Aggregation enhances luminescence and photosensitization properties of a hexaiodo-BODIPY†
P. P. Praveen
Kumar
,
Pranjali
Yadav
,
Asifkhan
Shanavas
 and
Prakash P.
Neelakandan
and
Prakash P.
Neelakandan
 *
*
Institute of Nano Science and Technology, Habitat Centre, Phase 10, (Sector 64), Mohali 160062, Punjab, India. E-mail: ppn@inst.ac.in
First published on 1st February 2020
Abstract
Intramolecular rotations are known to interfere with the excited-state processes resulting in luminescence quenching. Efforts aimed at restricting intramolecular rotations have led to the development of highly luminescent systems. Here we report the luminescence properties of a BODIPY molecule (I6) containing six iodo-substituents. The luminescence of I6 was highly quenched in the solution state whereas the conversion of this molecule into its nanoaggregates enhanced the emission intensity. Detailed photophysical studies and molecular simulations were employed to study the underlying mechanism which showed that the electron-withdrawing nature of iodine atoms and the peculiar structure of I6 contributed towards the anomalous luminescence behaviour. Further, the luminescence and photosensitization properties of I6 were exploited for cell imaging and photodynamic therapy. Thus, our simple but intelligent molecular design yielded a multi-faceted molecule having excellent photophysical properties for potential photobiological applications.
Introduction
Molecular motions are critical in determining luminescence efficiency of molecules. Intramolecular rotations about a C–C bond joining aromatic rings is a well-known non-radiative pathway in organic fluorophores. Any process that restricts the intramolecular rotations serves to hinder the non-radiative decays thus leading to enhanced emission. This principle served as the platform for the development of fluorophores exhibiting aggregation induced emission (AIE).1–5 The signature of the AIE phenomenon is a weak emission in dilute solutions which intensifies upon aggregation or in the solid state. AIE is typically exhibited by molecules having propeller-type structures that allow intramolecular rotations in dilute solutions which facilitates non-radiative decays. Upon aggregation, the intramolecular rotations are restricted thereby hindering the non-radiative decay processes and eventually leading to an enhancement in emission. Because of the inverted emission characteristics, AIE luminogens were readily accepted in biology and materials science. These materials are ideal for biomedical applications because of the superior luminescence features and excellent stability in the aggregated state.6–11Boron-dipyrromethene (BODIPY) dyes are commonly used as fluorescent indicators, bio labelling agents, photosensitizers and as active material in optoelectronics owing to their exceptional optical and electronic properties.12–19 BODIPYs are known for their sharp absorption and emission bands, high fluorescence quantum yields and small Stokes shifts in dilute solutions.20 However, because of the hydrophobic core, non-functionalized BODIPYs undergo aggregation in the aqueous medium and their benchmark photophysical properties are compromised under these conditions.21 The utility of simple BODIPY molecules are thus limited for biological applications.
Efforts are ongoing to develop new BODIPYs that retain their excellent photophysical properties in solution as well as in the aggregated/solid state.22 A commonly adopted strategy in this direction is to attach bulky molecules on the main backbone to reduce the intramolecular rotations.23–32 In another approach, BODIPYs are substituted with known moieties that exhibit the AIE phenomenon.33–39 It has also been shown that molecules with twisted BODIPY cores show interesting luminescence properties.40–42 Although a fair amount of success has been achieved in terms of luminescence properties, many of these strategies required multi-step synthetic routes which limits their widespread utility. Moreover, functional groups should be judiciously selected so as to improve cellular uptake and reduce toxicity if these molecules are to be employed for biological applications. Thus, design and synthesis of simple BODIPY dyes that retain their excellent photophysical properties under a variety of conditions remains a challenging area of research.
Herein we report the synthesis and photophysical properties of a simple BODIPY molecule (I6) bearing six iodine atoms. Because of the peculiar structure, I6 exhibited low emission in solution which could be turned-on by aggregation. Iodine atoms facilitated intersystem crossing thus allowing generation of singlet oxygen. Preliminary photobiological studies revealed that this molecule has the potential to be used as an efficient photosensitizer for photodynamic therapy (PDT).
