Easily readable palindromic sequence-defined polymers built by cascade thiol-maleimide Michael couplings†
Qiunan
Shi
a,
Xiaohuan
Cao
a,
Yajie
Zhang
a,
Suhua
Duan
a,
Lihua
Hu
b,
Yuxuan
Xu
c,
Jingqiu
Lu
a,
Zhihao
Huang
*a,
Zhengbiao
Zhang
 *ad and
Xiulin
Zhu
ae
*ad and
Xiulin
Zhu
ae
aState and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: zhhuang@suda.edu.cn; zhangzhengbiao@suda.edu.cn
bAnalysis and Testing Center, Soochow University, Suzhou 215123, China
cApplied Technology College of Soochow University, Soochow University, Suzhou 215325, China
dCollaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, China
eGlobal Institute of Software Technology, No. 5 Qingshan Road, Suzhou National Hi-Tech District, Suzhou 215163, China
First published on 8th September 2020
Abstract
Palindromic sequence-induced specific and genetic functions widely exist in biological systems. Nevertheless, the study of synthetic palindromic sequence-defined polymers has received scarce attention due to the challenge of efficient synthesis. Herein, we demonstrated cascade thiol-maleimide Michael couplings (CTMMC) as an efficient chemistry to access palindromic sequences. Taking bromomaleimide as the synthon, the CTMMC enabled the construction of an array of palindromic sequences at an accelerated growth rate via an iterative exponential growth strategy. Moreover, owing to the synergetic cleavages of two C–S bonds located in dithiosuccinimide linkages, the palindromic sequences were easily readable (i.e., decipherable) by tandem mass spectrometry. The CTMMC chemistry endowed structural versatility and diversity to the palindromic sequences, thereby uncovering many potential applications, such as anti-counterfeiting labeling and item identification like artificial “DNA”.
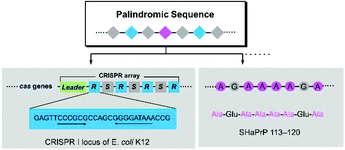
Palindromic sequences represent a unique and important class of sequence structures, which can be deciphered in either the forward or backward direction.1 In biological polymers, palindromic sequences have been extensively explored. For example, the double-stranded palindromic sequence repeats are often associated with the restriction endonuclease recognition site in the DNA of some bacteria. This discovery creates the fundamental of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology.2–6 Palindromic sequences are also frequently observed in proteins as single strands, e.g. the palindromic region AGAAAAGA (PrP113–120) in a prion, which dictate various properties, such as increase of the binding effect, promotion of aggregation, etc. (Fig. 1).7–12 Despite the fact that it is possible to create unique or improved properties/performances, man-made palindromic sequences are rarely explored due to the challenge in their synthesis.
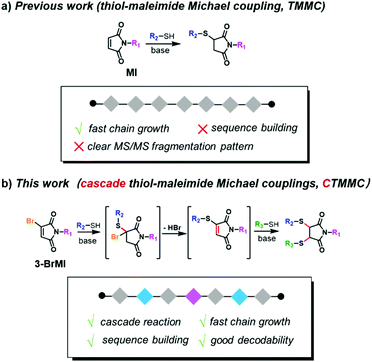
With regard to the efficient synthesis of sequence-defined discrete polymers, the reasonable combination of an optimized synthetic strategy and efficient chemistry is crucial.13–15 During the past years, significant progress on the synthetic strategy of sequence-defined polymers has been made, including solid-phase iterative synthesis,16–19 template approach,20 multicomponent reactions,21,22 single unit monomer insertion23,24 or even complex biomimetic molecular machines25–27 and iterative exponential growth (IEG) strategy.28–35 The IEG strategy (also termed the iterative convergent/divergent strategy) enabled fast chain growth in a “molecular doubling” manner. However, for the construction of defined sequences, IEG requires an efficient and orthogonal side chain installation during main chain growth.15 On the other hand, several robust and efficient reactions have been employed in these strategies, e.g. copper catalyzed azide–alkyne cycloaddition (CuAAC), sulfur-fluoride exchange reaction and thiol–ene addition.36 To date, a typical strategy for constructing palindromic sequence-defined polymers has been bidirectional growth. By repeating the process of deprotection-coupling37–39 or orthogonal coupling40–42 from a double functionalized core, two uniform sequence units could symmetrically add to both sides of the precursor in one bidirectional growth cycle; thus the palindromic sequence could be realized. Besides, in 2015, Johnson et al. demonstrated the elegant production of a palindromic sequence by the combination of IEG with side-chain functionalization via CuAAC-induced chain growth.43 The esterification or nucleophilic substitution-based side-chain functionalization enabled fast sequence construction via an IEG strategy. To meet sophisticated and demanding application scenarios, especially to mimic the functions of biological polymers, polymer chemists always pursue more efficient, metal-free, biocompatible and eco-friendly chemistry to access discrete and exquisite polymers. It has been well documented that thiol-maleimide Michael coupling (TMMC) is a “click” reaction which is widely used for bio-conjugation, crosslink and surface modification for biomedical materials,44–54 owing to its mild, efficient and physiology-compatible features. In our group, TMMC together with the IEG strategy has well demonstrated its robustness and versatility in constructing discrete polymers (Scheme 1a).55–57 For constructing symmetrical palindromic sequences, the IEG strategy could be a better option due to its exponential growth manner. Nevertheless, how to conveniently install varied side chains during fast main chain growth still remains a challenge.
