Enhanced thermoelectric properties of Sb2Te3/CH3NH3I hybrid thin films by post-annealing
Shuo
Chen
a,
Fu
Li
 a,
Yuexing
Chen
a,
Jingting
Luo
a,
Guangxing
Liang
a,
Yuexing
Chen
a,
Jingting
Luo
a,
Guangxing
Liang
 a,
Xianghua
Zhang
b,
Zhuanghao
Zheng
a,
Xianghua
Zhang
b,
Zhuanghao
Zheng
 *a and
Ping
Fan
a
*a and
Ping
Fan
a
aShenzhen Key Laboratory of Advanced Thin Films and Applications, College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060, China. E-mail: zhengzh@szu.edu.cn
bUniv Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes), UMR 6226, F-35000 Rennes, France
First published on 28th October 2019
Abstract
In this work, to improve the ZT value of Sb2Te3, a novel concept of inorganic–organic hybridization is presented and Sb2Te3/CH3NH3I hybrid thin films have been prepared via a post-annealing involved sequential sputtering/evaporation method. The results show that the crystallinity of the thin films is greatly improved after post-annealing with preferential orientations of (006) and (009) at an appropriate temperature, leading to an obvious enhancement of electrical conductivity. Additionally, the hybrid thin films show uniform nano-sized grainy structures and the organic component CH3NH3I has been successfully incorporated into the hybrid system in a stable state, thus resulting in an increase of the Seebeck coefficient and decrease of the thermal conductivity based on the energy filtering effect. As expected, a maximum room-temperature ZT value of 0.56 for the Sb2Te3/CH3NH3I hybrid thin film annealed at 573 K can be obtained. This outcome further demonstrates great potential for the inorganic–organic hybrid thin film in low temperature application scenarios.
Introduction
Thermoelectric (TE) materials can directly convert heat energy into electricity and have great potential applications in semiconductor cooling and power generation fields.1 The development of TE generators for small electronic devices as self-powered components has drawn tremendous research attention as they provide continuous power supply by using heat from other devices.2 However, conventional TE materials are relatively bulky and naturally brittle, thereby limiting the development of micro-TE devices. By contrast, thin film TE materials are lightweight and easily prepared on various types of substrates, offering the possibility for micro-sized TE devices with broad applications in bendable and miniature scenarios.3–6 The efficiency of the TE generator is determined by the properties of its intrinsic materials, which is closely related to a dimensionless figure of merit, expressed as ZT = SσT/κ, where S is the Seebeck coefficient, σ is the electrical conductivity, T is the absolute temperature and κ is the thermal conductivity.7 Thus, simultaneously modifying S, σ, and κ to obtain a TE material with a high ZT value is essential to realize an efficient conversion for widespread commercial TE device applications. Recently, a new concept of TE performance improvement involving inorganic–organic thin film TE nanocomposite materials has emerged. To be specific, combining the inorganic and organic components not only enhances the TE properties, thanks to an energy filtering effect, but also extends the flexibility of the inorganic material.8–11 For instance, Jin et al. fabricated Bi2Te3 thin film composites with highly ordered (0001)-textured nanocrystals on high-quality single-walled carbon nanotube (SWCNT) bundles using the sputtering method, demonstrating a high ZT value and superior flexibility.8 Zhang et al. prepared a p-type Bi0.5Sb1.5Te3/PEDOT:PSS hybrid thin film that has over 2000% enhancement in the power factor.9Classic antimony telluride-based thin films are well known as efficient thermoelectric materials that have been intensively studied and widely applied. For thin film deposition, various techniques such as sputtering,11–15 evaporation,16 