Results and discussion
The synthesis of the BODIPY molecule I6 was achieved as shown in Fig. 1a. 1,3,5-Tribenzene carbonyl chloride on condensation with 2,4-dimethylpyrrole followed by complexation with BF3·Et2O yielded the BODIPY B3 in moderate yields.43 Iodination of B3 with N-iodosuccinimide in dichloromethane gave I6 in 88% yield. Both B3 and I6 were characterized by NMR and high resolution mass spectrometry (Fig. S1, ESI†).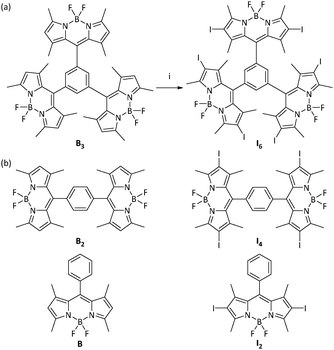 | ||
| Fig. 1 (a) Synthesis of I6. Reactions conditions. (i) N-Iodosuccinimide, dichloromethane, 25 °C, 4 h. (b) Chemical structures of related BODIPY molecules reported elsewhere.44,45 | ||
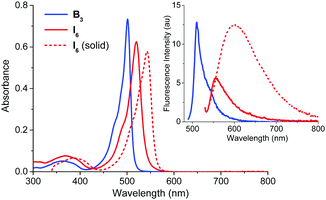
At the outset, we were interested in studying the photophysical properties of B3 and I6 under a variety of conditions. As shown in Fig. 2 and Table 1, B3 and I6 showed absorption maxima at 502 and 520 nm, respectively in acetonitrile whereas the corresponding fluorescence maxima were observed at 525 and 550 nm. B3 was observed to be negligibly fluorescent in solution (ϕf = 0.000543) whereas I6 was observed to be moderately fluorescent. The fluorescence quantum yield and lifetime of I6 were found to be 0.03 and 1.65 ns in acetonitrile (Fig. S2, ESI†), and the radiative (kr) and non-radiative (knr) decay constants were calculated to be 1.8 × 108 and 1.8 × 107 s−1, respectively. It is noteworthy that I6 was observed to be fluorescent in the solid state as well. The solid state absorption spectrum of I6 was slightly broader with a maximum at 542 nm and the emission maximum appeared at 604 nm. The fluorescence lifetime of I6 was significantly longer (τ = 4.71 ns) in the solid state as compared to that in solution. On the other hand, B3 was not observed to be emissive in the solid state. The bathochromically shifted absorption and emission peaks and longer fluorescence lifetime of I6 in the solid state indicates aggregation of the molecules in the solid state.
| λ abs (nm) | λ em (nm) | ϕ f | ϕ Δ | |||||
|---|---|---|---|---|---|---|---|---|
| a In solution. b As aggregates in aqueous medium. c Not reported. d Not determined as photo-bleaching was observed. | ||||||||
| B | 50145 | 55046 | 51045 | 50546 | 0.6445 | 0.00846 | ||
| B2 | 502 | 0.0247 | 0.071 | |||||
| B3 | 501 | 502 | 510 | 550 | 0.000543 | 0.013 | ||
| I2 | 53345 | 53745 | 0.0245 | 0.7345 | ||||
| I4 | 54044 | 0.6844 | ||||||
| I6 | 520 | 506 | 550 | 566 | 0.03 | 0.15 | 0.21 | 0.82 |
The absorption and emission properties of B3 and I6 are anomalous as compared to typical BODIPY dyes which are known for their sharp absorption and fluorescence spectra with high quantum yields that are typically independent of their environment. To gain insights into the luminescence behaviour of I6, we studied the effect of temperature and viscosity on the fluorescence of I6. When a solution of I6 in acetonitrile was heated from 5 to 60 °C, the absorption spectrum remained unaffected whereas the emission intensity decreased regularly (Fig. 3a). At 60 °C, the fluorescence emission intensity was ∼6 times lower as compared to that at 5 °C. The effect of viscosity on the absorption and emission spectra of I6 was then studied. When a highly viscous solvent such as glycerol was added to a solution of I6 in acetonitrile, we observed broadening of the absorption spectrum (Fig. 3b). Further, a new peak emerged at 463 nm and became prominent as the amount of glycerol was increased to 90%. Under similar conditions, the fluorescence intensity of I6 was observed to increase ∼17 times. However, we observed turbidity in the solution as the amount of glycerol was increased above 50% thereby indicating the aggregation of I6.
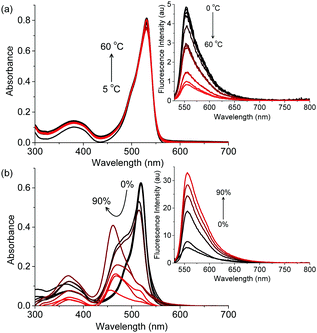 | ||
| Fig. 3 Changes in the absorption and (inset) emission spectra of I6 (4.5 μM) as a function of varying (a) temperature and (b) amount of glycerol in acetonitrile. λex is 520 nm. | ||
As glycerol was promoting aggregation of I6, we were intrigued to investigate whether other solvents induced similar changes. In this context, the effect of solvent polarity was evaluated by recording the absorption and emission of I6 in different solvents. We observed that in non-polar solvents I6 showed a red-shift of ∼20 nm in the absorption maximum as compared to that in polar solvents (Fig. S3 and S4, ESI†). A similar trend was observed in the emission behaviour as well wherein we observed a maximum at 545–550 nm in polar solvents whereas in non-polar solvents the emission maximum was red shifted by ∼40 nm. These observations indicated the non-polar character of the I6 molecule.