 | ||
| Scheme 1 (a) Previous work: discrete polymers by TMMC. (b) This work: palindromic sequences built by CTMMC. | ||
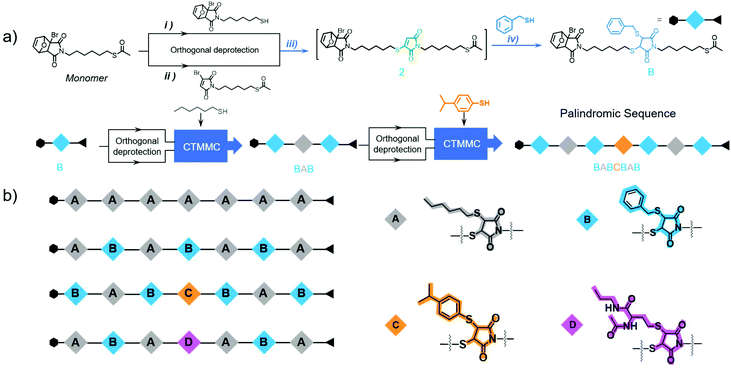
It was noted that thiol and bromomaleimide could undergo cascade thiol-maleimide Michael couplings (CTMMC) in an efficient way.58–60 Therefore, it was envisioned that bromomaleimide could successively couple with two thiols effectively, enabling both chain growth and side chain installation. Herein, by applying the IEG strategy, we demonstrated the CTMMC-enabled fast access to palindromic sequences. Through the CTMMC-integrated IEG strategy, the 1st TMMC allowed the main chain growth and the 2nd TMMC installed the pre-determined thiol-containing side chains. Variable side chains could be deliberately installed during chain growth, thus creating sequences on demand (Scheme 1b). This work opens a new way to create palindromic sequences for mimicking biological polymers and enriches the toolbox for the ultimate target of polymer synthesis, i.e., the development of novel polymeric materials with superior performance.
With inherent electron deficiency, bromomaleimide (3-BrMI) can readily react with thiol via TMMC.58 The reaction produced a bromide-substituted succinimide moiety, which was highly unstable and converted to maleimide by the removal of hydrogen bromide. The freshly generated maleimide was active toward the 2nd TMMC with a thiol-containing molecule, giving the dithiosuccinimide (DTS) moiety.60 This cascade thiol-maleimide Michael couplings (CTMMC) realized the efficient installation of side chains (2nd TMMC) during main chain growth (1st TMMC). Considering the high efficiency of the cascade reaction,61–67 CTMMC was proposed as an ideal chemistry for the construction of palindromic sequences via the iterative exponential growth (IEG) strategy. To verify the cascade behaviour, monomer 1 possessed a furan-protected bromomaleimide group and an acetyl-protected thiol group was designed (Scheme S1†). The orthogonal and quantitative deprotections produced the thiol-capped 1-SH and maleimide-capped 1-MA, respectively (Scheme S2 and Fig. S8, S9†), the degree of deprotection was monitored by 1H NMR. The CTMMC between 1-MA (1.0 equiv.) and 1-SH (2.2 equiv.) catalysed by triethylamine was explored in deuterochloroform and monitored by NMR spectrometry (Fig. 2a). Surprisingly, the 1st TMMC was completed within only 8 min and produced intermediate 2 exclusively, determined by the disappearance of the resonance at δ 6.87 (ppm) related to 3-BrMI (marked in green, Fig. 2b). The intermediate 2-SBr was not observed due to its high activity.68 The 2nd TMMC proceeded smoothly and afforded 3 with ∼70% yield after 25 h, as determined by NMR (Fig. 2b). The reaction kinetic plots of both 1st and 2nd TMMC clearly demonstrated the cascade manner (Fig. 2c). The structures of 2 and 3 were fully confirmed by 1H NMR (Fig. S10 and S11†).