electron-deposition17,18 and chemical vapor deposition19 have been applied successfully. As aforementioned, the construction of an inorganic–organic hybrid framework can greatly enhance its pristine TE properties. An important organic material CH3NH3I that originated from inorganic–organic perovskite solar cells20–23 was selected as the organic component in our previous work. Indeed, crystalline CH3NH3I-based materials have excellent electrical transport characteristics with a carrier mobility as high as 10 cm2 V−1 s−1, which is beneficial for thermoelectric materials to obtain more superior thermoelectric properties. The Sb2Te3/CH3NH3I hybrid thin film prepared using the multilayer deposition method has a dramatically enhanced the Seebeck coefficient and decreased thermal conductivity, resulting in a significant increase of ZT compared with the pure Sb2Te3 thin film.24 However, the obtained TE properties are still somewhat unsatisfactory and obviously lower than those of the bulk antimony telluride-based materials. For example, Luo et al. reported that a ZT value of over 1.7 for Sb2Te3 could be obtained under a high magnetic field,25 and a value of 1.5 was also achieved after FeTe2 doping as reported by Shin et al.26 Therefore, improving its ZT value especially for low temperature advantageous scenarios to broaden the scope of thin film based applications needs further exploration. In this work, high-quality inorganic–organic Sb2Te3/CH3NH3I hybrid thin films were prepared using magnetron sputtering in combination with the thermal evaporation method. The effect of post-annealing temperature on the TE properties was systematically investigated. As a result, a ZT value over 0.5 at room temperature for the hybrid thin film has been successfully obtained, surpassing that of the pure Sb2Te3 thin film and comparable to the maximum values of the Sb2Te3-based materials.
Experimental
Preparation of Sb2Te3/CH3NH3I hybrid thin films
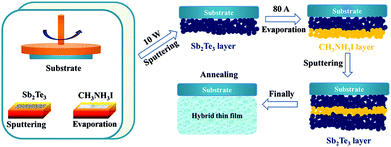
Firstly, methylammonium iodide (CH3NH3I) was synthesized using methylamine (CH3NH2, Sigma-Aldrich, 57 wt% in H2O) and hydroiodic acid (HI, Sigma-Aldrich, 40 wt% in H2O) as raw materials. A mixture of 28 ml CH3NH2 and 30 ml HI was loaded into a 250 ml flask and stirred at 0 °C for 2 hours to form a homogeneous precursor solution. Then it was transferred to a beaker and kept at a fixed temperature of 95 °C until the expected white CH3NH3I powder was obtained. The final purification process was carried out with diethyl ether three times and the high-purity crystalline CH3NH3I powder could be obtained after drying at 60 °C in a vacuum chamber for 10 hours.The Sb2Te3/CH3NH3I inorganic–organic hybrid thin films were prepared through a post-annealing involved sequential sputtering/evaporation method. A schematic illustration of the preparation process is shown in Fig. 1. Prior to deposition, 1.5 mm BK7 glass was sequentially cleaned with acetone, alcohol, and deionized water for 15 min, and then dried under N2 gas. The vacuum chamber was evacuated to an ultrahigh vacuum (2.0 × 10−4 Pa) prior to deposition. Initially, a high-purity Sb2Te3 (99.99%) sputtering target purchased from HZAM Co. Ltd was used; the Sb2Te3 layer was deposited onto the glass substrate with a thickness of approximately 200 nm by magnetron sputtering with a fixed power of 10 W. Then the CH3NH3I layer was evaporated onto the Sb2Te3 layer at a current of 100 A to a thickness of 100 nm. This evaporation process was carried out using a vacuum evaporating apparatus present in the same chamber and by using the as-prepared CH3NH3I powder as the source. Thereafter, another Sb2Te3 precursor layer was deposited onto the CH3NH3I layer to form a sandwich-structured inorganic–organic composite thin film. Finally, the thin films were post-annealed for 1 h under an Ar atmosphere with variable annealing temperatures.
