Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermoresponsive behavior of poly[trialkyl-(4-vinylbenzyl)ammonium] based polyelectrolytes in aqueous salt solutions†
Erno
Karjalainen
 *,
Narmin
Suvarli‡
and
Heikki
Tenhu
*,
Narmin
Suvarli‡
and
Heikki
Tenhu

Department of Chemistry, University of Helsinki, A.I. Virtasen aukio 1 (Chemicum), PL 55, 00014 Helsingin yliopisto, Finland. E-mail: erno.karjalainen@alumni.helsinki.fi
First published on 19th August 2020
Abstract
This contribution reports 16 new thermoresponsive systems based on poly[trialkyl-(4-vinylbenzyl)ammonium] chlorides. When salts are introduced into solution of the water-soluble polymers, thermoresponsive behavior is observed. The type of transition is dependent on the length of the alkyl chain: the polymer with an ethyl chain has an upper critical solution temperature (UCST) whereas the polymers with butyl or pentyl chains have a lower critical solution temperature (LCST). The magnitude of the effect of a given salt on the solution behavior is dependent on the type of the used electrolyte. With all polymers, the strength of the effect increases in the following order: NaH2PO4 < Na2CO3 < Na2SO4 < NaCl < sodium pentanesulfonate < NaNO3 < NaSCN < lithium trifluoromethanesulfonate < lithium bis(trifluoromethane) sulfonimide. For monovalent salts, the order follows a reversed Hofmeister series. The results can be used to design new thermosensitive systems by matching the alkyl content of a polycation with an effect of the small molecular salt. Nearly an unlimited number of thermoresponsive polymers can be designed following the approach introduced in this article.
Introduction
Two main types of thermoresponsive behavior of polymers in aqueous solutions exist: lower critical solution temperature (LCST) and upper critical solution temperature (UCST).1 Polymers with an LCST type behavior turn insoluble when the solution is heated whereas UCST polymers phase separate upon cooling. A number of neutral polymers with an LCST behavior in water have been reported.2 The most studied polymer with an LCST in water is poly(N-isopropyl acrylamide) (PNIPAm),1 although many other polymers with similar behavior are known.2Within UCST polymers, there are two main categories: polymers that have a UCST due to hydrogen bonding (HB) and ones with UCST that is based on Coulomb interactions.3,4 The HB UCST polymers are polymers with repeating units that are able to form strong hydrogen bonds with other repeating units of the polymer.3 When such polymers are heated above their transition temperatures, the polymer–polymer hydrogen bonds break and they are replaced by polymer–water bonds.5 Examples of polymers that have an HB based UCST in water include poly(N-acryloylglycinamide) (PNAGA),6 copolymers of acrylamide and acrylonitrile,7 polymethacrylamide,7 and poly(N-acryloylasparaginamide).8
The polymers that have a UCST based on Coulomb interactions typically contain oppositely charged groups in the same polymer chain.1 The polymers are insoluble at low temperatures due to electrostatic attraction between the opposite charges.9 The interactions can be weakened by increasing the temperature, which leads to solubilization of the polymers and thus to a UCST type cloud point (Tc).9 The cloud points are significantly affected by addition of salts.9 The presence of opposite charges can be achieved by polymerization of zwitterionic monomers.9
Many strong polyelectrolytes with an LCST or a UCST type transition in water have been reported.10 The first published example of a strong polyanion – or any strong polyelectrolyte – displaying an LCST type behavior was poly(tetrabutylphosphonium styrenesulfonate) (poly([P4444][SS])).11–15 The polymer has a Tc of 57 °C as a 10 wt% aqueous solution and the corresponding monomer has an LCST as well.11 The cloud point of poly([P4444][SS]) is heavily dependent on polymer concentration and presence of salts.12 Addition of salts may increase or decrease the cloud point, depending on the type of salt. Other sulfonate containing polyanions with large organic cations have been observed to undergo a salt sensitive LCST as well.13,16–21 Mechanistically, the polyanions separate from the solution and get buried inside the formed aggregates during the phase separation process of poly([P4444][SS]) and similar polymers while the small molecular cations tend to stay in the periphery.14,20 If the small molecular cation is divalent, then the anionic groups are located more on the surface of the aggregates.21 Weak polyanions with carboxylic acid groups have been shown to have a UCST type transition in certain aqueous systems,22–26 but no strong homopolyanion with a UCST in water has been described in the literature so far.