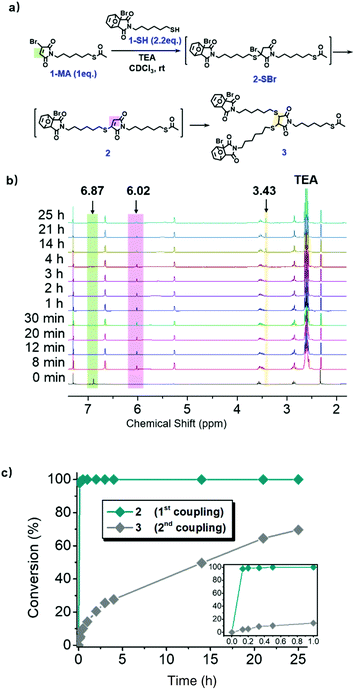 | ||
| Fig. 2 (a) The schematic illustration of CTMMC. (b) In situ1H NMR monitoring of CTMMC (300 MHz, CDCl3). (c) The kinetic plot of CTMMC in 1.0 h (inset) and 25 h. | ||
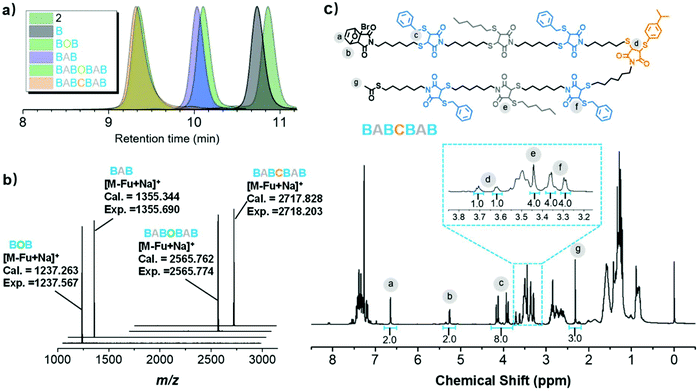
Starting from monomer 1, an array of palindromic sequences was built via the CTMMC-integrated IEG approach. For example, as described in Fig. 2a, intermediate 2 was generated from the TMMC (iii) between 1-SH (1.2 equiv.) and 1-MA (1.0 equiv.) (Fig. 3a). After about 30 minutes of the 1st TMMC, benzyl mercaptan (B, 2.0 equiv. to ensure the conversion of the intermediate) was added in situ and reacted with 2 to form Bvia the 2nd TMMC (iv). With B as the precursor, repeating i to iv and using hexyl mercaptan (A) as the thiol agent during the 2nd TMMC (iv), the palindromic sequence of BAB was created. In the next cycle, the sequence of BABCBAB was produced by incorporating p-isopropyl thiophenol (C) as the thiol agent during the 2nd TMMC. By tailored installation of different thiol agents during the 2nd TMMC, other palindromic sequences of AAAAAAA, ABABABA and ABADABA were successfully created in an IEG manner, which were validated by NMR, SEC and MALDI-TOF mass spectrometry (Fig. S12–S25 and S40–S53†). Taking BABCBAB as an example, the SEC traces including its discrete precursors, i.e., 2, B, BOB, BAB, BABOBAB and BABCBAB, are presented in Fig. 4a. Unimodal, symmetrical and narrow distributed SEC traces as well as an apparent molecular weight shift could be observed. The MALDI-TOF mass spectra of the discrete oligomers BOB, BAB, BABOBAB and BABCBAB are shown in Fig. 4b. A single MS peak signal agreeing well with the calculated value of the molecular mass ([M − Furan + Na]+) was evidenced. The proton NMR spectrum of BABCBAB is shown in Fig. 4c. Apparently, all the characteristic resonance could be perfectly assigned to the theoretical one. Specifically, the resonance of the protons of protected species at both terminals, i.e., furan and acetyl groups, could be easily identified at δ 6.64 (a), 5.26 (b) and 2.32 (g) ppm, implying the high fidelity of terminal protections. Furthermore, the complete disappearance of the proton resonance at δ 6.02 ppm belonging to the maleimide moiety resulting from the 1st TMMC indicated the success of the 2nd TMMC. The resonance between δ 3.70–3.65 (d), 3.44 (e) and 3.36–3.30 (f) ppm verified the formation of DTS through CTMMC. The results of the MALDI-TOF mass spectrometry and isolated yields are presented in Table 1 for a clear illustration. All the results confirmed the structural uniformity of these palindromic sequences, which were built in reasonable yields. It should be noted that CTMMC was not limited to the construction of palindromic sequences. We proved that more complex sequences were prepared by iterative and cross growth (Scheme S14†).