Characterization
The crystal structure of the thin films was investigated through X-ray diffraction (XRD, D/max2500, Rigaku Corporation) with Cu/Kα radiation at a voltage of 40 kV and a counting duration of 10° min−1 ranging from 10° to 70°. Morphologies and elemental composition of the Sb2Te3/CH3NH3I hybrid thin films were determined using a Zeiss supra 55 thermal field emission scanning electron microscope (SEM) equipped with an EDAX instrument. The room-temperature Raman scattering measurements were performed using a LabRam XploRA spectra system (Horiba Jobin Yvon). A steady-state spectrometer (Zolix Scan) equipped with a 325 nm HeCd laser as the excitation source was used to obtain the photoluminescence (PL) spectra. The chemical valence was determined by conducting X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi) with a monochromatic Al Kα X-ray source of 1486.6 eV. The electrical conductivity and the Seebeck coefficient were simultaneously measured at room temperature using a Potential-Seebeck-Microprobe (PSM, Quantum Design) with an estimated 7% instrument error. The carrier concentration and Hall mobility were characterized using a Hall-effect measurement system (HL5500PC, Nanometrics) with the Van der Pauw configuration at room temperature. The thermal conductivity at room temperature was determined by a transient hot-wire theory method (TC3000, Xiaxi Electronic Technology). To obtain a reliable value, each sample was measured ten times and the average value was used also with an estimated error of approximately 10%.Results and discussion
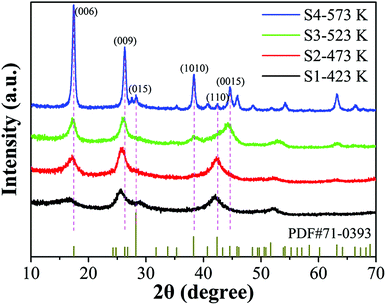
Fig. 2 shows the XRD patterns of the as-prepared Sb2Te3/CH3NH3I hybrid thin films with different post-annealing temperatures. The diffraction peaks belonging to sample S1 annealed at 423 K present a large full-width half maximum and low intensities, suggesting its poor crystallinity under this lower temperature condition. Then the temperature-induced thin film crystallinity improvement can also be observed with a preferential orientation of (006) and (009). When the post-annealing temperature reached up to 573 K, all the sharp and prominent peaks were in agreement with the JCPDS standard card (71-0393) of the hexagonal phase of Sb2Te3 without any detectable impurity. The absence of an observable second phase of CH3NH3I is probably due to its low content.To further investigate the crystalline nature and interior structures of the Sb2Te3/CH3NH3I hybrid thin films, Raman analysis was carried out using a 514.5 nm laser in the range from 50 cm−1 to 300 cm−1. As shown in Fig. 3a, no obvious peaks can be observed for sample S1, indicating its poor crystallinity, which is consistent with the XRD results. With increasing post-annealing temperature, some distinct peaks start to emerge and strengthen due to the improvement of thin film crystallinity. According to the theoretical calculations and previous experimental results, three major peaks located at 63, 106, and 159 cm−1 can match well with the Ag1, Eg2, and Ag2 vibration modes of Sb2Te3, respectively.27 A slight right shift of Eg2 for sample S4 is closely related to the incorporation of CH3NH3I after high temperature post-annealing. Moreover, an additional peak at 133 cm−1 can be identified as the vibration mode of the CH3NH3I based perovskite tetragonal crystal structure,27,28 implying the existence of this second phase. Photoluminescence spectroscopy is also an effective tool for organic component characterization. Fig. 3b shows the corresponding spectra of the hybrid thin films; one observable broad peak located at ∼770 nm belonging to CH3NH3I further confirms that the as-prepared thin film consists of inorganic–organic hybrid structures.29
 | ||
| Fig. 3 (a) Raman scattering spectra and (b) photoluminescence spectra of the hybrid thin films obtained at room temperature. | ||
Fig. 4 presents the SEM images of the thin films, where the temperature-induced surface morphology evolution has been clarified. A number of large clusters randomly dispersed on the surface can be observed for sample S1 (Fig. 4a), indicating that the organic components have not been fully incorporated when exposed to a low post-annealing temperature. Significant decomposition and diffusion of these clusters occurred with an increase in the annealing temperature, leading to uniform nano-sized grainy structures in the thin film. Importantly, the as-designed sandwich structure with a CH3NH3I layer embedded in the middle is really beneficial for this diffusion under post-annealing treatment. Upon further increasing the temperature to 573 K, as shown in Fig. 4d, sample S4 exhibits cocked plate-shaped features, implying that the Sb2Te3/CH3NH3I hybrid thin film has a preferential growth direction that is perpendicular to the substrate, which is also consistent with the XRD results with preferential orientations of (006) and (009). Moreover, the dense and well-shaped plates with an average size of ∼200 nm can benefit the charge transport properties and provide additional scattering centers for the propagation of phonons, thus resulting in reduced thermal conductivity and also an increased Seebeck coefficient. The representative I content belonging to the organic component of CH3NH3I has also been obtained by SEM coupled with EDS analysis; a slight decrease in the atomic percent from 1.90% to 1.50% with increasing post-annealing temperature further indicates that it is essential to control the temperature.