The first strong polycation that was reported to have an LCST type transition in water is poly(tributyl-4-vinylbenzylphosphonium 1-pentanesulfonate) (poly(TVBP-C5S)), which has a cloud point that is highly dependent on polymer concentration and addition of salts.27 The polymer forms particles in aqueous solution already at temperatures below the phase transition temperature and the aggregation of the particles at the cloud point is then responsible of the observed turbidity at the cloud point.28 Also, an analogous ammonium-based polycation, poly(tributyl-(4-vinylbenzyl)ammonium) undergoes an LCST type transition when it has 1-pentanesulfonate or 1-hexanesulfonate as its counterion.28 Poly([tripentyl(vinylbenzyl)phosphonium]chloride) has an LCST type cloud point in dilute NaCl solutions and the cloud point can be modified with salt concentration.29 A positively charged polypeptide with iodide as a counterion has been observed to have both LCST and UCST transitions.30 In addition, weakly cationic poly(dialkylaminoethyl methacrylate)s display a pH depdendent LCST type Tc.31
There are more reports about polycations that show a UCST type transition than about ones with an LCST. UCST transition for strong polycations was first shown for a series of imidazolium containing poly(vinyl ethers), which displayed salt and concentration dependent cloud points.32 All the polymers had tetrafluoroborate as their counterion. The BF4− ion often yields polyelectrolytes that are insoluble in water at room temperature.33,34 Many other small molecular ions can be used to turn polycations insoluble in water as well.33,35,36 Polycations with such ions that have a UCST type behavior in water as it has been shown for polycations with tetrafluoroborate,30,32,37–48 bis(trifluoromethane)sulfonimide (NTf2),49,50 or trifluoromethanesulfonate (OTf)49,51 as their counterions. Certain polycations have a UCST type transition with a halide,30,37,44,48,52,53 thiocyanate,44,53 or perchlorate44,53 as a counterion as well. These polycations usually have repeating units with high hydrocarbon content.
The UCST behavior can be achieved by synthesizing the polymers with the aforementioned counterions32,38–43,45–47,51 or by introducing the ions into solutions of water soluble polycations.44,49,50,53 The cloud points for cationic polymers synthesized with a hydrophobic anion have been observed to be highly concentration dependent.32,41–43,45–47 In this approach, increasing the polymer concentration naturally increases the counterion concentration as well, which may be the cause for the strong concentration dependency. When the counterion is introduced in situ, the effect of polymer concentration on the cloud point is small with low polymer concentration, but it is possible that the dependency becomes more significant with higher polymer concentrations.49,53 In both approaches, the transition temperature can be modified by introducing additional salts into the solution.30,32,37,41,43,45,49–53
A versatile class of polyelectrolytes with thermoresponsive behavior in aqueous systems are poly[trialkyl-(4-vinylbenzyl)ammonium] based polymers. Whether they display LCST or UCST type of behavior in water is dependent on the length of the alkyl chain connected to the ammonium group. Poly[tributyl-(4-vinylbenzyl)ammonium] cation with either 1-pentane sulfonate or 1-hexane sulfonate as an anion has an LCST type transition28 whereas poly[trimethyl(4-vinylbenzyl)ammonium trifluoromethanesulfonate] has a UCST in LiOTf solutions.51 It has been shown that a polycation may have a UCST type phase transition in the presence added salts,44,49,50,53 but no LCST transition induced in this way has been reported. The purpose of this work is to study the possibilities of inducing an LCST and to study the effect of the alkyl chain length along with the quality and quantity of added salts on the phase transition of poly[trialkyl(4-vinylbenzyl)ammonium] salts.
Results and discussion
Polymer synthesis
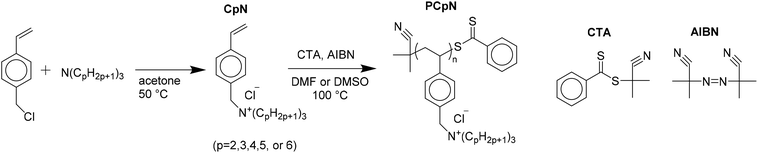
A series of trialkyl(4-vinylbenzyl)ammonium chloride monomers with three identical n-alkyl chains, with the chain length varying from ethyl to hexyl, were synthesized. All the synthetic and analytical details are reported in the associated ESI.† The monomers were polymerized to the corresponding poly[trialkyl(4-vinylbenzyl)ammonium chlorides] using RAFT polymerization technique54 with 2-cyano-2-propyl benzoditionate as the chain transfer agent (CTA) and AIBN as the initiator. RAFT was used as a polymerization method in order to achieve high monomer conversions and thus obtain more material for the solution property studies.The monomers are abbreviated as CpN (p = 2, 3, 4, 5, or 6) where p denotes the length of the n-alkyl chain, e.g. C4N refers to tributyl(4-vinylbenzyl)ammonium chloride. The corresponding polymers are analogously named as PCpNs. The synthesis and chemical structures of the studied compounds are illustrated in Scheme 1 and described more in detail in the ESI.† NMR spectroscopy was utilized to verify the structure and purity of the polymers. As an example, the NMR spectrum of PC3N and its corresponding monomer, C3N, are shown in Fig. 1. The rest of the polymer spectra are shown in the associated ESI (Fig. S1–S4†). During the synthesis, it was noticed that PC6N is insoluble in water. As the purpose of the work was to study the aqueous solution properties of the polymers, the said polymer was not analyzed further.