| Palindromic sequence | [M − Furan + Na]+m/zcal. (Da) | m/zexp. (Da) | Error (Da) | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield: Over a CTMMC-integrated IEG cycle (4 steps in total) and purified by column chromatography over silica gel. | ||||
| AAA | 1343.438 | 1343.856 | +0.418 | 59.8 |
| ABA | 1349.391 | 1349.499 | +0.108 | 54.7 |
| BAB | 1355.344 | 1355.690 | +0.346 | 51.8 |
| AAAAAAA | 2660.031 | 2660.401 | +0.370 | 33.6 |
| ABABABA | 2677.891 | 2678.046 | +0.155 | 23.6 |
| BABCBAB | 2717.828 | 2718.203 | +0.375 | 26.3 |
| ABADABA | 2771.965 | 2772.322 | +0.357 | 18.4 |
To mimic the biological palindromic sequence, the synthetic palindromic sequence should be readable or decipherable for fulfilling potential applications.7,69 The sequencing by tandem mass spectrometry (MS/MS) is popularly considered as a versatile and valid tool to read the sequence information stored in both biological and synthetic polymers. During MS/MS sequencing, the metastable polymer chains are fragmented into many sequence-induced species upon the imposed energy, such as a pulsed electric field or inert gas collision.70–72 The m/z analysis of the fragmented species enables the restoration of the sequence information. However, due to similar bond energies of the covalent bonds of the polymeric chain, the MS/MS sequencing frequently induced many irregular and secondary chain fragmentations, giving rise to many interfering and unattributable MS signals.73,74 On the other hand, both fragment pieces from the cleavage of each chemical bond are detected by MS/MS, which could further perturb the sequencing process. Therefore, for “easy-to-read” and predictable MS/MS signals, the selective cleavage of chemical bonds in repeat units to afford legible sequence-related MS signals is highly desirable. For example, Lutz et al. cleverly incorporated wieldy C–ON alkoxyamine bonds in each repeating unit to provide a clear and easily readable MS/MS fragmentation pattern.18,75,76 In order to probe the information readability of these palindromic sequences, the tetramers were subjected to MS/MS sequencing. Excitingly, the MS/MS spectra of tetramers showed clear fragmentation patterns, which were possibly due to the controlled cleavages on the C–S bonds of the DTS moiety (Fig. S56, S57 and Tables S2, S3†). To confirm this hypothesis, the computer calculation of the bond dissociation energy (BDE) was performed via the density functional theory method (Table S1†). According to the results, a synergetic cleavage mechanism was proposed (Fig. S54†). Specifically, during MALDI-TOF MS/MS sequencing, the cleavage of one C–S bond attached to the succinimide group gave rise to a carbon radical species. The driving force of stabilization causes the BDE of the adjacent C–S bond to significantly drop from 56.8 to 7.9 kcal mol−1.77 Thus, after rearrangement, a series of dominant fragments with stable maleimide terminus were observed in the tandem mass spectrum, resulting in the easy-to-read sequences.