 | ||
| Fig. 4 SEM images of the Sb2Te3/CH3NH3I hybrid thin films with different post-annealing temperatures of (a) 423 K, (b) 473 K, (c) 523 K and (d) 573 K. | ||
XPS analysis was further conducted to investigate the valence states of the three representative constituent elements Sb, Te and I. The corresponding high-resolution core level spectra obtained from all the hybrid thin films are shown in Fig. 5. A sharp peak located at 768.5 eV associated with Sb 3p3/2 is depicted in Fig. 5a, and no obvious modification can be observed by increasing the post-annealing temperature. Additionally, a high-resolution scan of the Te 3d region (Fig. 5b) shows peaks at about 572.6 eV and 583.1 eV, which effectively match the reported values of the binding energies of Te 3d5/2 and Te 3d3/2 of Bi2Te3, respectively.27 The other two peaks that synchronously exist toward higher binding energies at approximately 576.3 eV and 586.7 eV indicate the existence of oxidized states (Te4+) on the surface, which is reasonable to occur after post-annealing of this composite thin film.30 Finally, the spin–orbit-coupled doublet of the I 3d core levels of all the thin films is split into 3d5/2 (∼618.7 eV) and 3d3/2 (∼630.2 eV) with a separation binding energy value of 11.5 eV, which is the typical peak position and separation of the spin–orbit components for I−, further confirming that the organic component CH3NH3I has been incorporated into the hybrid system in a stable state.
In a subsequent study, for a better comparison, pure Sb2Te3 thin films with the same annealing treatment procedures have been prepared and denoted as P1, P2, P3 and P4, respectively. The Hall carrier concentration (n) and Hall carrier mobility (μ) of the pure Sb2Te3 thin films and the Sb2Te3/CH3NH3I hybrid thin films have been experimentally determined at room temperature and illustrated in Fig. 6. As shown in Fig. 6a, the pure Sb2Te3 thin film post-annealed at 423 K (sample P1) has a carrier concentration n as high as 9.91 × 1020 cm−3, which is comparable to that of the reported bulk materials. As the post-annealing temperature increases, a significant linear decrease in the n value can be observed, and it is closely related to the reduction of defect numbers after a more complete crystallization at high temperature. The temperature-induced grain growth and structure densification also lead to a reduction in the scattering of free electrons and therefore an increase in the carrier mobility. This phenomenon resulted in a remarkable increase in the carrier mobility μ from 0.08 cm2 V−1 s−1 at 423 K to 11.3 cm2 V−1 s−1 at 573 K for pure Sb2Te3 thin films. Importantly, for the Sb2Te3/CH3NH3I hybrid thin films, an obvious decrease in the Hall carrier concentration can be attributed to an enhanced scattering effect with the addition of the organic components as scattering centers. Meanwhile, the intrinsic n-type characteristic of CH3NH3I can also cause the recombination of partial electrons and holes, thus further reducing the carrier concentration of the hybrid thin film. A temperature-induced carrier mobility increase due to the improved crystallinity has also been observed in the hybrid thin films. Specifically, the μ values also show an increase of about 2–4 times compared with those of the pure Sb2Te3 thin films, and this is closely related to the formation of denser nanostructures in the presence of the organic component CH3NH3I. The enhanced carrier mobility (up to 44.4 cm2 V−1 s−1) leads to a longer recombination life-time for carriers, which is undoubtedly beneficial for the migration of the electron/hole.
