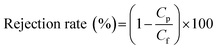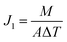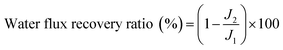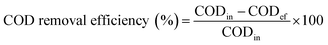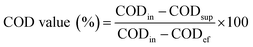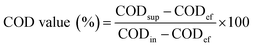Open Access Article
Open Access ArticleThe preparation of a modified PVDF hollow fiber membrane by coating with multiwalled carbon nanotubes for high antifouling performance†
MengJing Cao a,
Yan Zhang*a,
BoKang Zhangc,
ZiQi Liub,
XiangShan Mab and
ChangMing Chena
a,
Yan Zhang*a,
BoKang Zhangc,
ZiQi Liub,
XiangShan Mab and
ChangMing Chena
aKey Laboratory of Beijing for Water Quality Science and Water Environment Recovery Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: yzhang@bjut.edu.cn
bBeijing General Municipal Engineering Design & Research Institude Co.,Ltd., Beijing, 100082, Beijing, China
cGao'antun Reclaimed Water Plant of Beijing Drainage Group Co.,Ltd. Beijing, 100024, Bejing, China
First published on 8th January 2020
Abstract
In this study, an outer surface modified polyvinylidene fluoride (PVDF) hollow fiber membrane (HF-PVDF-CNT) was prepared by coating with dopamine (PD) and multiwalled carbon nanotubes (CNTs), to solve the problems of the instability of pure CNT mats fabricated by filter coating methods and membrane fouling in wastewater treatment. The modified membrane was assessed and characterized by various methods, including studies of its top surface and cross-sectional morphology, wettability, functional groups and electrical conductivity. The CNT material stability was evaluated during backwashing. The antifouling and filtering abilities of the unmodified and modified membranes were tested by monitoring the change in TMP and the rejection performance for different contaminants during filtration in bovine serum albumin solution (BSA), sodium alginate solution (SA) and humic acid solution (HA). Furthermore, HF-PVDF-CNT and electro-assisted HF-PVDF-CNT membranes were employed as the basic separation units in an anaerobic membrane bioreactor (AnMBR) system and an anaerobic electrochemical membrane bioreactor (AnEMBR) system, respectively. Characterization of the HF-PVDF-CNT membrane indicated that the CNT mats exhibited good stability, electrical conductivity and wettability. In filtration experiments using BSA, SA and HA solutions, the HF-PVDF-CNT membrane showed an obvious improvement compared with the HF-PVDF membrane in antifouling performance. During its application in the AnMBR and AnEMBR systems, the electro-assisted HF-PVDF-CNT membrane had greater effects than the HF-PVDF-CNT membrane on reducing fouling.
1. Introduction
An anaerobic membrane bioreactor (AnMBR) is a technology which successfully combines anaerobic biological processes and membrane technology to achieve the separation of hydraulic retention time and sludge age. Compared with other sewage treatment processes, AnMBR technology offers advantages, such as higher effluent quality, less excess sludge formation, reduced space requirements and the generation of value-added products. However, the important issue of membrane fouling limits the practical application and development of AnMBR systems in wastewater treatment, while also being a key current problem in environmental science and engineering.1The main factors causing membrane fouling are the adsorption and accumulation of extracellular polymeric substances (EPS)2 and soluble microbial products (SMP)3 on the membrane surfaces, resulting in an increase in sludge viscosity and membrane filtration resistance. The major components of EPS and SMP are proteins, polysaccharides and humic acid. The properties of the membrane materials are a main factor affecting the fouling of membranes. There are many distinct differences in membrane fouling resistance capabilities because of differences in physical and chemical membrane properties, such as membrane materials, porosity, hydrophilicity and surface roughness. This suggests that changes in membrane properties are an effective way to mitigate the fouling of membranes.
By far, polyvinylidene fluoride hollow fiber membranes (HF-PVDF) have been the most widely used due to their stable chemical properties, low cost and outstanding antifouling ability.4,6 However, the hydrophobic surfaces of the HF-PVDF membrane tend to be susceptible to membrane fouling.7 Therefore, hydrophilic groups are used to enhance the wettability of HF-PVDF membranes to reduce the tendency for irreversible pollution and increase the membrane service life.8 Hydrophilic modification of the HF-PVDF membrane is a feasible method to reduce membrane fouling.