To further validate the good decodability of these synthetic palindromic sequences, ABADABA was sequenced by MS/MS spectrometry (Fig. 5). It found that the MALDI-TOF MS/MS spectrum of ABADABA displayed a clear fragmentation pattern (Fig. 5b and c). The MS/MS-induced synergetic cleavages of the C–S bonds of DTS broke the polymer chain and led to distinct sequence-related signals in the MS/MS spectrum, e.g., the first fragment at m/z 607.475 assigned to [A-ω + Na]+ (named q2qs2) and the homolog series of q3qs3 at m/z 942.816 ([B-A-ω + Na]+), q4qs4 at m/z 1272.054 ([A-B-A-ω + Na]+), q5qs5 at m/z 1701.374 ([D-A-B-A-ω + Na]+), q6qs6 at m/z 2030.645 ([A-D-A-B-A-ω + Na]+), and q7qs7 at m/z 2365.825 ([B-A-D-A-B-A-ω + Na]+) could be easily identified with the concomitant fragmentation signals ([M + 32]+) marked with asterisks as shown in Table S7.† Thus, the sequence information α-A-B-A-D-A-B-A-ω could be explicitly deciphered (Fig. 5c) from the dominant and unidirectional MS/MS signals. Interestingly, the structural information of the side chain pendent on the DTS moiety could also be decoded by calculating the m/z interval with the precursor ion. For example, the m/z intervals 118.775 and 124.176 Da implied the hexyl mercaptan (A) and benzyl mercaptan (B)-derived side chains, respectively. This information could be decoded and the intervals 218.367 and 184.225 Da revealed unit D (marked in red in Fig. 5c). To demonstrate the “easy-to-read” characteristic of the palindromic sequence, a “sequence unknown” sample with a given MS/MS-induced fragmentation pattern (Table S8†) could be easily deciphered within 30 minutes. The details of the deciphering process are described in the ESI (Fig. S62†). Therefore, owing to the unique DTS moiety, the clear and unidirectional fragmentation patterns greatly facilitated the sequencing of these palindromic sequences (Tables S2–S7†). Such easily readable palindromic sequences could be used as artificial DNA for anti-counterfeit purposes.78 As a proof-of-concept, an anti-counterfeit inkjet ink labelled by a DTS-incorporated palindromic sequence-defined macromolecule was illustrated (Fig. S63†), highlighting its good potential in anti-counterfeit applications.
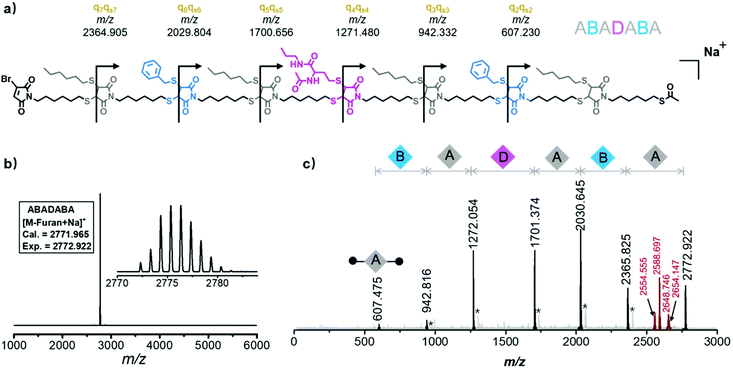 | ||
| Fig. 5 (a) Illustration of the theoretical fragmentations of ABADABA. (b) The MALDI-TOF MS spectrum and (c) MALDI-TOF MS/MS spectrum of ABADABA. *Signals arise from concomitant fragmentations, detailed in the ESI (Table S7†). | ||
Conclusions
In summary, a straightforward and efficient CTMMC combined IEG strategy was demonstrated for constructing palindromic sequence-defined polymers. This chemistry allows fast chain growth with convenient side chain installations. An array of palindromic sequences with different side chains was successfully constructed. Moreover, tandem MS sequencing provided a unidirectional, clear and predictable fragmentation pattern due to the synergetic cleavages of DTS moieties, endowing the palindromic sequence with good decodability. This work offered an example for constructing palindromic sequences by applying cascade chemistry, establishing an efficient platform for constructing precision polymers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21925107 and 21674072), the China Post-doctoral Science Foundation (2020M671571), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Program of Innovative Research Team of Soochow University.Notes and references
- T. Cavalier-Smith, Nature, 1974, 250, 467–470 CrossRef CAS.
- S. Al-Attar, E. R. Westra, J. van der Oost and S. J. Brouns, Biol. Chem., 2011, 392, 277–289 CAS.
- S. Camporesi and G. Cavaliere, Pers. Med., 2016, 13, 575–586 CrossRef CAS.
- N. V. Munshi, Circulation, 2016, 134, 777–779 CrossRef.
- M. Naeimi Kararoudi, S. S. Hejazi, E. Elmas, M. Hellstrom, M. Naeimi Kararoudi, A. M. Padma, D. Lee and H. Dolatshad, Front. Immunol., 2018, 9, 1711 CrossRef.