 | ||
| Fig. 6 Hall-effect measurements for the pure Sb2Te3 thin films and the Sb2Te3/CH3NH3I hybrid thin films: (a) Hall carrier concentration and (b) Hall carrier mobility. | ||
Fig. 7 shows the room temperature thermoelectric properties of all the pure Sb2Te3 thin films and Sb2Te3/CH3NH3I hybrid thin films. The corresponding values with attached error bars are determined by measuring the values with estimated instrument errors and measuring errors. Fig. 7a presents the experimentally determined electrical conductivity σ of all the samples. A similar positive relationship between σ and post-annealing temperature can be observed for both pure Sb2Te3 thin films and Sb2Te3/CH3NH3I hybrid thin films according to the typical expression of σ = neμ, where n is the carrier concentration, μ is the carrier mobility and e is the unit charge.31 Thus, carrier mobility is the dominant factor that accounts for the increase in electrical conductivity in the presence of a decreased carrier concentration as depicted previously. Moreover, the maximum σ values are 4.25 × 104 S m−1 and 3.1 × 104 S m−1 for the Sb2Te3 thin film and Sb2Te3/CH3NH3I hybrid thin film post-annealed at 573 K, respectively. This slight decrease is mainly caused by the decreased n value after the incorporation of the organic component, which cannot be fully compensated for by the enhanced μ value. The Seebeck coefficients S of the thin films remain positive at these four different annealing temperatures, indicating their P-type nature, even after the incorporation of the organic component (Fig. 7b). A normal quasi-linear positive relationship between the S values and the post-annealing temperatures for pure Sb2Te3 thin films can be explained by the reduction of some ionized impurities and improvement of crystallinity with increasing temperature. By contrast, the S values are obviously increased after the incorporation of CH3NH3I and reach a maximum value of 432.6 μV K−1 at 473 K for the Sb2Te3/CH3NH3I hybrid thin film. This improvement of S should partially result from the reduced carrier concentration based on the Pisarenko relation.32 Additionally, band bending at the interfaces between the inorganic–organic inclusions can produce a significant scattering effect that might preferentially scatter low energy charge carriers, which can also enhance the density of states and effective mass of the carriers, and thus are beneficial for the increase of S values.
The total thermal conductivity k measured at room temperature is shown in Fig. 7c. The k value of the pure Sb2Te3 thin film post-annealed at 423 K (sample P1) is 0.97 W m−1 K−1, which is lower than that of the bulk Sb2Te3 owing to its poor crystallinity. With increasing post-annealing temperature, a significant increase in the k value can be observed and it could inevitably diminish the improvement of the final ZT value. By contrast, all the Sb2Te3/CH3NH3I hybrid thin films have a lower k value, especially an interesting value as low as 0.8 W m−1 K−1 for the highly crystalline inorganic–organic hybrid thin film post-annealed at 523 K (sample S3). Explanatorily, after the incorporation of the organic component CH3NH3I, it can scatter more phonons thanks to the existence of organic particles and defects. Moreover, the added abundant nanoscale or mesoscale boundaries between the inorganic and organic structures will further increase the effective scattering of phonons. Overall, this decrease in the hybrid structure induced thermal conductivity will be undoubtedly beneficial for the improvement of the ZT value. As shown in Fig. 7d, the calculated ZT values can be increased up to 8.5 times, 6.5 times and 3.1 times when comparing the hybrid thin films and pure Sb2Te3 thin films with annealing treatments at 473 K, 523 K and 573 K, respectively. Notably, a maximum ZT value of 0.56 at room temperature for the Sb2Te3/CH3NH3I hybrid thin film is comparable with the maximum values of the Sb2Te3-based thermoelectric materials.19,21 Furthermore, a ZT value of 0.15 for the Sb2Te3/CH3NH3I hybrid thin film annealed at 473 K is comparable with 0.18 for the pure Sb2Te3 thin film annealed at 573 K. This obvious 100 K temperature difference further demonstrates great potential for the inorganic–organic hybrid thin film in low temperature application scenarios.