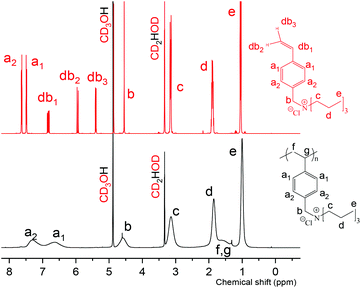 | ||
| Fig. 1 NMR-spectra of PC3N (bottom, black) and the corresponding monomer C3N (top, red). Both spectra have been measured in deuterated methanol. | ||
Attempts to measure the molecular weight distribution of the PCpN series with size exclusion chromatography were made in DMF with LiBr and in aqueous NaNO3, but no data could be obtained, presumably due to strong interactions between the polymer and the column walls. The complexity of determining the molecular weight distributions of ammonium polycations has been observed for other systems too.50,55
He et al. showed that using organic solvents with salts is an accurate method for determining the molecular weights of water-insoluble imidazolium based polycations.56 It seems that the approach is not feasible for ammonium polycations that are soluble in water, as no polymer elution could be detected in DMF with LiBr. The method by He et al. relies on standards that have been made by reacting a series of poly(4-vinylbenzylchlorides) with butyl imidazole to obtain polycations of known degrees of polymerization and with the same chemical structures than the samples in order to make reliable standards for the molecular weight determination; use of neutral polymers as standards would have produced unreliable results due to different hydrodynamic volumes of neutral polymers and polyelectrolytes.
Before choosing the synthetic route describe in Scheme 1, the authors tried a similar reaction between poly(4-vinylbenzylchloride) and tributylamine, as He et al. did with butyl imidazole.56 Several attempts were made, but the conversions of the reactions were never quantitative and thus the product was never a true homopolyelectrolyte. Therefore, the authors chose the route starting from electrolyte monomers as the method for this study. The non-quantitative conversions make it infeasible to determine the molecular weights with self-synthesized polycation standards, even if a method for elution could be found. Therefore, alternative methods for molecular weight determination are required.
The NMR signals arising from the CTA are masked by the signals of the repeating units of the polymer and thus determining the molecular weight by NMR end-group analysis is not feasible. Because of this, the number average molecular weights of the polymers were estimated by measuring the absorbance of CTA at 310 nm in methanol as a function of concentration and the extinction coefficient was determined (Fig. S5†). As the monomers do not absorb at the measured wavelength (Fig. S6†), it is reasonable to assume that all absorption for the polymer solution at 310 nm arises from the CTA. The measured molecular weights along with the theoretical molecular weights are reported in Table 1. The measured molecular weights agree with the theoretical values. Calculation of molecular weight from the UV spectra is explained in more detail in the ESI.†
| Polymer | p | [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTA] [CTA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]b [I]b |
Solvent | Conversionc (%) |
M
n,th.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (kg mol−1) d (kg mol−1) |
M
n, UV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (kg mol−1) e (kg mol−1) |
DPf | [1 mg mL−1]g (mM) |
|---|---|---|---|---|---|---|---|---|
| a Length of the alkyl chain. b Ratio between initial concentrations of monomer (M), chain transfer agent (CTA), and initiator (I). c Determined by NMR spectroscopy directly for the reaction mixture. d Theoretical number average molecular weight, calculated as conversion × ([M]/[I]) + M(CTA). e Number average molecular weight determined by UV spectroscopy. f Degree of polymerization, calculated from Mn, UV. g The concentration of repeating units in a 1 mg mL−1 solution of the polymer. | ||||||||
| PC2N | 2 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
DMSO | 76 | 38.8 | 34.2 | 134 | 3.95 |
| PC3N | 3 | 201![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
DMF | 77 | 46.0 | 38.4 | 129 | 3.37 |
| PC4N | 4 | 183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
DMF | 82 | 51.1 | 61.8 | 182 | 2.96 |
| PC5N | 5 | 197![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
DMF | 96 | 72.2 | 69.1 | 181 | 2.63 |
Using the CTA as a standard for determining molecular weight is akin to the use of NMR end group analysis as a tool in determining molecular weights. The method tends to produce values that are within 20% from the ones obtained by NMR.57 Therefore, the reported molecular weights should be taken only as indicative, but it is safe to assume that the values are within the right range and all polymers have similar degrees of polymerization. Since only one batch of each polymer is studied and thus all the solutions of a given polymer contain the same molecular weight, possible molecular weight dependency is cancelled out. Some error may also arise from scattering of the solutions as well, but this is insignificant as the baseline is essentially at zero in the UV measurements (Fig. S6†).