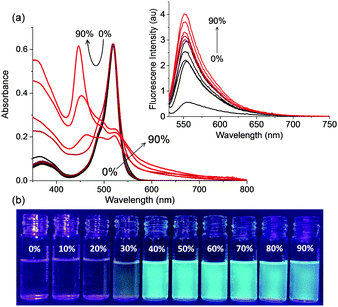
Our next objective was to study the aggregation of I6 in acetonitrile–water mixtures. I6, which showed an absorption peak at 520 nm in acetonitrile, exhibited significantly broadened and red-shifted absorption upon increasing water content. In 90% water–acetonitrile mixture, the long-wavelength absorption band was observed to redshift by 7 nm and a new sharp absorption peak appeared at 445 nm (Fig. 4a). The fluorescence intensity of I6 was observed to increase with the addition of water and we noticed ∼13-times enhancement in the fluorescence in 90% water–acetonitrile mixture. The fluorescence quantum yield of I6 determined in 90% water–acetonitrile mixture was found to be ϕf = 0.075 which was 4-times higher than that in acetonitrile. Moreover, these changes were significant enough to result in visual changes in the fluorescence of I6 and we observed a change in colour of I6 from transparent pale green in acetonitrile to bluish-green in 90% water–acetonitrile mixture. Under similar experimental conditions, we also studied the effect of aggregation on the photophysical properties of B3. However, in contrast to the results obtained with I6, B3 exhibited negligible changes in the absorption and emission spectra in acetonitrile–water mixtures thereby ruling out aggregation induced emission (Fig. S5, ESI†).
Since heavy atoms like bromine and iodine are known to enhance the intersystem crossing efficiency of organic dyes,48–51 we inferred that triplet excited states will be formed in I6. The formation of triplet excited states can facilitate generation of reactive oxygen species thus making I6 suitable as a photosensitizer for photodynamic therapy. To test this hypothesis, we studied the photosensitization properties of I6 in acetonitrile using 1,3-diphenylisobenzofuran (DPBF) as a trapping agent for singlet oxygen. On photoirradiation of a mixture of I6 and DPBF in acetonitrile, we observed a gradual decrease in the absorbance of DPBF at 420 nm whereas the absorbance of I6 at 520 nm remained unperturbed (Fig. S6 and S7, ESI†). These results indicate that I6 was photostable under our experimental conditions and that it sensitizes the generation of singlet oxygen. The presence of singlet oxygen was further confirmed by recording its characteristic emission at 1290 nm52 in acetonitrile (Fig. S8, ESI†). The efficiency of singlet oxygen generation of I6 was quantified by calculating the quantum yield using methylene blue as a reference standard and was obtained as ϕΔ = 0.21 (Fig. S9, ESI†).
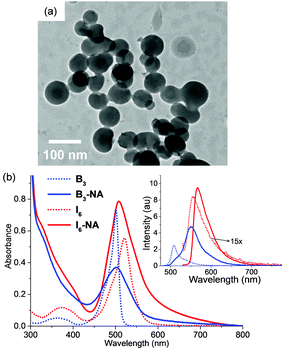
As I6 exhibited enhanced emission in acetonitrile–water mixtures and was capable of generating singlet oxygen, we were interested in studying if nanoaggregates of I6 could be synthesized for studying the photosensitization properties in the aqueous medium. These nanoaggregates would be dispersible in the aqueous medium and would show superior fluorescence and photosensitization properties thereby making it an ideal active ingredient for image-guided PDT. Nanoaggregates of I6 (denoted hereafter as I6-NA) were synthesized by the re-precipitation method wherein a concentrated solution of I6 in acetone was added to large excess of water and the nanoaggregates were collected by centrifugation (see the experimental section for the detailed procedure). I6-NA was dispersed in water and characterized by dynamic light scattering (DLS) and microscopy. As shown in Fig. S10 (ESI†), DLS analysis showed an average particle size of 85.6 ± 3.1 nm for I6-NA. Similarly, high resolution transmission electron microscopy measurements (Fig. 5a) showed the presence of spherical particles and the particle size corroborated well with that obtained from DLS experiments.
It is assumed that I6 undergoes spontaneous self-assembly during the nanoaggregate formation the evidence for which was obtained from 19F NMR spectroscopy. As shown in Fig. S11 (ESI†), I6 in CDCl3 showed a multiplet from δ –145.94 to –146.28 ppm corresponding to the –BF2 moieties. On the other hand, we observed a broad singlet at –119.28 ppm in the 19F NMR spectrum of I6-NA in D2O. Although a comparison of the NMR spectra in organic and aqueous media are inappropriate, the peak broadening and the downfield shift indicates the aggregation of I6 in I6-NA. The formation of I6-NA was also accompanied by changes in the absorption and emission spectra of I6 (Fig. 5b). I6-NA showed a broad absorption peak that was blue shifted by 13 nm as compared to I6 in acetonitrile whereas the emission maximum for I6-NA was observed at 568 nm. The fluorescence quantum yield and lifetime of I6-NA were observed to be 0.15 and 3.12 ns, respectively in water which were substantially higher as compared to that of I6 in acetonitrile. Similarly, the radiative (kr = 4.8 × 108 s−1) and non-radiative (knr = 4.8 × 107 s−1) decay constants were also observed to be higher in the aggregated state as compared to that in acetonitrile. Nanoaggregates of B3 (denoted as B3-NA) were also synthesized by following a similar procedure. Fig. S10 (ESI†) and Fig. 5b shows the characterization data and the absorption and emission spectra of B3-NA. It is remarkable to note that B3-NA was observed to be negligibly fluorescent with a quantum yield of 0.013 and this observation was in stark contrast to the fluorescence properties of I6-NA.
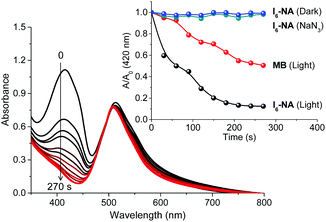
Next, we were interested in studying the photosensitization properties of the nanoaggregates in water. Similar to the experiments carried out in acetonitrile, the singlet oxygen generation ability of I6-NA was studied using DPBF as a singlet oxygen trap. As shown in Fig. 6, we observed a gradual decrease in the absorbance of DPBF at 410 nm upon photoirradiating a mixture of I6-NA and DPBF. It is noteworthy that I6-NA was highly efficient in generating singlet oxygen as compared to I6 and the quantum yield of singlet oxygen generation was found to be 0.82 (Fig. S9, ESI†). The formation of singlet oxygen was further confirmed by monitoring the emission of 1O2 at 1280 nm in water (Fig. S8, ESI†). Further, control experiments carried out in dark and in the presence of sodium azide conclusively proved the photosensitized generation of singlet oxygen by I6-NA.
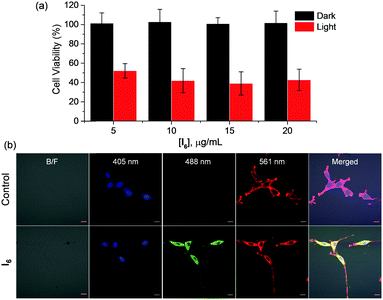
Our results indicate that I6-NA generates singlet oxygen efficiently in the aqueous medium upon photoirradiation. The efficacy of I6-NA as photodynamic agents was tested against C6-glioblastoma cell line upon photoirradiation. When the cells were incubated with different concentrations of I6-NA such as 5, 10 and 20 μg mL−1 and photoirradiated for 10 minutes, we observed up to ∼60% cell death (Fig. 7a). On the other hand, cells incubated with similar concentrations of I6-NA and kept in dark conditions were observed to be almost 100% viable thereby confirming photosensitized generation of singlet oxygen only in light conditions. Since the nanoaggregates were fluorescent, their cellular localization was studied by incubating I6-NA with C6 cells followed by fixation and treatment with DAPI/Phalloidin-TRITC (nuclear/cytoskeletal labels) prior to imaging by confocal microscopy. As I6-NA exhibited an emission that tailed up to ∼750 nm, we could image the cells in the green and red channels. It was observed that I6-NA localized in the cytoplasm around the nuclear region when simultaneously scanned with 405/488/561 nm excitations. This was exemplified in the merged image of Fig. 7b wherein the I6-NA appears yellow around the blue region. Phalloidin-TRITC assisted in marking the boundary of the cells and its emission was clearly limited only to the cell surface as seen in control.
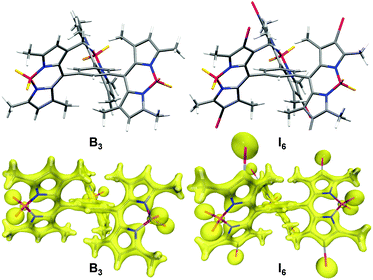
BODIPYs are known to be highly emissive in solution with many molecules exhibiting fluorescence quantum yield close to unity.16,20,53–55 However, B3 is an exception and it was observed to be negligibly fluorescent. The peculiar structure of B3 could have contributed to its non-emissive character. Molecular simulations indicate that the BODIPY moieties are oriented orthogonally as compared to the central phenyl ring (Fig. 8). As compared to the previously reported monomeric and dimeric BODIPYs B and B2 (Fig. 1b), BODIPY cores in B3 are arranged in close proximity with an average distance of 7.649 Å between the BODIPY units. It is known that the presence of multiple chromophores in close proximity often leads to exciton coupling between dyes thereby resulting in significant changes in the absorption and emission properties.56–59 Thus, the presence of three BODIPY units around the central phenyl ring plausibly promotes intramolecular interactions thereby resulting in quenching of fluorescence. The observation of negligible difference in the absorbance but a significant red-shift in the emission maximum of B3 as compared to B and B2 (Table 1) suggests excited state interactions. Further, intramolecular rotations about the C–C bond joining the BODIPY unit to the phenyl ring contribute towards the quenching effect.
