G. and M. B. B. wish to acknowledge the Alexander von Humboldt Foundation for financial support.References
- M. Tolba, T. M. Millan and I. Rummel-Bulska, Montreal protocol on substances that deplete the ozone layer, US Government Printing Office, Washington, DC, 1987, vol. 26, pp. 128–136 Search PubMed.
- T. Sandler, Oxf. Econ. Pap., 2017, 69(2), 345–364 Search PubMed.
- S. A. Barrett, Oxf. Econ. Pap., 1994, 46, 878–894 CrossRef.
- K. Pittel, D. Rübbelke and M. Altemeyer-Bartscher, Handbook of Climate Change Mitigation, Springer, New York, NY, 2012 Search PubMed.
- United Nations Environment Program (UNEP), Handbook for the International Treaties for the Protection of the Ozone Layer, UNEP, Nairobi, Kenya, 6th edn, 2003 Search PubMed.
- G. Hayman and R. D. Derwent, Environ. Sci. Technol., 1997, 31, 327–336 CrossRef CAS.
- S. A. Montzka, G. S. Dutton, P. Yu, E. Ray, R. W. Portmann, J. S. Daniel and J. D. Nance, Nature, 2018, 557, 413–417 CrossRef CAS PubMed.
- A. Butle, J. S. Daniel, R. W. Portmann, A. Ravishankara, P. J. Young, D. W. Fahey and K. H. Rosenlof, Environ. Res. Lett., 2016, 11, 064017 CrossRef.
- A. Mellouki, G. L. Bras and H. Sidebottom, Chem. Rev., 2003, 103, 5077–5096 CrossRef CAS PubMed.
- J. Calvert, A. Mellouki, J. Orlando, M. Pilling and T. Wallington, The Mechanisms of Atmospheric Oxidation of Oxygenates, Oxford, New York, 2011 Search PubMed.
- S. Yang, J. G. Wang, K. Ogino, S. Valiyaveettil and C. K. Ober, Chem. Mater., 2000, 12, 33–40 CrossRef CAS.
- J. R. Lee, F. L. Jin, S. J. Park and J. M. Park, Surf. Coat. Technol., 2004, 180, 650–654 CrossRef.
- L. V. Ravanstein, W. Ming, R. D. V. D. Grampel, R. V. D. Linde, G. D. White, T. Loontjens, P. C. Thüne and J. W. Niemantsverdriet, Macromolecules, 2004, 37, 408–413 CrossRef.
- A. Vitale, R. Bongiovanni and B. Amenduri, Chem. Rev., 2015, 115, 8835–8866 CrossRef CAS PubMed.
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. de Voogt, A. A. Jensen, K. Kannan, S. A. Mabury and S. P. J. van Leeuwen, Integr. Environ. Assess. Manage., 2011, 7(4), 513–541 CrossRef CAS PubMed.
- F. Boschet, G. Kostov, B. Ameduri, T. Yoshida and K. Kosuke, J. Polym. Sci., 2010, 48, 1029–1037 CrossRef CAS.
- S. Srivastava, Des. Monomers Polym., 2009, 12, 1–18 CrossRef CAS.
- N. M. L. Hansen, M. Gerstenberg, D. M. Haddleton and S. Hvilsted, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 8097–8111 CrossRef CAS.
- F. Boschet, G. Kostov, B. Ameduri, T. Yoshida and K. Kosuke, J. Polym. Sci., 2010, 48, 1029–1037 CrossRef CAS.
- P. E. Keipert, Artificial cells, blood substitutes, and immobilization biotechnology, 1995, vol. 23, pp. 381–394 Search PubMed.
- A. McCulloch, J. Fluorine Chem., 2003, 123, 21–29 CrossRef CAS.
- C. M. Butt, C. J. Young, S. A. Mabury, M. D. Hurley and T. Wallington, J. Phys. Chem. A, 2009, 113(13), 3155–3161 CrossRef CAS PubMed.
- C. M. Tovar and M. A. Teruel, Atmos. Environ., 2014, 94, 489–495 CrossRef CAS.
- C. B. Rivela, C. M. Tovar, M. A. Teruel, I. Barnes, P. Wiesen and M. B. Blanco, Chem. Phys. Lett., 2019, 714, 190–196 CrossRef CAS.
- I. Barnes, K. H. Becker and N. Mihalopoulos, J. Atmos. Chem., 1994, 18, 267–289 CrossRef CAS.
- E. S. C. Kwok and R. Atkinson, Atmos. Environ., 1995, 29, 1685–1695 CrossRef CAS.
- M. B. Blanco, R. Tacconne, S. Lane and M. A. Teruel, Chem. Phys. Lett., 2006, 429, 389–394 CrossRef CAS.
- M. B. Blanco, I. Bejan, I. Barnes, P. Wiesen and M. A. Teruel, J. Phys. Chem. A, 2009, 113, 5958–5965 CrossRef CAS PubMed.
- R. Atkinson, Chem. Rev., 1986, 86, 169–201 CrossRef.
- J. P. Colomer, M. B. Blanco, A. B. Peñeñory, I. Barnes, P. Wiesen and M. A. Teruel, RSC Adv., 2016, 6, 53723–53729 RSC.
- T. Yamada, A. El-Sinawi, M. Siraj, P. H. Taylor, J. Peng, X. Hu and P. Marshall, J. Phys. Chem. A, 2001, 105, 7588–7597 CrossRef CAS.
- Z. Safaei, A. Shiroudi, E. Zahedi and M. Sillanpää, Phys. Chem. Chem. Phys., 2019, 21, 8445–8456 RSC.
- M. Mozurkewich and S. W. Benson, J. Phys. Chem., 1984, 88, 6429–6435 CrossRef CAS.
- S. Dhanya, K. K. Pushpa and P. D. Naik, Curr. Sci., 2012, 102, 452–459 CAS.
- R. Hein, P. J. Crutzen and M. Heimann, Global Biogeochem. Cycles, 1997, 11, 43–76 CrossRef CAS.
- M. Beychok, 2013, http://www.eoearth.org/view/article/170859.
- C. B. Rivela, M. B. Blanco and M. A. Teruel, Atmos. Environ., 2018, 178, 206–217 CrossRef CAS.
- O. W. Wingenter, M. K. Kubo, N. J. Blake, T. W. Smith, D. R. Blake and F. S. Rowland, J. Geophys. Res.: Atmos., 1996, 101, 4331–4340 CrossRef CAS.
- M. B. Blanco, I. Bejan, I. Barnes, P. Wiesen and M. A. Teruel, Environ. Sci. Technol., 2010, 44, 7031–7036 CrossRef CAS PubMed.
- H. Frank, D. Renschen, A. Klein and H. Scholl, J. High Resolut. Chromatogr., 1995, 18, 83–88 CrossRef CAS.
- D. Zehavi and J. N. Seiber, Anal. Chem., 1996, 68, 3450–3459 CrossRef CAS.
- D. G. Richey, C. T. Driscoll and G. E. Likens, Environ. Sci. Technol., 1997, 31, 1723–1727 CrossRef CAS.
- C. E. Wujcik, D. Zehavi and J. N. Seiber, Chemosphere, 1998, 36, 1233–1245 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |