Transition-metal-free direct C-3 cyanation of quinoxalin-2(1H)-ones with ammonium thiocyanate as the “CN” source†
Jiayang
Wang
a,
Bin
Sun
a,
Liang
Zhang
b,
Tengwei
Xu
b,
Yuanyuan
Xie
b and
Can
Jin
 *b
*b
aCollaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou 310014, P. R. China
bCollege of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310014, P. R. China. E-mail: jincan@zjut.edu.cn
First published on 19th November 2019
Abstract
A novel avenue of C–CN bond construction was facilely achieved via the TBHP-mediated oxidative coupling reaction between quinoxalin-2(1H)-one derivatives and ammonium thiocyanate without any metal catalyst. Applying this protocol, a variety of 3-cyanoquinoxalin-2(1H)-one derivatives were prepared in moderate to good yields with good functional-group tolerance under mild conditions. Additionally, this methodology featured a broad substrate scope, excellent regioselectivity and operational simplicity.
As a common functional group, cyanide plays an important role in natural products, chemistry, biology and medicine.1 Meanwhile, the cyano functional group is highly versatile in organic synthesis, acting as a privileged precursor of carboxyl, carbonyl, amines, amides and other heterocyclic groups.2 Thus, developing convenient and efficient methods for the installation of cyano groups into biological molecules is of great interest. In the past decade, transition metal-catalyzed C–H cyanation with various cyanide sources has been applied to construct C–CN bonds. However, most of the procedures required expensive and complex metals such as ruthenium,3 rhodium4 or palladium.5 Moreover, some cyanide sources involved in the process, like KCN, NaCN, TMSCN, CuCN and Zn(CN)2,6 are highly toxic. Although several mild cyanating agents have been developed for C–H cyanation such as diaminomaleonitrile,7 aryl cyanates,8 tosyl cyanide,9 cyanobenziodoxole,10 aryl(cyano)iodonium triflates,11N-cyano-N-phenyl-p-toluenesulfonamide,12tert-butyl isonitrile,13 cyanocarbonyls14 and Zhdankin's reagent,15 these cyanide sources should also be prepared from highly toxic precursors, and most of them were demonstrated to be easily decomposed. Therefore, the development of novel, environmentally friendly and metal-free C–H cyanation using low toxic and easily available cyano sources on a broad substrate scope is still highly desirable.
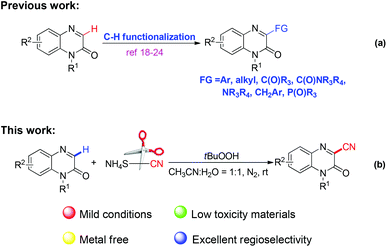
The quinoxalin-2(1H)-one skeleton is a crucial structural motif since it is endowed with significant biological activities and outstanding pharmaceutical properties such as anti-angiogenesis, antitumor, antidiabetic, anti-inflammatory, antimicrobial and aldose reductase inhibitor activities.16 The direct C–H functionalization at the C-3 position of available quinoxalin-2(1H)-ones has served as an ideal and powerful method to afford C-3 substituted quinoxalin-2(1H)-ones. Consequently, much effort has been made in the preparation of quinoxalin-2(1H)-one derivatives,17 including C-3 arylation,18 alkylation,19 acylation,20 amidation,21 amination,22 benzylation23 and phosphonation24 (Scheme 1, a). Despite the significance of these investigations, to the best of our knowledge, the synthesis of quinoxalin-2(1H)-ones bearing a cyano substituent at the C3 position via direct C–H bond functionalization has not been studied. With our ongoing studies on developing green methods for C–H functionaliztions,25 herein, we report a TBHP-mediated oxidative C–H cyanation of quinoxalin-2(1H)-ones utilizing inexpensive and low toxicity ammonium thiocyanate as the cyanide source under metal-/base-/ligand-free conditions.
In the preliminary experiment, we chose the cyanation of 1-methylquinoxalin-2(1H)-one 1aa as a model reaction to optimize the reaction conditions. The transformation was initially carried out in the presence of TBHP (70% solution in water) in CH3CN at room temperature under a N2 atmosphere by employing ammonium thiocyanate 2 as the cyanide source, and the corresponding product 3aa was obtained, even though in a relatively low yield. Subsequently, a variety of other commonly used solvents such as toluene, DMF, EtOAc, acetone and H2O were then studied, while this transformation could not proceed well in these screened solvents (Table 1, entries 2–6). To our delight, it was observed that adding an appropriate amount of water was beneficial for this reaction, and the yield of 3aa could be sharply increased to 68% when MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was employed as the solvent (Table 1, entries 7–10). Replacing aqueous tBuOOH with anhydrous tBuOOH resulted in a lower reaction efficiency (Table 1, entry 11). Next, a series of oxidants including O2, oxone, K2S2O8, PhI(OAc)2, PhI(OTf)2 and DTBP were examined, while all of these oxidants completely inhibited this reaction (Table 1, entries 12–17). The effect of the TBHP loading was also screened. As a result, 4.0 eq. was found to be the best choice and the yield could reach 72% (Table 1, entries 18–20). In addition, other cyanide sources such as NaSCN or KSCN were also demonstrated to be suitable “CN” sources for this reaction system (Table 1, entries 21–22). In light of the higher reactivity, NH4SCN was believed to be more suitable as a “CN” source. Subsequently, the effect of temperature was also tested. The experimental results indicated that raising the temperature from room temperature to 50 °C would exhibit a negative effect on this reaction, and the yield of 3aa was decreased from 72% to 58% (Table 1, entry 23). Furthermore, the experiment results revealed that shortening or prolonging the reaction time also failed to give better results (Table 1, entry 24). Thus, it could be concluded that the optimized reaction should be performed in the presence of TBHP (70% solution in water) in a mixed solvent (CH3CN/H2O = 1
1) was employed as the solvent (Table 1, entries 7–10). Replacing aqueous tBuOOH with anhydrous tBuOOH resulted in a lower reaction efficiency (Table 1, entry 11). Next, a series of oxidants including O2, oxone, K2S2O8, PhI(OAc)2, PhI(OTf)2 and DTBP were examined, while all of these oxidants completely inhibited this reaction (Table 1, entries 12–17). The effect of the TBHP loading was also screened. As a result, 4.0 eq. was found to be the best choice and the yield could reach 72% (Table 1, entries 18–20). In addition, other cyanide sources such as NaSCN or KSCN were also demonstrated to be suitable “CN” sources for this reaction system (Table 1, entries 21–22). In light of the higher reactivity, NH4SCN was believed to be more suitable as a “CN” source. Subsequently, the effect of temperature was also tested. The experimental results indicated that raising the temperature from room temperature to 50 °C would exhibit a negative effect on this reaction, and the yield of 3aa was decreased from 72% to 58% (Table 1, entry 23). Furthermore, the experiment results revealed that shortening or prolonging the reaction time also failed to give better results (Table 1, entry 24). Thus, it could be concluded that the optimized reaction should be performed in the presence of TBHP (70% solution in water) in a mixed solvent (CH3CN/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature under a N2 atmosphere for 30 h by employing ammonium thiocyanate 2 as the cyanide source.
1) at room temperature under a N2 atmosphere for 30 h by employing ammonium thiocyanate 2 as the cyanide source.
| Entry | Oxidant (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1aa (0.4 mmol), 2 (1.2 mmol), oxidant (1.2 mmol), solvent (3.0 mL), rt, N2 atmosphere, 30 h. b Isolated yield. c Anhydrous tBuOOH (5.5 M solution in decane). d KSCN was added instead of NH4SCN. e NaSCN was added instead of NH4SCN. f 50 °C. g 24 h. h 36 h. NR = no reaction. | |||
| 1 | tBuOOH (3) | CH3CN | 32 |
| 2 | tBuOOH (3) | Toluene | 15 |
| 3 | tBuOOH (3) | DMF | Trace |
| 4 | tBuOOH (3) | EtOAc | NR |
| 5 | tBuOOH (3) | Acetone | NR |
| 6 | tBuOOH (3) | H2O | 23 |
| 7 | tBuOOH (3) | CH3CN/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
44 |
| 8 | tBuOOH (3) | CH3CN/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
62 |
| 9 | tBuOOH (3) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
68 |
| 10 | tBuOOH (3) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
42 |
| 11c | tBuOOH (3) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
63 |
| 12 | O2 (balloon) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 13 | Oxone (3) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 14 | K2S2O8 (3) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 15 | PhI(OAc)2 (3) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 16 | PhI(OTf)2 (3) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 17 | DTBP (3) |
CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
NR |
| 18 | tBuOOH (2) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
54 |
| 19 | tBuOOH (4) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
72 |
| 20 | tBuOOH (5) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
67 |
| 21 | tBuOOH (4) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
52d |
| 22 | tBuOOH (4) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
43e |
| 23 | tBuOOH (4) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
58f |
| 24 | tBuOOH (4) | CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
54g, 66h |
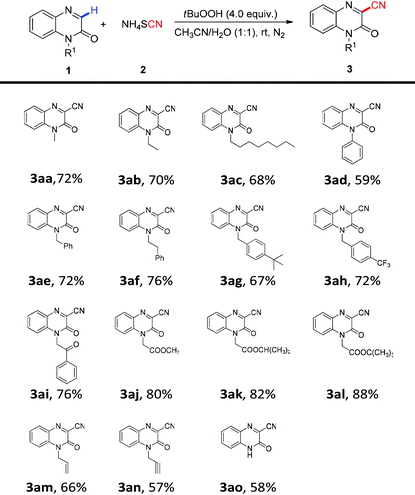
With the optimized conditions in hand, a range of quinoxalin-2(1H)-ones were applied to yield the corresponding 3-cyanoquinoxalin-2(1H)-one derivatives. The effects of the N-substituents of quinoxalin-2(1H)-one on this transformation were examined (Table 2). To our delight, the N-substituted quinoxalin-2(1H)-one analogues 1aa–1ao showed good reactivity with ammonium thiocyanate 2 under the optimal conditions to give the anticipated products 3aa–3ao in 57–88% yields. For N-alkyl groups, it was found that the short chain alkyl (methyl, ethyl) and long chain alkyl (octyl) groups were all compatible for this reaction and gave the desired products 3aa–3ac in 68% to 72% yields. In addition, the substrates with N-aryl groups such as phenyl 1ad were also tolerated and gave the cyanated product 3ad in moderate yield.
a All reactions were performed with 1 (0.3 mmol), 2 (0.9 mmol) and tBuOOH (70% solution in water, 1.2 mmol) in 2 ml of CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature under a N2 atmosphere for 30 h. 1) at room temperature under a N2 atmosphere for 30 h.
|
|---|
|
Quinoxalin-2(1H)-ones 1ae–1al containing a phenyl or an ester group on N-alkyl could also undergo this coupling process smoothly to give the corresponding products 3ae–3al in moderate to excellent yields. It was worth noting that the N-protecting group containing an alkenyl or an alkynyl group proved to be well tolerated for this transformation, providing the coupling products 3am and 3an in 66% and 57% yields, respectively, whereas the alkenyl or alkynyl group was not affected during the process. Notably, the unprotected quinoxalin-2(1H)-one 1ao also displayed a high reactivity in the reaction process, furnishing the desired product 3ao in satisfactory yield.
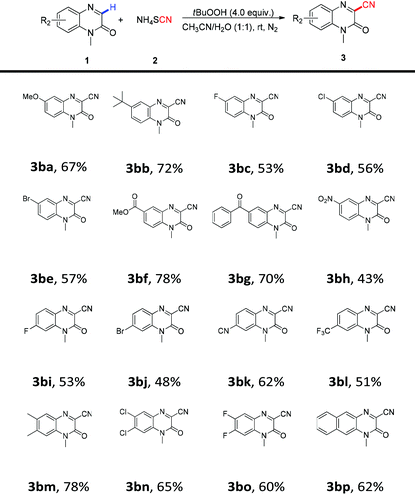
Subsequently, the effects of different substituents on the phenyl ring of quinoxalin-2(1H)-ones were studied, and the results are summarized in Table 3. The experimental results indicated that substrates bearing either electron-donating or electron-withdrawing groups were all compatible with satisfactory efficiency, affording the corresponding products 3ba–3bp in 32% to 81% yields. For example, the electron-donating groups at the 6-positon of the benzene ring, such as methoxy 3ba and t-butyl 3bb, could provide the desired products in 67% and 72% yields. Additionally, quinoxalin-2(1H)-ones containing electron-withdrawing groups such as bromo, chloro, fluoro, ester, acyl and nitro groups at the 6-positon of the benzene ring proceeded smoothly to deliver the corresponding products 3bc–3bh in moderate to good yields (43–78%). Subsequently, quinoxalin-2(1H)-ones with substituents at the 7-position of the benzene ring were also investigated, and the experimental results revealed that the substituted position did not affect the efficiency of the reaction, and 7-substituted quinoxalin-2(1H)-ones also engaged in this reaction efficiently and gave the desired products 3bi–3bl in moderate yields. Moreover, it was noted that the disubstituted quinoxalin-2(1H)-ones containing two electron-withdrawing or electron-donating groups at 6- and 7-positions could also undergo this coupling process well and the expected products 3bm–3bo were obtained in good yields. Finally, 1-methylbenzo[g]quinoxalin-2(1H)-one 1bp was also tested under the standard conditions, and we found that the target product 3bp was obtained in an acceptable yield.
a All reactions were performed with 1 (0.3 mmol), 2 (0.9 mmol) and tBuOOH (70% solution in water, 1.2 mmol) in 2 ml of CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature under a N2 atmosphere for 30 h. 1) at room temperature under a N2 atmosphere for 30 h.
|
|---|
|
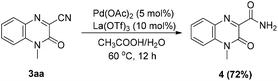
Further transformation of product 3aa was investigated to illustrate the applicability of this reaction (Scheme 2). When product 3aa was treated with 5 mol% Pd(OAc)2 and 10 mol% La(OTf)3 in AcOH/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (3 mL) at 60 °C for 12 h,26 1-methyl-1,2-dihydroquinoxalin-2-one-3-carboxamide 4 was obtained in 72% isolated yield. The experimental results indicated that 3-cyanoquinoxalin-2(1H)-one could be conveniently converted into quinoxalinone-3-carboxamides, which exhibited a wide range of medicinal and biological properties.27
1) (3 mL) at 60 °C for 12 h,26 1-methyl-1,2-dihydroquinoxalin-2-one-3-carboxamide 4 was obtained in 72% isolated yield. The experimental results indicated that 3-cyanoquinoxalin-2(1H)-one could be conveniently converted into quinoxalinone-3-carboxamides, which exhibited a wide range of medicinal and biological properties.27
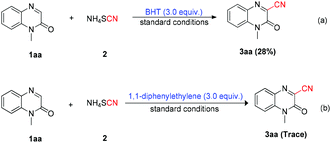
In order to study the mechanism of the target product formation, some control experiments were carried out. As a result, it was found that the yield of product 3aa decreased greatly from 72% to 28% when 3.0 equiv. of BHT (2,6-ditert-butyl-4-methylphenol) was applied to a model reaction system (Scheme 3a). We continued to examine the mechanistic studies and found that the addition of 1,1-diphenylethylene (3.0 equiv.) resulted in trace product formation (Scheme 3b).
These results indicate that a radical mechanism may be involved in this oxidative coupling process.
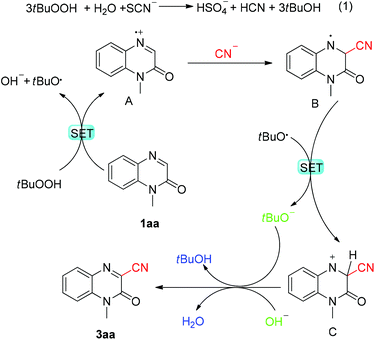
On the basis of the above-mentioned results and literature reports,28 a plausible catalytic cycle is proposed and depicted in Scheme 4. Initially, 1-methylquinoxalin-2(1H)-one 1aa was oxidized by tBuOOH to generate radical cation A, accompanied by the formation of a hydroxide anion and a tert-butyloxy radical. Subsequently, the cyanide ion that was generated by the oxidation of thiocyanate ions as suggested in eqn (1) was regioselectively added to the C-3 position of intermediate A, affording nitrogen radical intermediate B, which then underwent the single-electron transformation (SET) process to produce nitrogen cation C. Finally, the expected coupling product 3aa was obtained after deprotonation.
Conclusions
In summary, we have developed a novel and environmentally friendly direct C–H cyanidation of quinoxalin-2(1H)-ones by employing ammonium thiocyanate as the cyanide source under mild conditions. This transformation exhibited an excellent substrate scope and a variety of 3-cyanoquinoxalin-2(1H)-ones with different functional groups were obtained in moderate to excellent yields under the optimized conditions. In addition, the strategy featured room temperature, low toxicity, excellent regioselectivity and operational simplicity, thus affording an attractive approach to 3-cyanoquinoxalin-2(1H)-ones.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21606202). We are also grateful to the College of Pharmaceutical Sciences, the Zhejiang University of Technology and the Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals for the financial help.Notes and references
- (a) R. Letienne, Y. Verscheure, M. Perez, B. L. Grand, F. C. Colpaert and G. W. John, J. Pharmacol. Exp. Ther., 2003, 305, 749 CrossRef CAS PubMed; (b) F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902 CrossRef CAS PubMed; (c) L.-C. Campeau, Q. Chen, D. Gauvreau, M. Girardin, K. Belyk, P. Maligres, G. Zhou, C. Gu, W. Zhang, L. Tan and P. D. O'Shea, Org. Process Res. Dev., 2016, 20, 1476 CrossRef CAS; (d) D. Murdoch and S. J. Keam, Drugs, 2005, 65, 2379 CrossRef CAS PubMed; (e) Y. Ozoe, S. Ishikawa, S. Tomiyama, F. Ozoe, T. Kozaki and J. G. Scott, ACS Symp. Ser., 2007, 948, 39 CrossRef CAS; (f) E. Muñoz-Islas, S. Gupta, L. R. Jiménez-Mena, J. Lozano-Cuenca, A. Sánchez-López, D. Centurión, S. Mehrotra, A. MaassenVanDenBrink and C. M. Villalón, Br. J. Pharmacol., 2006, 149, 82 CrossRef PubMed.
- (a) C. W. Liskey, X. Liao and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 11389 CrossRef CAS PubMed; (b) Á. L. Fuentes de Arriba, E. Lenci, M. Sonawane, O. Formery and D. J. Dixon, Angew. Chem., Int. Ed., 2017, 56, 3655 CrossRef PubMed; (c) Y. Huang, Y. Yu, Z. Zhu, C. Zhu, J. Cen, X. Li, W. Wu and H. Jiang, J. Org. Chem., 2017, 82, 7621 CrossRef CAS PubMed; (d) L.-Y. Liu, K.-S. Yeung and J.-Q. Yu, Chem. – Eur. J., 2019, 25, 2199 CrossRef CAS PubMed; (e) A. M. Nauth, N. Otto and T. Opatz, Adv. Synth. Catal., 2015, 357, 3424 CrossRef CAS.
- (a) K. H. V. Reddy, G. Satish, V. P. Reddy, B. S. P. A. Kumar and Y. V. D. Nageswar, RSC Adv., 2012, 2, 11084 RSC; (b) A. Mishra, T. K. Vats and I. Deb, J. Org. Chem., 2016, 81, 6525 CrossRef CAS PubMed.
- (a) T.-J. Gong, B. Xiao, W.-M. Cheng, W. Su, J. Xu, Z.-J. Liu, L. Liu and Y. Fu, J. Am. Chem. Soc., 2013, 135, 10630 CrossRef CAS PubMed; (b) M. Chaitanya, D. Yadagiri and P. Anbarasan, Org. Lett., 2013, 15, 4960 CrossRef CAS PubMed; (c) M. Chaitanya and P. Anbarasan, Org. Lett., 2015, 17, 3766 CrossRef CAS PubMed; (d) A. B. Khemnar, D. N. Sawant and B. M. Bhanage, Tetrahedron Lett., 2013, 54, 2682 CrossRef CAS; (e) P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 519 CrossRef CAS PubMed; (f) D. N. Sawant, Y. S. Wagh, P. J. Tambade, K. D. Bhatte and B. M. Bhanage, Adv. Synth. Catal., 2011, 353, 781 CrossRef CAS.
- (a) X. Jia, D. Yang, W. Wang, F. Luo and J. Cheng, J. Org. Chem., 2009, 74, 9470 CrossRef CAS PubMed; (b) X. Jia, D. Yang, S. Zhang and J. Cheng, Org. Lett., 2009, 11, 4716 CrossRef CAS PubMed; (c) L. Liu, J. Li, J. Xu and J.-t. Sun, Tetrahedron Lett., 2012, 53, 6954 CrossRef CAS; (d) T. Zou, X. Feng, H. Liu, X. Yu, Y. Yamamotoac and M. Bao, RSC Adv., 2013, 3, 20379 RSC; (e) B. Mariampillai, J. Alliot, M. Li and M. Lautens, J. Am. Chem. Soc., 2007, 129, 15372 CrossRef CAS PubMed; (f) D. Zhang, H. Sun, L. Zhang, Y. Zhou, C. Li, H. Jiang, K. Chen and H. Liu, Chem. Commun., 2012, 48, 2909 RSC; (g) P. Y. Yeung, C. M. So, C. P. Lau and F. Y. Kwong, Org. Lett., 2011, 13, 648 CrossRef CAS PubMed; (h) J. Richardson and S. P. Mutton, J. Org. Chem., 2018, 83, 4922 CrossRef CAS PubMed.
- (a) H.-J. Cristau, A. Ouali, J.-F. Spindler and M. Taillefer, Chem. – Eur. J., 2005, 11, 2483 CrossRef CAS PubMed; (b) K. Kima and S. H. Hong, Adv. Synth. Catal., 2017, 359, 2345 CrossRef; (c) H.-Q. Do and O. Daugulis, Org. Lett., 2010, 12, 2517 CrossRef CAS PubMed; (d) Y. Ping, Q. Ding, Z. Chen and Y. Peng, J. Organomet. Chem., 2016, 815, 59 CrossRef; (e) X. Zhang, A. Xia, H. Chen and Y. Liu, Org. Lett., 2017, 19, 2118 CrossRef CAS; (f) J. R. Coombs, K. J. Fraunhoffer, E. M. Simmons, J. M. Stevens, S. R. Wisniewski and M. Yu, J. Org. Chem., 2017, 82, 7040 CrossRef CAS PubMed; (g) H. Yu, R. N. Richey, W. D. Miller, J. Xu and S. A. May, J. Org. Chem., 2011, 76, 665 CrossRef CAS PubMed.
- S. Goswami, A. Manna, A. K. Maity, S. Paul, A. K. Das, M. K. Das, P. Saha, C. K. Quah and H.-K. Fun, Dalton Trans., 2013, 42, 12844 RSC.
- (a) J. Qiu, D. Wu, P. G. Karmaker, G. Qi, P. Chen, H. Yin and F.-X. Chen, Org. Lett., 2017, 19, 4018 CrossRef CAS PubMed; (b) N. Sato, Tetrahedron Lett., 2002, 43, 6403 CrossRef CAS; (c) N. Sato and Q. Yue, Tetrahedron, 2003, 59, 5831 CrossRef CAS.
- (a) T. Hoshikawa, S. Yoshioka, S. Kamijo and M. Inoue, Synthesis, 2013, 45, 874 CrossRef CAS; (b) S. Kamijo, T. Hoshikawa and M. Inoue, Org. Lett., 2011, 13, 5928 CrossRef CAS; (c) R. Akula, Y. Xiong and H. Ibrahim, RSC Adv., 2013, 3, 10731 RSC.
- (a) T. Nagata, H. Matsubara, K. Kiyokawa and S. Minakata, Org. Lett., 2017, 19, 4672 CrossRef CAS PubMed; (b) B. Ma, X. Lin, L. Lin, X. Feng and X. Liu, J. Org. Chem., 2017, 82, 701 CrossRef CAS PubMed; (c) R. Chowdhury, J. Schörgenhumer, J. Novacek and M. Waser, Tetrahedron Lett., 2015, 56, 1911 CrossRef CAS PubMed; (d) Y.-F. Wang, J. Qiu, D. Kong, Y. Gao, F. Lu, P. G. Karmaker and F.-X. Chen, Org. Biomol. Chem., 2015, 13, 365 RSC; (e) M. Chen, Z.-T. Huang and Q.-Y. Zheng, Org. Biomol. Chem., 2015, 13, 8812 RSC.
- (a) X. Wang and A. Studer, J. Am. Chem. Soc., 2016, 138, 2977 CrossRef CAS PubMed; (b) Z. Shu, W. Ji, X. Wang, Y. Zhou, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2014, 53, 2186 CrossRef CAS; (c) D. Zhu, D. Chang and L. Shi, Chem. Commun., 2015, 51, 7180 RSC.
- (a) M. Chaitanya and P. Anbarasan, J. Org. Chem., 2015, 80, 3695 CrossRef CAS; (b) J. Li and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 3635 CrossRef CAS PubMed; (c) Y. Ping, Q. Ding and Y. Peng, ACS Catal., 2016, 6, 5989 CrossRef CAS; (d) W. Liu and L. Ackermann, Chem. Commun., 2014, 50, 1878 RSC; (e) Y. Yang and P. Liu, ACS Catal., 2015, 5, 2944 CrossRef CAS; (f) N. K. Mishra, T. Jeong, S. Sharma, Y. Shin, S. Han, J. Park, J. S. Oh, J. H. Kwak, Y. H. Jung and I. S. Kim, Adv. Synth. Catal., 2015, 357, 1293 CrossRef CAS; (g) J. Cui, J. Song, Q. Liu, H. Liu and Y. Dong, Chem. – Asian J., 2018, 13, 482 CrossRef CAS PubMed.
- (a) X. Jiang, J.-M. Wang, Y. Zhang, Z. Chen, Y.-M. Zhu and S.-J. Ji, Tetrahedron, 2015, 71, 4883 CrossRef CAS; (b) X. Hong, H. Wang, G. Qian, Q. Tan and B. Xu, J. Org. Chem., 2014, 79, 3228 CrossRef CAS PubMed; (c) S. Xu, X. Huang, X. Hong and B. Xu, Org. Lett., 2012, 14, 4614 CrossRef CAS PubMed.
- (a) A. Brahma, B. Musio, U. Ismayilova, N. Nikbin, S. B. Kamptmann, P. Siegert, G. E. Jeromin, S. V. Ley and M. Pohl, Synlett, 2016, 27, 262 CAS; (b) J. Vicario, J. M. Ezpeleta and F. Palacios, Adv. Synth. Catal., 2012, 354, 2641 CrossRef CAS.
- T. Yang, H. Zhu and W. Yu, Org. Biomol. Chem., 2016, 14, 3376 RSC.
- (a) S. Pirasa, M. Lorigaa, A. Cartaa, G. Pagliettia, M. P. Costib and S. Ferrari, Heterocycl. Chem., 2006, 43, 541 CrossRef; (b) J. Dudash, Jr., Y. Zhang, J. B. Moore, R. Look, Y. Liang, M. P. Beavers, B. R. Conway, P. J. Rybczynski and K. T. Demarest, Bioorg. Med. Chem. Lett., 2005, 15, 4790 CrossRef PubMed; (c) B. Wu, Y. Yang, X. Qin, S. Zhang, C. Jing, C. Zhu and B. Ma, ChemMedChem, 2013, 8, 1913 CrossRef CAS PubMed; (d) S. N. Khattab, S. A. H. Abdel Moneim, A. A. Bekhit, A. M. E. Massry, S. Y. Hassan, A. El-Faham, H. E. A. Ahmed and A. Amer, Eur. J. Med Chem., 2015, 93, 308 CrossRef CAS PubMed; (e) D. Chen, Z.-J. Wang and W. Bao, J. Org. Chem., 2010, 75, 5768 CrossRef CAS PubMed; (f) L. Shi, H. Zhou, J. Wu and X. Li, Mini-Rev. Org. Chem., 2015, 12, 96 CrossRef CAS; (g) J. Quinn, C. Guo, L. Ko, B. Sun, Y. He and Y. Li, RSC Adv., 2016, 6, 22043 RSC; (h) S. A. Galal, S. H. M. Khairat, F. A. F. Ragab, A. S. Abdelsamie, M. M. Ali, S. M. Soliman, J. Mortier, G. Wolber and H. I. El Diwani, Eur. J. Med. Chem., 2014, 86, 122 CrossRef CAS PubMed; (i) D. Chen and W. Bao, Adv. Synth. Catal., 2010, 352, 955 CrossRef CAS; (j) R. Liu, Z. Huang, M. G. Murray, X. Guo and G. Liu, J. Med. Chem., 2011, 54, 5747 CrossRef CAS PubMed.
- (a) L.-Y. Xie, T.-G. Fang, J.-X. Tan, B. Zhang, Z. Cao, L.-H. Yang and W.-M. He, Green Chem., 2019, 21, 3858 RSC; (b) P. Mao, J. Zhu, J. Yuan, L. Yang, Y. Xiao and C. Zhang, Chin. J. Org. Chem., 2019, 39, 1529 CrossRef.
- (a) A. Carrër, J. D. Brion, S. Messaoudi and M. Alami, Org. Lett., 2013, 15, 5607 CrossRef PubMed; (b) B. Ramesh, C. R. Reddy, G. R. Kumar and B. V. S. Reddy, Tetrahedron Lett., 2018, 59, 628 CrossRef CAS; (c) X. Qin, X. Hao, H. Han, S. Zhu, Y. Yang, B. Wu, S. Hussain, S. Parveen, C. Jing, B. Ma and C. Zhu, J. Med. Chem., 2015, 58, 1254 CrossRef CAS PubMed; (d) A. Carrër, J.-D. Brion, M. Alami and S. Messaoudi, Adv. Synth. Catal., 2014, 356, 3821 CrossRef; (e) K. Yin and R. Zhang, Org. Lett., 2017, 19, 1530 CrossRef CAS PubMed; (f) K. Yin and R. Zhang, Synlett, 2017, 28, 597 Search PubMed; (g) J. Yuan, S. Liu and L. Qu, Adv. Synth. Catal., 2017, 359, 4197 CrossRef CAS; (h) S. Toonchue, L. Sumunnee, K. Phomphrai and S. Yotphan, Org. Chem. Front., 2018, 5, 1928 CAS; (i) S. Paul, H. D. Khanal, C. D. Clinton, S. H. Kim and Y. R. Lee, Org. Chem. Front., 2019, 6, 231 RSC; (j) L. Wang, P. Bao, W. Liu, S. Liu, C. Hu, H. Yue, D. Yang and W. Wei, Chin. J. Org. Chem., 2018, 38, 3189 CrossRef CAS.
- (a) J. Fu, J. Yuan, Y. Zhang, Y. Xiao, P. Mao, X. Diaoa and L. Qu, Org. Chem. Front., 2018, 5, 3382 RSC; (b) J. Yuan, J. Fu, J. Yin, Z. Dong, Y. Xiao, P. Maoa and L. Qu, Org. Chem. Front., 2018, 5, 2820 RSC; (c) C. Jin, Z. Yan, B. Sun and J. Yang, Org. Lett., 2019, 21, 2064 CrossRef CAS PubMed; (d) W. Wei, L. Wang, H. Yue, P. Bao, W. Liu, C. Hu, D. Yang and H. Wang, ACS Sustainable Chem. Eng., 2018, 6, 17252 CrossRef CAS; (e) L.-Y. Xie, L.-L. Jiang, J.-X. Tan, Y. Wang, X.-Q. Xu, B. Zhang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 14153 CrossRef CAS.