The photophysical behaviour of I6 in acetonitrile was on expected lines and was comparable to I2 and I4. It was recently reported that I4 having four iodine atoms fared poorly against I2 having two iodines in terms of singlet oxygen generation.44 This observation was rationalized on the basis of the competition between heavy atom effect and configuration of I4. Our results support this observation wherein I6 exhibited a further lower singlet oxygen generation efficiency of 0.21 in acetonitrile as compared to 0.73 and 0.68 for I2 and I4, respectively. However, we observed a dramatic change in the photophysical behaviour of I6 upon aggregation: the fluorescence was turned-on and a substantial increase was observed in the generation of singlet oxygen. We propose a model for the aggregation of I6 as depicted in Fig. 9 for explaining the unforeseen behaviour of the aggregates of I6. Because of the peculiar geometry consisting of the orthogonal BODIPY units, the chromophore (BODIPY) in the I6 molecule cannot form aggregates in a conventional manner wherein a large number of the chromophores self-assemble through non-covalent interactions (Fig. 9a). Instead, the self-assembly is limited to the formation of H-type dimers with respect to the chromophore units. The observation of blue-shifted absorption in glycerol and in acetonitrile–water mixture exemplifies this proposition. However, the aggregation restricts the rotation of the BODIPY units and enhances the emission intensity and the efficiency of singlet oxygen generation.60 It may also be noted that the increased absorption cross-section of I6-NA in water as compared to I6 in acetonitrile also contributes to the enhanced 1O2 generation efficiency.
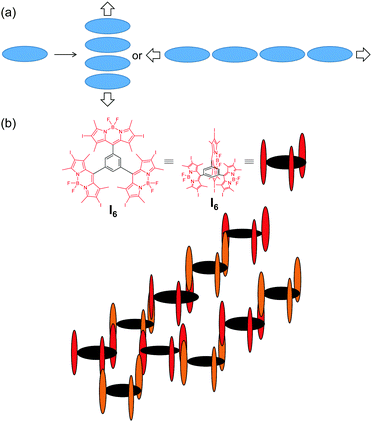 | ||
| Fig. 9 Schematic representation of aggregation in (a) conventional planar molecules and (b) I6. The alternate molecules of I6 are represented in different colours for better visualization. | ||
It is intriguing to note that despite having similar structures, the aggregates of B3 and I6 exhibited extremely different fluorescence properties. The key to the remarkable photophysical properties of I6-NA is the presence of the iodo-substituents. It is proposed that the iodo-groups force the BODIPY units to stack in an off-set manner because of their steric bulkiness. Moreover, iodine atoms are known to form halogen bonds61 and thus would promote intermolecular interactions. This in turn would reduce the intramolecular excitonic interactions between the individual BODIPY units in I6 as compared to that in B3 thereby resulting in an enhanced fluorescence and photosensitization efficiency. The progressive red-shift in the emission spectrum of I6 from solution to the aggregated state in water and further to the solid state could also be rationalized on the basis of halogen bonding which would be stronger in the solid state as compared to that in I6-NA in water due to the competition from water molecules.
Conclusions
We synthesised a hexaiodinated BODIPY molecule with interesting photophysical properties. The molecule showed low fluorescence in acetonitrile whereas the emission was enhanced significantly in the aggregated state. Similarly, the photosensitization properties were also observed to be more efficient in the aggregated state as compared to that in solution. Photobiological studies showed that this molecule was capable of functioning as a photosensitizer and imaging agent for photodynamic therapy. Molecular simulations indicated that the peculiar arrangement of the BODIPY units and the presence of iodine atoms as responsible for its emission characteristics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Science and Engineering Research Board, New Delhi (PDF/2017/001221). We thank Mr Aritra Mukhopadhyaya (INST) and Dr Md. Ehesan Ali (INST) for molecular simulations, Prof. Sameer Sapra, Department of Chemistry, IIT Delhi for help with luminescence measurements and SAIF (Panjab University, Chandigarh) for analytical facilities.References
- Y. Chen, J. W. Y. Lam, R. T. K. Kwok, B. Liu and B. Z. Tang, Aggregation-Induced Emission: Fundamental Understanding and Future Developments, Mater. Horiz., 2019, 6, 428–433 RSC.
- Y. Li, S. Liu, T. Han, H. Zhang, C. Chuah, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Sparks Fly When AIE Meets with Polymers, Mater. Chem. Front., 2019, 3, 2207–2220 RSC.
- Z. He, C. Ke and B. Z. Tang, Journey of Aggregation-Induced Emission Research, ACS Omega, 2018, 3, 3267–3277 CrossRef CAS PubMed.
- H. Wang, E. Zhao, J. W. Y. Lam and B. Z. Tang, AIE Luminogens: Emission Brightened by Aggregation, Mater. Today, 2015, 18, 365–377 CrossRef CAS.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- H.-B. Cheng, Y. Li, B. Z. Tang and J. Yoon, Assembly Strategies of Organic-Based Imaging Agents for Fluorescence and Photoacoustic Bioimaging Applications, Chem. Soc. Rev., 2020, 49, 21–31 RSC.
- K. C. Chong, F. Hu and B. Liu, AIEgen Bioconjugates for Specific Detection of Disease-Related Protein Biomarkers, Mater. Chem. Front., 2019, 3, 12–24 RSC.
- H. Wang and G. Liu, Advances in Luminescent Materials with Aggregation-Induced Emission (AIE) Properties for Biomedical Applications, J. Mater. Chem. B, 2018, 6, 4029–4042 RSC.
- H. Gao, X. Zhao and S. Chen, AIEgen-Based Fluorescent Nanomaterials: Fabrication and Biological Applications, Molecules, 2018, 23, 419 CrossRef PubMed.