Conclusions
In summary, an effective magnetron sputtering in combination with the thermal evaporation method has been used to prepare inorganic–organic Sb2Te3/CH3NH3I hybrid thermoelectric thin films. The post-annealing process induced crystallization and surface morphology evolution as well as the construction of a stable hybrid framework of the thin films can be observed. Moreover, upon increasing the annealing temperature, the Sb2Te3/CH3NH3I hybrid thin films exhibit an obvious improvement in the electrical conductivity and Seebeck coefficient. Thanks to its effective scattering of phonons in the hybrid structure, a decrease of thermal conductivity is also beneficial for the TE performance improvement. As a result, a ZT value as high as 0.56 at room temperature for the Sb2Te3/CH3NH3I hybrid thin film has been successfully obtained, which totally surpasses the ZT value of a pure Sb2Te3 thin film and is comparable with the maximum values of the Sb2Te3-based thermoelectric materials, further demonstrating its great application potential in low temperature scenarios.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 11604212), the Shenzhen Key Lab Fund (ZDSYS 20170228105421966), and the Natural Science Foundation of SZU (grant no. 85304/00000297).Notes and references
- P. J. He and T. M. Tritt, Science, 2017, 357, 1369 Search PubMed.
- Y. Wang, L. Yang, X. L. Shi, X. Shi, L. D. Chen, M. S. Dargusch, J. Zou and Z. G. Chen, Adv. Mater., 2019, 31, 1807916 CrossRef.
- Z. Y. Lu, M. Layani, X. X. Zhao, L. P. Tan, T. Sun, S. F. Fan, Q. Y. Yan, S. Magdassi and H. H. Hng, Small, 2014, 10, 3551 CrossRef CAS.
- A. L. Hansen, T. Dankwort, M. Winkler, J. Ditto, D. C. Johnson, J. D. Koenig, K. Bartholomé, L. Kienle and W. Bensch, Chem. Mater., 2014, 26, 6518 CrossRef CAS.
- X. Mu, H. Zhou, D. He, W. Zhao, P. Wei, W. Zhu, X. Nie, H. Liu and Q. Zhang, Nano Energy, 2017, 33, 55 CrossRef CAS.
- W. Zhu, Y. Deng and L. Cao, Nano Energy, 2017, 34, 463 CrossRef CAS.
- C. Zhang, X. A. Fan, J. Hu, C. Jiang, Q. Xiang, G. Li, Y. Li and Z. He, Adv. Eng. Mater., 2017, 19, 1600696 CrossRef.
- Q. Jin, S. Jiang, Y. Zhao, D. Wang, J. Qiu, D. M. Tang, J. Tan, D. M. Sun, P. X. Hou, X. Q. Chen, K. P. Tai, N. Gao, C. Liu, H. M. Cheng and X. Jiang, Nat. Mater., 2019, 18, 62 CrossRef CAS.
- T. Zhang, K. Li, C. Li, S. Ma, H. H. Hng and L. Wei, Adv. Electron. Mater., 2017, 3, 1770017 Search PubMed.
- H. Shi, C. Liu, Q. Jiang and J. Xu, Adv. Electron. Mater., 2015, 1, 1500017 CrossRef.
- F. Li, J. T. Luo, Z. H. Zheng, G. X. Liang, A. H. Zhong, Y. X. Chen and P. Fan, J. Mater. Sci., 2019, 54, 9565 CrossRef CAS.