Carbon nanotubes (CNTs) have also been considered for removing chemicals and microbes from water owing to their high mechanical strength, high hydrophilic characteristics, large specific surface area and encouraging electrical conductivity.9–11 If CNTs and HF-PVDF membrane could be combined, the original hydrophobic HF-PVDF membrane could be turned into a hydrophilic and conductive membrane. Both characteristics are helpful for reducing the adhesion of pollutants on the membrane surfaces. On the one hand, due to the interconnected CNTs, as-constructed porous and hydrophilic membranes with a mesh-like structure are usually beneficial for water-transport.1 A high flux may be obtained even at a low operating pressure because of these properties. On the other hand, anaerobic electrochemical membrane bioreactor systems (AnEMBR) are the best certified way to mitigate fouling resistance due to the conductivity of the membranes. A negative voltage is applied to enhance membrane separation performance in AnEMBR systems. Higher energy gas yields and larger electrostatic repulsion at the membrane surface protect against colloids scaling on the membrane surface in AnEMBR systems.9,12–14 Thus, a combination of CNTs and HF-PVDF membranes to fabricate polymer composite membranes offers promising opportunities to relieve membrane fouling.
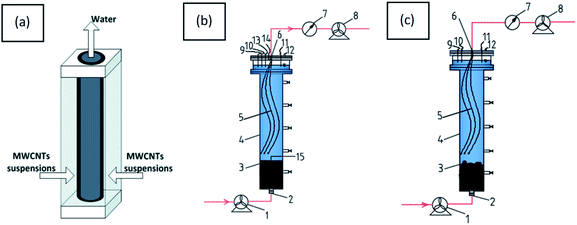
Recently, filtration coating as a form of direct loading to fabricate CNT polymer composite membranes has attracted a lot of research attention.15,16 Apart from the filter coating method, in situ polymerization17 and self-assembly18 are other preparation methods for CNT polymer composite membranes. Compared with the above methods, CNT mats prepared by the filter coating method take full advantage of CNTs at the membrane/water surface because the CNTs are not impregnated in the membrane polymer matrix.16 Therefore, the filter coating method was employed to modify the HF-PVDF membrane in the study. Unfortunately, CNT mats exhibit poor stability and easily detach from surfaces in real-world membrane systems, as shown for the outer surface of HF-PVDF and lamellar membranes.19 Therefore, how to effectively improve adhesion between the support layer and CNT mats in order to avoid CNTs shedding due to flow shear force, is an important issue in surface modification.
Dopamine (PD) can be easily integrated onto the surface of organic materials. Organic membrane surfaces modified by PD retain their original structures and exhibit good hydrophilicity.20,21 Beyond that, PD can be adhered firmly onto CNTs surfaces without ruining the sidewalls and generating harmful waste in mild environments22 and the introduction of a PD coating can greatly enhance the bioactivity and promote the dispersibility of CNTs.23 Based on this information, PD coated CNTs provide potential applications in organic membrane modification. Zhu et al. found that dispersions of PD and single-walled CNTs can be used to produce strongly hydrophilic and independent flat ultrathin membranes by vacuum-filtration for rejection desalination.24 However, it is very difficult to prepare a HF-PVDF membrane coated with CNTs by vacuum-filtration. Interestingly, functionalized CNTs can directly decorate onto the PVDF membrane surfaces by dopamine copolymerization, and the modified membrane shows great promise for applications in oil/water emulsion separation, as reported by Yang et al.5 But few applications to membrane fouling in wastewater have been reported.
In this study, a new and simple method to generate backwashable CNT mats on the outer surface of HF-PVDF membranes (HF-PVDF-CNT) is described. The aim is to overcome the shortcomings of the detachment of CNTs from the outer surface of the HF-PVDF membrane by filtration coating and the membrane fouling problem in wastewater treatment. The characteristics of the HF-PVDF-CNT membrane were studied by scanning electron microscopy (SEM), infrared spectroscopy (FTIR), and static contact angle and conductivity tests. The stability of the CNT mats was confirmed by UV-vis analysis during backwashing. The antifouling performance of unmodified and modified membranes was assessed via filtration in a bovine serum albumin solution (BSA), sodium alginate solution (SA), and humic acid solution (HA). The HF-PVDF-CNT and electro-assisted HF-PVDF-CNT membranes were then applied in AnMBR and AnEMBR systems to analyze water quality and antifouling properties.
2. Materials and methods
2.1 Base materials
2.2 Experimental methods
2.3 Measuring methods
where Cp and Cf are the pollutant concentrations in the permeate and feed, respectively.
The membrane water flux was calculated from the following equation:31
The flux recovery ratio was calculated by means of the following expression:32
Soluble chemical oxygen demand (SCOD) and chemical oxygen demand (COD) concentrations were determined by standard methods.33 COD removal efficiency, the contribution of biological removal rate to the COD value and the contribution of membrane separation rate to the COD value were calculated as follows:
contribution of membrane separation rate to the
where CODin, CODef, CODsup are the COD concentrations of influent, effluent and supernatant.
The transmembrane pressure (TMP) of the membrane modules was monitored with a pressure transducer (JYB-3151, Beijing, China) to explore the antifouling performance. UV absorbance measurements at 254 nm (UV254) and total organic carbon (TOC) were applied to serve as simple and reliable surrogate parameters to monitor the removal of HA. So UV-vis analysis was employed to analyze the concentration of HA. The protein content was estimated by the Coomassie bright blue method.34 The polysaccharide content in SA solutions was determined via the sulfuric acid–phenol method.35
3. Results and discussion
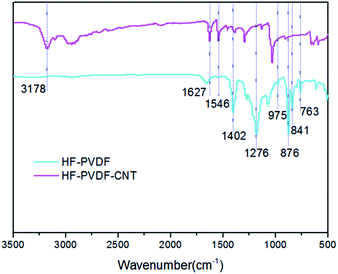
3.1 Characteristics of HF-PVDF and HF-PVDF-CNT membranes
| Sample | J (L m−2 h−1) | ε (%) |
|---|---|---|
| HF-PVDF | 5670 ± 15 | 34.87 ± 5 |
| HF-PVDF-CNT | 637 ± 20 | 47.51 ± 4 |
3.2 Stability analysis of CNT mats towards backwashing
The stability of CNT mats of the HF-PVDF-CNT membrane before and after backwashing was tested by UV-visible analysis. During the whole backwashing process, there was only 0.20 mg of CNTs detected in the backwashing water, accounting for 0.54% of the total load of CNTs (see Table S4†). This may be because the greatest stress experienced by the CNT mats loaded on the outer surface of membranes was the tensile hoop stress, when trying to expand the materials during backwashing.40 It may be speculated that the dopamine induced firm adhesion between the membrane surface and CNTs or among CNTs. The adhesion stress was more effective than the tensile cyclic stress, in preventing CNTs from shedding. However, the mechanical properties of the HF-PVDF-CNT membrane were poor. Slight stretching or rotation of the membrane resulted in severe shedding of CNTs from the CNT mats on the membrane surface.3.3 Anti-fouling ability of the HF-PVDF-CNT membrane
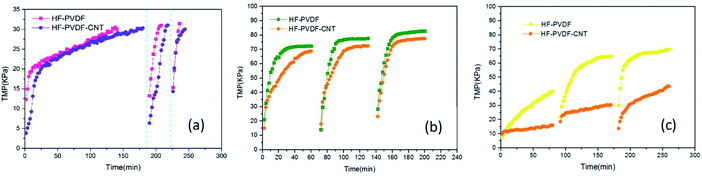 | ||
| Fig. 6 Relationships between TMP and time when BSA (a), SA (b) or HA (c) solution was filtered by the HF-PVDF and HF-PVDF-CNT membranes. | ||
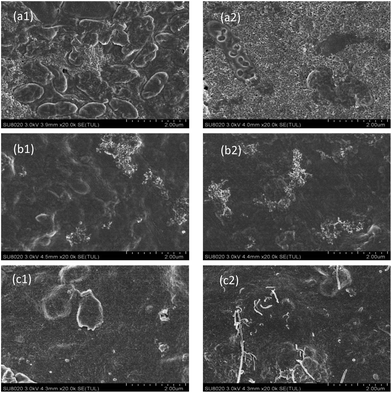 | ||
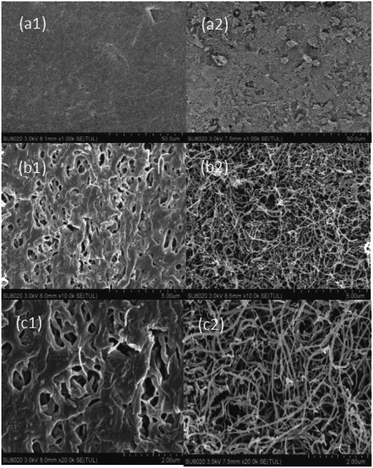
| Fig. 7 SEM images of the HF-PVDF membrane polluted by BSA (a1), SA (b1) or HA (c1) solution; SEM images of the HF-PVDF-CNT membrane polluted by BSA (a2), SA (b2) or HA (c2) solution. | ||
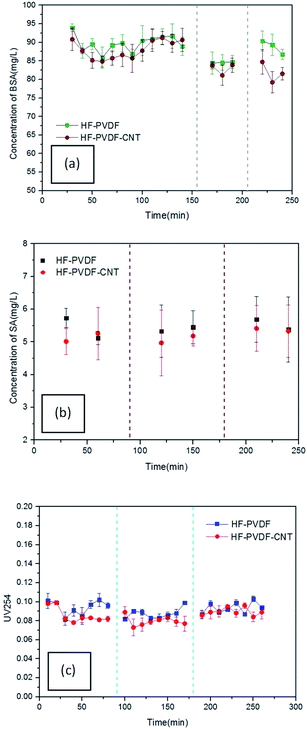
Meanwhile, the BSA concentration of the filtered water through the HF-PVDF-CNT membrane was significantly lower than the that through the HF-PVDF membrane, as shown in Fig. 8(a). This may be ascribed to the charges on the modified membrane surface and the CNT mats. pH 4.7 is the isoelectric point of BSA. The negatively charged BSA molecules affect membrane fouling because of the electrostatic interactions among organic foulants and between organic foulants and the membrane surface directly.42 In addition, the complex porosity structure on the surface of HF-PVDF-CNT membranes helped to trap the BSA molecules and increased the rejection.
In addition, the rejection rate of SA with the HF-PVDF-CNT membrane was improved (Fig. 8(b)), as we expected. The HF-PVDF-CNT membrane had a greater effect on the osmosis separation compared with the HF-PVDF membrane. This could be ascribed to the zwitterionic molecular structure45 and highly hydrophilic CNT mats on the membrane surfaces.
Furthermore, it was found that the UV254 values of most effluent samples for the HF-PVDF-CNT membrane were lower than those for the HF-PVDF membrane (Fig. 8(c)). The average effluent UV254 values during filtration of every cycle through the HF-PVDF membrane were 0.094, 0.088 and 0.094, respectively, while the average effluent UV254 values during filtration of each cycle through the HF-PVDF-CNT membrane were 0.085, 0.080 and 0.089, respectively. For the two membrane types, the UV254 values of the effluent generated from the second cycle were the lowest, while the UV254 values of the effluent during the first and third cycles were poor. The variation trend of the UV254 values in effluent for all three cycles was consistent with the findings reported by Lowe et al.47 When the HA solution was filtered in the first cycle, HA molecules were at a high concentration and could easily pass through the membrane pores. With extended filtration time, the formation of cake layers on the membrane surfaces led to shrinkage and reduction of the pores. And the organic substance in the membrane filtered water decreased accordingly. Backwashing after filtration did not prevent or slow down the formation of cake layers or irreversible pollutants. When the layers of pollutants on the membrane surfaces became thick enough, a large concentration difference was formed around the membrane. Furthermore, concentration polarization was caused on the membrane surfaces. The organic concentration in the effluent increased gradually with the accelerated migration of HA molecules.
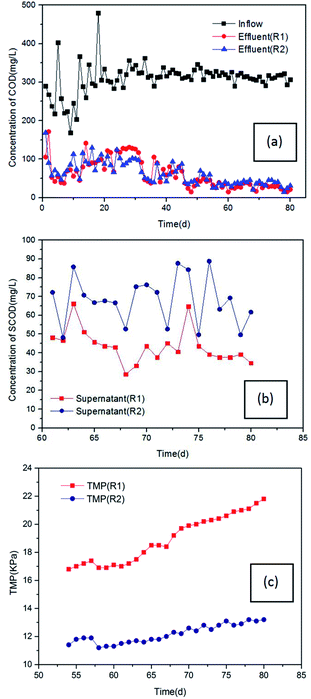
3.4 Application of HF-PVDF and HF-PVDF-CNT membranes in MBRs
4. Conclusions
In summary, a novel and simple technique to fabricate CNT conductive composite membranes was introduced in our study. The method involves filtering a PD/CNTs dispersion through a HF-PVDF membrane operating in dead-end mode from the outside-in. The characteristics of the modified membrane were described. The antifouling performance with different contaminants was investigated compared with the unmodified membrane. The obtained results show that:(1) The CNT mats on the modified membrane surfaces were very stable during hydraulic backwashing. In addition, the CNT mats endowed the unmodified membrane with high hydrophilicity and conductivity.
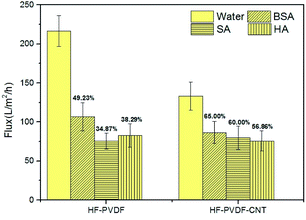
(2) The antifouling and rejection performance of the modified membrane was significantly improved compared with the unmodified membrane during the filtration of BSA, SA or HA solution. Furthermore, the modified membrane is superior in terms of membrane water permeation flux recovery compared with the unmodified membrane. This is further evidence of the high antifouling performance of the modified membrane.
(3) The electro-assisted CNT mats on the outer surface of the modified membrane exhibited high antifouling and separation performance. The results confirmed that biofouling could be mitigated with the application of a voltage in the AnEMBR system.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding support from the National Natural Science Foundation of China (51478015).References
- G. Skouteris, D. Hermosilla, P. López, C. Negro and Á. Blanco, Chem. Eng. J., 2012, 198, 138–148 CrossRef.
- A. Ramesh, D. J. Lee, M. L. Wang, J. P. Hsu, R. S. Juang, K. J. Hwang, J. C. Liu and S. J. Tseng, Sep. Sci. Technol., 2006, 41, 1345–1370 CrossRef CAS.
- A. Ramesh, D. Lee and J. Lai, Appl. Microbiol. Biotechnol., 2007, 74, 699–707 CrossRef CAS.
- M. Tao, F. Liu and L. Xue, J. Mater. Chem., 2012, 22, 9131–9137 RSC.
- X. Yang, Y. He, G. Zeng, X. Chen, H. Shi, D. Qing, F. Li and Q. Chen, Chem. Eng. J., 2017, 321, 245–256 CrossRef CAS.
- N. Yamato, K. Kimura, T. Miyoshi and Y. Watanabe, J. Membr. Sci., 2006, 280, 911–919 CrossRef CAS.
- J.-G. Choi, T.-H. Bae, J.-H. Kim, T.-M. Tak and A. Randall, J. Membr. Sci., 2002, 203, 103–113 CrossRef CAS.
- V. Gekas, K. M. Persson, M. Wahlgren and B. Sivik, J. Membr. Sci., 1992, 72, 293–302 CrossRef CAS.
- X. Xie, C. Criddle and Y. Cui, Energy Environ. Sci., 2015, 8, 3418–3441 RSC.
- B. J. Hinds, N. Chopra, T. Rantell, R. Andrews, V. Gavalas and L. G. Bachas, Science, 2004, 303, 62–65 CrossRef CAS PubMed.
- A. López-Lorente, B. Simonet and M. Valcárcel, Anal. Chem., 2010, 82, 5399–5407 CrossRef PubMed.
- L. Liu, J. Liu, B. Gao, F. Yang and S. Chellam, J. Membr. Sci., 2012, 394, 202–208 CrossRef.
- A. Ding, Y. Yang, G. Sun and D. Wu, Chem. Eng. J., 2016, 283, 260–265 CrossRef CAS.
- Y. Yang, S. Qiao, R. Jin, J. Zhou and X. Quan, J. Membr. Sci., 2018, 553, 54–62 CrossRef CAS.
- S. Madaeni, S. Zinadini and V. Vatanpour, Sep. Purif. Technol., 2011, 80, 155–162 CrossRef CAS.
- G. S. Ajmani, D. Goodwin, K. Marsh, D. H. Fairbrother, K. J. Schwab, J. G. Jacangelo and H. Huang, Water Res., 2012, 46, 5645–5654 CrossRef CAS PubMed.
- J. C. Hoepfner, M. R. Loos and S. H. Pezzin, J. Appl. Polym. Sci., 2019, 136, 48146 CrossRef.
- Y. Liu, Y. Su, J. Cao, J. Guan, L. Xu, R. Zhang, M. He, Q. Zhang, L. Fan and Z. Jiang, Nanoscale, 2017, 9, 7508–7518 RSC.
- M. Gallagher, H. Huang, K. Schwab, D. Fairbrother and B. Teychene, J. Membr. Sci., 2013, 446, 59–67 CrossRef CAS.
- Z.-Y. Xi, Y.-Y. Xu, L.-P. Zhu, Y. Wang and B.-K. Zhu, J. Membr. Sci., 2009, 327, 244–253 CrossRef CAS.
- J. Jiang, L. Zhu, L. Zhu, B. Zhu and Y. Xu, Langmuir, 2011, 27, 14180–14187 CrossRef CAS PubMed.
- B. Fei, B. Qian, Z. Yang, R. Wang, W. Liu, C. Mak and J. H. Xin, Carbon, 2008, 46, 1795–1797 CrossRef CAS.
- P. Yan, J. Wang, L. Wang, B. Liu, Z. Lei and S. Yang, Appl. Surf. Sci., 2011, 257, 4849–4855 CrossRef CAS.
- Y. Zhu, W. Xie, S. Gao, F. Zhang, W. Zhang, Z. Liu and J. Jin, Small, 2016, 12, 5034–5041 CrossRef CAS.
- S. Kang, A. Asatekin, A. M. Mayes and M. Elimelech, J. Membr. Sci., 2007, 296, 42–50 CrossRef CAS.
- F. Li, Q. Tian, B. Yang, L. Wu and C. Deng, Desalination, 2012, 286, 34–40 CrossRef CAS.
- J. Na and Z. Yonggang, 2010 3rd International Conference on Environment and Computer Science, Kunming, 2011 Search PubMed.
- K. P. Katuri, C. M. Werner, R. J. Jimenez-Sandoval, W. Chen, S. Jeon, B. E. Logan, Z. Lai, G. L. Amy and P. E. Saikaly, Environ. Sci. Technol., 2014, 48, 12833–12841 CrossRef CAS.
- J. Xu and Z.-L. Xu, J. Membr. Sci., 2002, 208, 203–212 CrossRef CAS.
- L. J. van der Pauw, Philips Res. Rep., 1958, 20, 220–224 Search PubMed.
- V. Vatanpour, S. S. Madaeni, R. Moradian, S. Zinadini and B. Astinchap, J. Membr. Sci., 2011, 375, 284–294 CrossRef CAS.
- V. Vatanpour, S. S. Madaeni, L. Rajabi, S. Zinadini and A. A. Derakhshan, J. Membr. Sci., 2012, 401, 132–143 CrossRef.
- American Public Health Association, American Water Works Association and Water Environmental Federation, Standard methods for the examination of water and wastewater, American Public Health Association (APHA), Washington, DC, 2005 Search PubMed.
- J. J. Sedmak and S. E. Grossberg, Anal. Biochem., 1977, 79, 544–552 CrossRef CAS PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. t. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- J. T. Arena, B. McCloskey, B. D. Freeman and J. R. McCutcheon, J. Membr. Sci., 2011, 375, 55–62 CrossRef CAS.
- Q. Wan, J. Tian, M. Liu, G. Zeng, Q. Huang, K. Wang, Q. Zhang, F. Deng, X. Zhang and Y. Wei, Appl. Surf. Sci., 2015, 346, 335–341 CrossRef CAS.
- J. T. Arena, S. S. Manickam, K. K. Reimund, B. D. Freeman and J. R. McCutcheon, Desalination, 2014, 343, 8–16 CrossRef CAS.
- K. Gethard, O. Sae-Khow and S. Mitra, ACS Appl. Mater. Interfaces, 2010, 3, 110–114 CrossRef PubMed.
- A. Gijsbertsen-Abrahamse, E. Cornelissen and J. Hofman, Desalination, 2006, 194, 251–258 CrossRef CAS.
- P. Heinemann, J. Howell and R. Bryan, Desalination, 1988, 68, 243–250 CrossRef CAS.
- H. Matsumoto, Y. Koyama and A. Tanioka, J. Colloid Interface Sci., 2003, 264, 82–88 CrossRef CAS.
- D. M. Kanani, X. Sun and R. Ghosh, J. Membr. Sci., 2008, 315, 1–10 CrossRef CAS.
- M. Herzberg, S. Kang and M. Elimelech, Environ. Sci. Technol., 2009, 43, 4393–4398 CrossRef CAS.
- M. Hadidi and A. L. Zydney, J. Membr. Sci., 2014, 452, 97–103 CrossRef CAS.
- K. Ghosh and M. Schnitzer, Soil Sci., 1980, 129, 266–276 CrossRef CAS.
- J. Lowe and M. M. Hossain, Desalination, 2008, 218, 343–354 CrossRef CAS.
- C.-W. Jung, H.-J. Son and L.-S. Kang, Desalination, 2006, 197, 154–164 CrossRef CAS.
- Y.-K. Wang, W.-W. Li, G.-P. Sheng, B.-J. Shi and H.-Q. Yu, Water Res., 2013, 47, 5794–5800 CrossRef CAS PubMed.
- Y. Yang, S. Qiao, R. Jin, J. Zhou and X. Quan, Environ. Sci. Technol., 2018, 53, 1014–1021 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07542a |
| This journal is © The Royal Society of Chemistry 2020 |