- J. Mei, Y. Huang and H. Tian, Progress and Trends in AIE-Based Bioprobes: A Brief Overview, ACS Appl. Mater. Interfaces, 2018, 10, 12217–12261 CrossRef CAS PubMed.
- J. Qian and B. Z. Tang, AIE Luminogens for Bioimaging and Theranostics: From Organelles to Animals, Chem, 2017, 3, 56–91 CAS.
- M. A. Filatov, Heavy-Atom-Free BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer, Org. Biomol. Chem., 2020, 18, 10–27 RSC.
- A. Turksoy, D. Yildiz and E. U. Akkaya, Photosensitization and Controlled Photosensitization with BODIPY Dyes, Coord. Chem. Rev., 2019, 379, 47–64 CrossRef CAS.
- P. Kaur and K. Singh, Recent Advances in the Application of BODIPY in Bioimaging and Chemosensing, J. Mater. Chem. C, 2019, 7, 11361–11405 RSC.
- C. S. Kue, S. Y. Ng, S. H. Voon, A. Kamkaew, L. Y. Chung, L. V. Kiew and H. B. Lee, Recent Strategies to Improve Boron Dipyrromethene (BODIPY) for Photodynamic Cancer Therapy: An Updated Review, Photochem. Photobiol. Sci., 2018, 17, 1691–1708 RSC.
- L. Jean-Gérard, W. Vasseur, F. Scherninski and B. Andrioletti, Recent Advances in the Synthesis of [a]-Benzo-Fused BODIPY Fluorophores, Chem. Commun., 2018, 54, 12914–12929 RSC.
- J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, The Triplet Excited State of Bodipy: Formation, Modulation and Application, Chem. Soc. Rev., 2015, 44, 8904–8939 RSC.
- A. Kamkaew, S. Hui Lim, H. Boon Lee, L. Voon Kiew, L. Yong Chung and K. Burgess, BODIPY Dyes in Photodynamic Therapy, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- N. Boens, V. Leen and W. Dehaen, Fluorescent Indicators Based on BODIPY, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- A. Loudet and K. Burgess, BODIPY Dyes and Their Derivatives:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Syntheses and Spectroscopic Properties, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
Syntheses and Spectroscopic Properties, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed. - J. Bañuelos, BODIPY Dye, the Most Versatile Fluorophore Ever?, Chem. Rec., 2016, 16, 335–348 CrossRef PubMed.
- Z. Liu, Z. Jiang, M. Yan and X. Wang, Recent Progress of BODIPY Dyes with Aggregation-Induced Emission, Front. Chem., 2019, 7, 712 CrossRef PubMed.
- C. Yu, Z. Huang, W. Gu, Q. Wu, E. Hao, Y. Xiao, L. Jiao and W.-Y. Wong, A Novel Family of AIE-Active Meso-2-Ketopyrrolyl BODIPYs: Bright Solid-State Red Fluorescence, Morphological Properties and Application as Viscosimeters in Live Cells, Mater. Chem. Front., 2019, 3, 1823–1832 RSC.
- C. Duan, Y. Zhou, G.-G. Shan, Y. Chen, W. Zhao, D. Yuan, L. Zeng, X. Huang and G. Niu, Bright Solid-State Red-Emissive BODIPYs: Facile Synthesis and Their High-Contrast Mechanochromic Properties, J. Mater. Chem. C, 2019, 7, 3471–3478 RSC.
- R. P. Paitandi, R. S. Singh, B. K. Dwivedi, V. D. Singh and D. S. Pandey, Time Dependent Aggregation Induced Emission Enhancement and the Study of Molecular Packing in Closely Related Azo-Phenol BODIPY Species, Dalton Trans., 2018, 47, 3785–3795 RSC.
- H. Gao, Y. Gao, C. Wang, D. Hu, Z. Xie, L. Liu, B. Yang and Y. Ma, Anomalous Effect of Intramolecular Charge Transfer on the Light Emitting Properties of BODIPY, ACS Appl. Mater. Interfaces, 2018, 10, 14956–14965 CrossRef CAS PubMed.
- Q. Li and Y. Qian, Aggregation-Induced Emission Enhancement and Cell Imaging of a Novel (Carbazol-N-Yl)Triphenylamine–BODIPY, New J. Chem., 2016, 40, 7095–7101 RSC.
- S. Zhang, Y. Wang, F. Meng, C. Dai, Y. Cheng and C. Zhu, Circularly Polarized Luminescence of AIE-Active Chiral O-BODIPYs Induced via Intramolecular Energy Transfer, Chem. Commun., 2015, 51, 9014–9017 RSC.
- T. T. Vu, M. Dvorko, E. Y. Schmidt, J.-F. Audibert, P. Retailleau, B. A. Trofimov, R. B. Pansu, G. Clavier and R. Méallet-Renault, Understanding the Spectroscopic Properties and Aggregation Process of a New Emitting Boron Dipyrromethene (BODIPY), J. Phys. Chem. C, 2013, 117, 5373–5385 CrossRef CAS.