- C. Richter, J. T. Chang and P. C. Fineran, Viruses, 2012, 4, 2291–2311 CrossRef CAS.
- K. Hettiarachchi, S. Ridge, D. W. Thomas, L. Olson, C. R. Obi and D. Singh, J. Pept. Res., 2001, 57, 151–161 CrossRef CAS.
- M. Giel-Pietraszuk, M. Hoffmann, S. Dolecka, J. Rychlewski and J. Barciszewski, J. Protein Chem., 2003, 22, 109–113 CrossRef CAS.
- V. A. Wagoner, M. Cheon, I. Chang and C. K. Hall, Proteins, 2011, 79, 2132–2145 CrossRef CAS.
- N. Prasanth, M. K. Vaishnavi and K. Sekar, J. Biosci., 2013, 38, 173–177 CrossRef CAS.
- L. Thoma, E. Sepulveda, A. Latus and G. Muth, Front. Microbiol., 2014, 5, 499 Search PubMed.
- L. Ning, Q. Wang, Y. Zheng, H. Liu and X. Yao, Mol. Biosyst., 2015, 11, 647–655 RSC.
- J. F. Lutz, M. Ouchi, D. R. Liu and M. Sawamoto, Science, 2013, 341, 1238149 CrossRef.
- J. F. Lutz, J. M. Lehn, E. W. Meijer and K. Matyjaszewski, Nat. Rev. Mater., 2016, 1, 16024 CrossRef CAS.
- S. C. Solleder, R. V. Schneider, K. S. Wetzel, A. C. Boukis and M. A. R. Meier, Macromol. Rapid Commun., 2017, 38, 1600711 CrossRef.
- J. Sun, X. Liao, A. M. Minor, N. P. Balsara and R. N. Zuckermann, J. Am. Chem. Soc., 2014, 136, 14990–14997 CrossRef CAS.
- A. Al Ouahabi, M. Kotera, L. Charles and J. F. Lutz, ACS Macro Lett., 2015, 4, 1077–1080 CrossRef CAS.
- R. K. Roy, A. Meszynska, C. Laure, L. Charles, C. Verchin and J. F. Lutz, Nat. Commun., 2015, 6, 7237 CrossRef CAS.
- G. Cavallo, A. Al Ouahabi, L. Oswald, L. Charles and J. F. Lutz, J. Am. Chem. Soc., 2016, 138, 9417–9420 CrossRef CAS.
- J. Niu, R. Hil and D. R. Liu, Nat. Chem., 2013, 5, 282–292 CrossRef CAS.
- S. C. Solleder and M. A. R. Meier, Angew. Chem., Int. Ed., 2014, 53, 711–714 CrossRef CAS.
- S. C. Solleder, D. Zengel, K. S. Wetzel and M. A. R. Meier, Angew. Chem., Int. Ed., 2016, 55, 1204–1207 CrossRef CAS.
- D. Y. Oh, M. Ouchi, T. Nakanishi, H. Ono and M. Sawamoto, ACS Macro Lett., 2016, 5, 745–749 CrossRef CAS.
- Z. X. Huang, B. B. Noble, N. Corrigan, Y. Y. Chu, K. Satoh, D. S. Thomas, C. J. Hawker, G. Moad, M. Kamigaito, M. L. Coote, C. Boyer and J. T. Xu, J. Am. Chem. Soc., 2018, 140, 13392–13406 CrossRef CAS.
- B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189–193 CrossRef CAS.
- G. De Bo, S. Kuschel, D. A. Leigh, B. Lewandowski, M. Papmeyer and J. W. Ward, J. Am. Chem. Soc., 2014, 136, 5811–5814 CrossRef CAS.
- G. De Bo, M. A. Y. Gall, M. O. Kitching, S. Kuschel, D. A. Leigh, D. J. Tetlow and J. W. Ward, J. Am. Chem. Soc., 2017, 139, 10875–10879 CrossRef CAS.
- K. Takizawa, H. Nulwala, J. Hu, K. Yoshinaga and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5977–5990 CrossRef CAS.
- K. Takizawa, C. Tang and C. J. Hawker, J. Am. Chem. Soc., 2008, 130, 1718–1726 CrossRef CAS.
- S. Binauld, D. Damiron, L. A. Connal, C. J. Hawker and E. Drockenmuller, Macromol. Rapid Commun., 2011, 32, 147–168 CrossRef CAS.
- F. A. Leibfarth, J. A. Johnson and T. F. Jamison, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 10617–10622 CrossRef CAS.
- Y. Jiang, M. R. Golder, H. V. Nguyen, Y. Wang, M. Zhong, J. C. Barnes, D. J. Ehrlich and J. A. Johnson, J. Am. Chem. Soc., 2016, 138, 9369–9372 CrossRef CAS.
- M. R. Golder, Y. Jiang, P. E. Teichen, H. V. Nguyen, W. Wang, N. Milos, S. A. Freedman, A. P. Willard and J. A. Johnson, J. Am. Chem. Soc., 2018, 140, 1596–1599 CrossRef CAS.
- C. H. Ju, C. C. Meng, J. H. Ma, X. Y. Zhang and S. T. Ding, Chem. Commun., 2020, 56, 3955–3958 RSC.
- J. M. Lee, M. B. Koo, S. W. Lee, H. Lee, J. Kwon, Y. H. Shim, S. Y. Kim and K. T. Kim, Nat. Commun., 2020, 11, 1–9 Search PubMed.
- C. J. Yang, J. P. Flynn and J. Niu, Angew. Chem., Int. Ed., 2018, 57, 16194–16199 CrossRef CAS.
- N. Zydziak, F. Feist, B. Huber, J. O. Mueller and C. Barner-Kowollik, Chem. Commun., 2015, 51, 1799–1802 RSC.
- N. Zydziak, W. Konrad, F. Feist, S. Afonin, S. Weidner and C. Barner-Kowollik, Nat. Commun., 2016, 7, 13672 CrossRef.
- M. Van De Walle, K. De Bruycker, T. Junkers, J. P. Blinco and C. Barner-Kowollik, ChemPhotoChem, 2019, 3, 225–228 CrossRef CAS.
- W. Konrad, F. R. Bloesser, K. S. Wetzel, A. C. Boukis, M. A. R. Meier and C. Barner-Kowollik, Chem. – Eur. J., 2018, 24, 3413–3419 CrossRef CAS.
- W. Konrad, C. Fengler, S. Putwa and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2019, 58, 7133–7137 CrossRef CAS.
- B. Zhao, Z. Gao, Y. Zheng and C. Gao, J. Am. Chem. Soc., 2019, 141, 4541–4546 CrossRef CAS.
- J. C. Barnes, D. J. Ehrlich, A. X. Gao, F. A. Leibfarth, Y. Jiang, E. Zhou, T. F. Jamison and J. A. Johnson, Nat. Chem., 2015, 7, 810–815 CrossRef CAS.
- L. J. Wong, M. Kavallaris and V. Bulmus, Polym. Chem., 2011, 2, 385–393 RSC.
- T. N. Gevrek, T. Bilgic, H. A. Klok and A. Sanyal, Macromolecules, 2014, 47, 7842–7851 CrossRef CAS.
- H. Moon, J. Lee, J. Min and S. Kang, Biomacromolecules, 2014, 15, 3794–3801 CrossRef CAS.
- P. M. Kharkar, K. L. Kiick and A. M. Kloxin, Polym. Chem., 2015, 6, 5565–5574 RSC.
- P. Chakma, L. H. R. Possarle, Z. A. Digby, B. R. Zhang, J. L. Sparks and D. Konkolewicz, Polym. Chem., 2017, 8, 6534–6543 RSC.
- N. Bellassai, A. Marti, G. Spoto and J. Huskens, J. Mater. Chem. B, 2018, 6, 7662–7673 RSC.
- P. Chakma, Z. A. Digby, J. Via, M. P. Shulman, J. L. Sparks and D. Konkolewicz, Polym. Chem., 2018, 9, 4744–4756 RSC.
- L. E. Jansen, L. J. Negron-Pineiro, S. Galarza and S. R. Peyton, Acta Biomater., 2018, 70, 120–128 CrossRef CAS.
- F. Jivan, N. Fabela, Z. Davis and D. L. Alge, J. Mater. Chem. B, 2018, 6, 4929–4936 RSC.
- J. Ramos-Soriano, J. J. Reina, B. M. Illescas, J. Rojo and N. Martin, J. Org. Chem., 2018, 83, 1727–1736 CrossRef CAS.