- (a) X. Zeng, C. Liu, X. Wang, J. Zhang, X. Wang and Y. Hu, Org. Biomol. Chem., 2017, 15, 8929 RSC; (b) J.-W. Yuan, J.-H. Fu, S.-N. Liu, Y.-M. Xiao, P. Mao and L.-B. Qu, Org. Biomol. Chem., 2018, 16, 3203 RSC.
- J. Yuan, S. Liu, Y. Xiao, P. Mao, L. Yang and L. Qu, Org. Biomol. Chem., 2019, 17, 876 RSC.
- (a) A. V. Gulevskaya, O. N. Burov, A. F. Pozharskii, M. E. Kletskii and I. N. Korbukova, Tetrahedron, 2008, 64, 696 CrossRef CAS; (b) Y. Li, M. Gao, L. H. Wang and X. L. Cui, Org. Biomol. Chem., 2016, 14, 8428 RSC; (c) A. Gupta, M. S. Deshmukh and N. Jain, J. Org. Chem., 2017, 82, 4784 CrossRef CAS PubMed; (d) T. T. Hoang, T. A. To, V. T. T. Cao, A. T. Nguyen, T. T. Nguyen and N. T. S. Phan, Catal. Commun., 2017, 101, 20 CrossRef CAS; (e) W. Wei, L. Wang, P. Bao, Y. Shao, H. Yue, D. Yang, X. Yang, X. Zhao and H. Wang, Org. Lett., 2018, 20, 7125 CrossRef CAS PubMed.
- L. Hu, J. Yuan, J. Fu, T. Zhang, L. Gao, Y. Xiao, P. Mao and L. Qu, Eur. J. Org. Chem., 2018, 4113 CrossRef CAS.
- M. Gao, Y. Li, L. Xie, R. Chauvin and X. Cui, Chem. Commun., 2016, 52, 2846 RSC.
- (a) C. Jin, Z. Yan, B. Sun and J. Yang, Org. Lett., 2019, 21, 2064 CrossRef CAS PubMed; (b) B. Sun, J. Deng, D. Li, C. Jin and W. Su, Tetrahedron Lett., 2018, 59, 4364 CrossRef CAS; (c) B. Sun, Y. Wang, D. Li, C. Jin and W. Su, Org. Biomol. Chem., 2018, 16, 2902 RSC; (d) B. Sun, S. Yin, X. Zhuang, C. Jin and W. Su, Org. Biomol. Chem., 2018, 16, 6017 RSC; (e) B. Sun, Z. Yan, C. Jin and W. Su, Synlett, 2018, 29, 2432 CrossRef CAS; (f) C. Jin, X. Zhuang, B. Sun, D. Li and R. Zhu, Asian J. Org. Chem., 2019, 8, 1490 CrossRef CAS; (g) C. Jin, X. Zhang, B. Sun, Z. Yan and T. Xu, Synlett, 2019, 30, 1585 CrossRef CAS; (h) Z. Yan, B. Sun, X. Zhang, X. Zhuang, J. Yang, W. Su and C. Jin, Chem. – Asian J., 2019, 14, 3344 CrossRef CAS PubMed; (i) J. Wang, B. Sun, L. Zhang, T. Xu, Y. Xie and C. Jin, Asian J. Org. Chem., 2019, 8, 1942 CrossRef CAS; (j) B. Sun, J. Yang, L. Zhang, R. Shi, X. Zhang, T. Xu, X. Zhuang, R. Zhu, C. Yu and C. Jin, Asian J. Org. Chem., 2019, 8, 2058 CrossRef CAS.
- S. Zhang, H. Xu, C. Lou, A. M. Senan, Z. Chen and G. Yin, Eur. J. Org. Chem., 2017, 1870 CrossRef CAS.
- (a) I. R. Ager, A. C. Barnes, G. F. Danswan, P. W. Hairsine, D. P. Kay, P. D. Kennewell, S. S. Matharu, P. Miller, P. Robson, D. A. Rowlands, W. R. Tully and R. Westwood, J. Med. Chem., 1988, 31, 1098 CrossRef CAS PubMed; (b) P. Marqués-Gallego, M. A. Gamiz-Gonzalez, F. R. Fortea-Pérez, M. Lutz, A. L. Spek, A. Pevec, B. Kozlevčar and J. Reedijk, Dalton Trans., 2010, 39, 5152 RSC.
- (a) J. Yuan, J. Fu, J. Yin, Z. Dong, Y. Xiao, P. Maoa and L. Qu, Org. Chem. Front., 2018, 5, 2820 RSC; (b) A. Wagner and A. R. Ofial, J. Org. Chem., 2015, 80, 2848 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qo01055f |
| This journal is © the Partner Organisations 2020 |