- S. Choi, J. Bouffard and Y. Kim, Aggregation-Induced Emission Enhancement of a Meso-Trifluoromethyl BODIPY via J-Aggregation, Chem. Sci., 2013, 5, 751–755 RSC.
- L. Gai, H. Lu, B. Zou, G. Lai, Z. Shen and Z. Li, Synthesis and Spectroscopic Properties of Bodipy Dimers with Effective Solid-State Emission, RSC Adv., 2012, 2, 8840–8846 RSC.
- D. Zhang, Y. Wen, Y. Xiao, G. Yu, Y. Liu and X. Qian, Bulky 4-Tritylphenylethynyl Substituted Boradiazaindacene: Pure Red Emission, Relatively Large Stokes Shift and Inhibition of Self-Quenching, Chem. Commun., 2008, 4777–4779 RSC.
- J. Zhu, J. Zou, Z. Zhang, J. Zhang, Y. Sun, X. Dong and Q. Zhang, An NIR Triphenylamine Grafted BODIPY Derivative with High Photothermal Conversion Efficiency and Singlet Oxygen Generation for Imaging Guided Phototherapy, Mater. Chem. Front., 2019, 3, 1523–1531 RSC.
- R. S. Singh, A. Kumar, S. Mukhopadhyay, G. Sharma, B. Koch and D. S. Pandey, An Unconventional Mechanistic Insight on Aggregation Induced Emission in Novel Boron Dipyrromethenes and Their Rational Biological Realizations, J. Phys. Chem. C, 2016, 120, 22605–22614 CrossRef CAS.
- M. H. Chua, Y. Ni, M. Garai, B. Zheng, K.-W. Huang, Q.-H. Xu, J. Xu and J. Wu, Towards Meso-Ester BODIPYs with Aggregation-Induced Emission Properties: The Effect of Substitution Positions, Chem. – Asian J., 2015, 10, 1631–1634 CrossRef CAS PubMed.
- R. S. Singh, R. K. Gupta, R. P. Paitandi, M. Dubey, G. Sharma, B. Koch and D. S. Pandey, Morphological Tuning via Structural Modulations in AIE Luminogens with the Minimum Number of Possible Variables and Their Use in Live Cell Imaging, Chem. Commun., 2015, 51, 9125–9128 RSC.
- S. Mukherjee and P. Thilagar, Fine-Tuning Dual Emission and Aggregation-Induced Emission Switching in NPI–BODIPY Dyads, Chem. – Eur. J., 2014, 20, 9052–9062 CAS.
- Z. Li, M. Zheng, X. Guan, Z. Xie, Y. Huang and X. Jing, Unadulterated BODIPY-Dimer Nanoparticles with High Stability and Good Biocompatibility for Cellular Imaging, Nanoscale, 2014, 6, 5662–5665 RSC.
- R. Hu, C. F. A. Gómez-Durán, J. W. Y. Lam, J. L. Belmonte-Vázquez, C. Deng, S. Chen, R. Ye, E. Peña-Cabrera, Y. Zhong and K. S. Wong, et al., Synthesis, Solvatochromism, Aggregation-Induced Emission and Cell Imaging of Tetraphenylethene-Containing BODIPY Derivatives with Large Stokes Shifts, Chem. Commun., 2012, 48, 10099–10101 RSC.
- X.-F. Zhang, BODIPY Photosensitizers Based on PET and Heavy Atom Effect: A Comparative Study on the Efficient Formation of Excited Triplet State and Singlet Oxygen in BODIPY Dimers and Monomers, J. Photochem. Photobiol., A, 2018, 355, 431–443 CrossRef CAS.
- M. T. Whited, N. M. Patel, S. T. Roberts, K. Allen, P. I. Djurovich, S. E. Bradforth and M. E. Thompson, Symmetry-Breaking Intramolecular Charge Transfer in the Excited State of Meso-Linked BODIPY Dyads, Chem. Commun., 2011, 48, 284–286 RSC.
- M. Bröring, R. Krüger, S. Link, C. Kleeberg, S. Köhler, X. Xie, B. Ventura and L. Flamigni, Bis(BF2)-2,2′-Bidipyrrins (BisBODIPYs): Highly Fluorescent BODIPY Dimers with Large Stokes Shifts, Chem. – Eur. J., 2008, 14, 2976–2983 CrossRef PubMed.
- Y. Liu, N. Song, L. Chen and Z. Xie, Triple-BODIPY Organic Nanoparticles with Particular Fluorescence Emission, Dyes Pigm., 2017, 147, 241–245 CrossRef CAS.
- J. Zou, Z. Yin, K. Ding, Q. Tang, J. Li, W. Si, J. Shao, Q. Zhang, W. Huang and X. Dong, BODIPY Derivatives for Photodynamic Therapy: Influence of Configuration versus Heavy Atom Effect, ACS Appl. Mater. Interfaces, 2017, 9, 32475–32481 CrossRef CAS PubMed.