- W. Y. Lee, N. W. Park, S. G. Yoon and S. K. Lee, J. Nanosci. Nanotechnol., 2016, 7, 7567 CrossRef.
- I. Hilmi, A. Lotnyk, J. W. Gerlach, P. Schumacher and B. Rauschenbach, APL Mater., 2017, 5, 050701 CrossRef.
- V. D. Das, N. Soundararajan and M. Pattabi, J. Mater. Sci., 1987, 10, 3522 Search PubMed.
- N. Hatsuta, D. Takemori and M. Takashiri, J. Alloys Compd., 2016, 685, 147 CrossRef CAS.
- G. Bulman, P. Barletta, J. Lewis, N. Baldasaro, M. Manno, A. Bar-Cohen and B. Yang, Nat. Commun., 2016, 7, 10302 CrossRef CAS.
- Z. H. Zheng, P. Fan, J. T. Luo, G. X. Liang and D. P. Zhang, J. Electron. Mater., 2013, 42, 3421 CrossRef CAS.
- W. Jang, J. Lee, C. In, H. Choi and A. Soon, ACS Appl. Mater. Interfaces, 2017, 9, 42050 CrossRef CAS.
- S. Shen, W. Zhu, Y. Deng, H. Zhao, Y. Peng and C. Wang, Appl. Surf. Sci., 2017, 414, 197 CrossRef CAS.
- C. Cho, B. Stevens, J. H. Hsu, R. Bureau, D. A. Hagen, O. Regev, C. Yu and J. C. Grunlan, Adv. Mater., 2015, 19, 2996 CrossRef.
- H. Shi, C. Liu, J. Xu, H. Song, B. Lu, F. Jiang, W. Zhou, G. Zhang and Q. Jiang, ACS Appl. Mater. Interfaces, 2013, 5, 12811 CrossRef CAS.
- L. Wang, X. Jia, D. Wang, G. Zhu and J. Li, Synth. Met., 2013, 181, 79 CrossRef CAS.
- S. N. Patel, A. M. Glaudell, D. Kiefer and M. L. Chabinyc, ACS Macro Lett., 2016, 5, 268 CrossRef CAS.
- E. S. Kim, J. Y. Hwang, K. H. Lee, H. Ohta, Y. H. Lee and S. W. Kim, Adv. Mater., 2017, 29, 1604899 CrossRef.
- Y. Luo, J. Yang, Q. Jiang, L. Fu, Y. Xiao, W. X. Li, D. Zhang, Z. Zhou and Y. Cheng, Nano Energy, 2015, 15, 709 CrossRef CAS.
- W. H. Shin, O. W. Roh, B. Ryu, H. J. Chang, H. S. Kim, S. Lee, W. S. Seo and K. Ahn, ACS Appl. Mater. Interfaces, 2018, 104, 3689 CrossRef.
- Z. Zheng, P. Fan, J. Luo, G. Liang, H. Ma, X. Zhang, C. Yang and Y. Q. Fu, Nanoscale, 2018, 10, 13511–13519 RSC.
- B. Park, S. M. Jain, X. Zhang, A. Hagfeldt, G. Boschloo and T. Edvinsson, ACS Nano, 2015, 9, 2088 CrossRef CAS.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019 CrossRef CAS.
- T. S. Su, Y. W. Yin, M. L. Teng, Z. Z. Gong, M. J. Zhang and X. G. Li, J. Appl. Phys., 2013, 114, 183901 CrossRef.
- T. Zhu, Z. Xu, J. He, J. Shen, S. Zhu, L. Hu, T. M. Tritt and X. Zhao, J. Mater. Chem. A, 2013, 1, 11589 RSC.
- H. Wang, Z. M. Gibbs, Y. Takagiwa and G. J. Snyder, Energy Environ. Sci., 2014, 7, 804 RSC.
| This journal is © the Partner Organisations 2020 |