- Y. Zhou, Y. C. Qu, Q. Yu, H. Chen, Z. B. Zhang and X. L. Zhu, Polym. Chem., 2018, 9, 3238–3247 RSC.
- Z. H. Huang, J. F. Zhao, Z. M. Wang, F. Y. Meng, K. S. Ding, X. Q. Pan, N. C. Zhou, X. P. Li, Z. B. Zhang and X. L. Zhu, Angew. Chem., Int. Ed., 2017, 56, 13612–13617 CrossRef CAS.
- Z. M. Wang, Z. H. Huang, N. C. Zhou, X. H. Dong, X. L. Zhu and Z. B. Zhang, Polym. Chem., 2017, 8, 2346–2352 RSC.
- B. L. Liu, Q. N. Shi, L. H. Hu, Z. H. Huang, X. L. Zhu and Z. B. Zhang, Polym. Chem., 2020, 11, 1702–1707 RSC.
- L. M. Tedaldi, M. E. B. Smith, R. I. Nathani and J. R. Baker, Chem. Commun., 2009, 6583–6585 RSC.
- L. Castaneda, Z. V. F. Wright, C. Marculescu, T. M. Tran, V. Chudasama, A. Maruani, E. A. Hull, J. P. M. Nunes, R. J. Fitzmaurice, M. E. B. Smith, L. H. Jones, S. Caddick and J. R. Baker, Tetrahedron Lett., 2013, 54, 3493–3495 CrossRef CAS.
- J. Youziel, A. R. Akhbar, Q. Aziz, M. E. Smith, S. Caddick, A. Tinker and J. R. Baker, Org. Biomol. Chem., 2014, 12, 557–560 RSC.
- L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS.
- K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS.
- A. Padwa and S. K. Bur, Tetrahedron, 2007, 63, 5341–5378 CrossRef CAS.
- K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993–3009 RSC.
- Y. Wang, H. Lu and P. F. Xu, Acc. Chem. Res., 2015, 48, 1832–1844 CrossRef CAS.
- M. Porel, D. N. Thornlow, N. N. Phan and C. A. Alabi, Nat. Chem., 2016, 8, 590–596 CrossRef CAS.
- Z. Zhang, Y. Z. You and C. Y. Hong, Macromol. Rapid Commun., 2018, 39, 1800362 CrossRef.
- R. Kalish, A. Smith and E. Smutny, Tetrahedron Lett., 1971, 12, 2241–2244 CrossRef.
- C. Deng, X. Lv, J. Li, Y. Liu, G. Du, R. L. Amaro and L. Liu, Biotechnol. Bioeng., 2019, 116, 5–18 CrossRef CAS.
- S. M. Weidner and S. Trimpin, Anal. Chem., 2010, 82, 4811–4829 CrossRef CAS.
- C. D. Calvano, G. Ventura, M. Trotta, G. Bianco, T. R. I. Cataldi and F. Palmisano, J. Am. Soc. Mass Spectrom., 2017, 28, 125–135 CrossRef CAS.
- L. C. Nye, J. F. Hitzenberger, M. M. Roubelakis, M. Orfanopoulos and T. Drewello, Int. J. Mass Spectrom., 2019, 436, 59–64 CrossRef CAS.
- M. Porel and C. A. Alabi, J. Am. Chem. Soc., 2014, 136, 13162–13165 CrossRef CAS.
- J. A. Amalian, T. T. Trinh, J. F. Lutz and L. Charles, Anal. Chem., 2016, 88, 3715–3722 CrossRef CAS.
- C. Laure, D. Karamessini, O. Milenkovic, L. Charles and J. F. Lutz, Angew. Chem., Int. Ed., 2016, 55, 10722–10725 CrossRef CAS.
- A. Al Ouahabi, J. A. Amalian, L. Charles and J. F. Lutz, Nat. Commun., 2017, 8, 967 CrossRef.
- K. S. Ding, Y. J. Zhang, Z. H. Huang, B. L. Liu, Q. N. Shi, L. H. Hu, N. C. Zhou, Z. B. Zhang and X. L. Zhu, Eur. Polym. J., 2019, 119, 421–425 CrossRef CAS.
- M. J. Altamimi, J. C. Greenwood, K. Wolff, M. E. Hogan, A. Lakhani, G. P. Martin and P. G. Royall, Int. J. Pharm., 2019, 571, 118656 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional characterization data. See DOI: 10.1039/d0py01088j |
| This journal is © The Royal Society of Chemistry 2020 |