- W. Li, L. Li, H. Xiao, R. Qi, Y. Huang, Z. Xie, X. Jing and H. Zhang, Iodo-BODIPY: A Visible-Light-Driven, Highly Efficient and Photostable Metal-Free Organic Photocatalyst, RSC Adv., 2013, 3, 13417–13421 RSC.
- K. Naim, S. T. Nair, P. Yadav, A. Shanavas and P. P. Neelakandan, Supramolecular Confinement within Chitosan Nanocomposites Enhances Singlet Oxygen Generation, ChemPlusChem, 2018, 83, 418–422 CrossRef CAS PubMed.
- K. Mochizuki, L. Shi, S. Mizukami, M. Yamanaka, M. Tanabe, W.-T. Gong, A. F. Palonpon, S. Kawano, S. Kawata and K. Kikuchi, et al., Nonlinear Fluorescence Imaging by Photoinduced Charge Separation, Jpn. J. Appl. Phys., 2015, 54, 042403 CrossRef.
- Y. P. Rey, D. G. Abradelo, N. Santschi, C. A. Strassert and R. Gilmour, Quantitative Profiling of the Heavy-Atom Effect in BODIPY Dyes: Correlating Initial Rates, Atomic Numbers, and 1O2 Quantum Yields, Eur. J. Inorg. Chem., 2017, 2170–2178 CrossRef CAS.
- V. Lakshmi, M. R. Rao and M. Ravikanth, Halogenated Boron-Dipyrromethenes: Synthesis, Properties and Applications, Org. Biomol. Chem., 2015, 13, 2501–2517 RSC.
- K. N. Solov’ev and E. A. Borisevich, Intramolecular Heavy-Atom Effect in the Photophysics of Organic Molecules, Phys.-Usp., 2005, 48, 231 CrossRef.
- S. P. McGlynn, J. Daigre and F. J. Smith, External Heavy-Atom Spin-Orbital Coupling Effect. IV. Intersystem Crossing, J. Chem. Phys., 1963, 39, 675–679 CrossRef CAS.
- Z. Wang, X. Hong, S. Zong, C. Tang, Y. Cui and Q. Zheng, BODIPY-Doped Silica Nanoparticles with Reduced Dye Leakage and Enhanced Singlet Oxygen Generation, Sci. Rep., 2015, 5, 12602 CrossRef CAS PubMed.
- R. S. Llano, E. A. Zaballa, J. Bañuelos, C. F. A. G. Durán, J. L. B. Vázquez, E. P. Cabrera and I. L. Arbeloa, Tailoring the Photophysical Signatures of BODIPY Dyes: Toward Fluorescence Standards across the Visible Spectral Region, in Photochemistry and Photophysics – Fundamentals to Applications, IntechOpen, 2018, pp. 21–40 Search PubMed.
- A. Nagai, K. Kokado, J. Miyake and Y. Chujo, Quantum Yield and Morphology Control of BODIPY-Based Supramolecular Self-Assembly with a Chiral Polymer Inhibitor, Polym. J., 2010, 42, 37–42 CrossRef CAS.
- L. Wang, Y. Zhang and Y. Xiao, Meso-Alkoxy BODIPYs with a Good Balance between Larger Stokes Shifts and Higher Fluorescence Quantum Yields, RSC Adv., 2013, 3, 2203–2206 RSC.
- A. Oliden-Sánchez, R. Sola-Llano, J. Bañuelos, I. García-Moreno, C. Uriel, J. C. López and A. M. Gómez, Tuning the Photonic Behavior of Symmetrical Bis-BODIPY Architectures: The Key Role of the Spacer Moiety, Front. Chem., 2019, 7, 801 CrossRef PubMed.
- T. Brixner, R. Hildner, J. Köhler, C. Lambert and F. Würthner, Exciton Transport in Molecular Aggregates – From Natural Antennas to Synthetic Chromophore Systems, Adv. Energy Mater., 2017, 7, 1700236 CrossRef.
- J. Ahrens, A. Scheja, R. Wicht and M. Bröring, Excitonic Coupling in Acyclic and Cyclic Dithioaryl-Linked BODIPY DYEmers, Eur. J. Org. Chem., 2016, 2864–2870 CrossRef CAS.
- M. Kasha, H. R. Rawls and E.-B. M. Ashraf, The Exciton Model in Molecular Spectroscopy, Pure Appl. Chem., 1965, 11, 371–392 CAS.
- R. Hu, E. Lager, A. Aguilar-Aguilar, J. Liu, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, Y. Zhong, K. S. Wong and E. Peña-Cabrera, et al., Twisted Intramolecular Charge Transfer and Aggregation-Induced Emission of BODIPY Derivatives, J. Phys. Chem. C, 2009, 113, 15845–15853 CrossRef CAS.
- P. Xu, Q. Qiu, X. Ye, M. Wei, W. Xi, H. Feng and Z. Qian, Halogenated Tetraphenylethene with Enhanced Aggregation-Induced Emission: An Anomalous Anti-Heavy-Atom Effect and Self-Reversible Mechanochromism, Chem. Commun., 2019, 55, 14938–14941 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization and spectroscopy data. See DOI: 10.1039/d0qm00010h |
| This journal is © the Partner Organisations 2020 |