Solution properties
The solution behavior of the synthesized polycations was studied in the presence of several salts. Lithium trifluoromethanesulfonate (LiOTf) and lithium bis(trifluoromethane) sulfonimide (LiNTf2) were chosen since several polycations have been shown to display a UCST type transition in the presence of the anions of the salts.49–51 Sodium 1-pentanesulfonate (NaPS) was used since its anion has been used for phosphonium and ammonium based polycations, which have a LCST type behavior.27,28 The polymers reported in the literature have been synthesized with the pentanesulfonate as their counterion. The aim of this study was to see how the concentration of the anion alters the phase transition temperature while keeping the polymer concentration constant. Sodium dodecanesulfonate (SDS) was utilized as a longer alkyl chain analogue for NaPS.Since it is known that LCST behavior of PNIPAm can be influenced with anions from the Hofmeister series,58–60 several anions from the series were used to see if the same holds for strong polycations as well. The order of strength of the LCST decreasing effect for the Hofmeister anions for PNIPAm follows the following order: CO32− > SO42− > H2PO4− > F− > Cl− > Br− ≈ NO3− > I− > ClO4− > SCN−.59,60 The anions left from Cl− are known as kosmotropes whereas the ones on the right are referred as chaotropes. The series is based on concentration and the higher ionic strength of solutions with the divalent anions is typically not taken into account. In this study, both concentration and ionic strength are considered (please see discussion below).
In order to study anions from different parts of the series, the Hofmeister anions that were used in this study were CO32−, SO42−, H2PO4−, Cl−, NO3−, and SCN−. All the anions were used as their sodium salts. The effect of sulfate was studied both with Na2SO4 and Mg2SO4 in order to observe the effect of ionic strength without increasing the concentration of the anion. The salts were introduced into solutions of the original polymers with chloride counterions and constant polymer concentration of 1 mg mL−1. This has been shown to be a versatile approach in varying the UCST behavior of strong polycations and thus it was chosen to be the approach for the present study as well.49,50
The solution properties of the polymers were studied by means of turbidity measurements, which were supported by differential scanning calorimetry (DSC). The exact experimental details are reported in the ESI.† The definitions for the turbidity-based cloud points (Fig. S7†) along with DSC-based temperatures of maximum heat capacity (Tmax) and transition enthalpy (ΔH) (Fig. S8†) are given as ESI† as well.
The concentration range of interest for all salts was initially found by mixing polymer and salt solutions, which were then visually inspected. After the approximate range was found, the exact phase transition temperatures were determined with turbidity measurement. It is worth nothing that with low enough salt concentrations, all the polymers were soluble over the whole temperature range. This means that none of the polymers have has an observable phase transition in a dilute solution without added electrolytes.
The concentration of repeating units – and thus the concentration of chloride anions brought in by the polymer – is between 2.6 mM and 4.0 mM in 1 mg mL−1 solutions of the polymers (Table 1). In majority of the samples, the salt concentrations that are required for any detectable effect are much higher than the polymer concentration. The lowest concentration of NaCl that was required to cause any noticeable effect in transmittance of the solutions was 150 mM (with PC5N, please see discussion below). Therefore, it is safe to assume that the amount of the chloride ions that come into the solutions as counterions with the polycations is insignificant from the perspective of the phenomena studied in this contribution.
When PC2N was studied with LiNTf2, no phase transition could be observed in the turbidity measurements; the turbidity simply decreases when the concentration of LiNTf2 increases. Similar monotonous and temperature independent decrease in transmittance with increasing concentration of LiNTf2 has been observed for other polycations as well.49,50 Polycations with NTf2 as their counterion are typically insoluble in water at room temperature.33,35 Thus the increase in turbidity (decrease in transmittance) is probably due to increase in particle size or increasing difference in refractive indices of the polymer particles and the solvent as the ion exchange proceeds. Either way, the interactions between water and polymer become less and less favorable with increasing concentration. Gradual increase in aggregate size with increasing concentration LiNTf2 has been observed for a diblock copolymer with a strong polycation block.61,62 This indicates that similar phenomenon takes place for PC2N as well, but this is mere speculation and unimportant for the subject of this paper.
When concentration of LiNTf2 in PC2N solution is varied with a constant NaCl concentration of 500 mM, the picture changes: a UCST type transition appears (Fig. 2). The polymers in solutions form associates at low temperatures, which is evidenced by transmittance values well below 100%. The associates then have an apparent LCST type transition. With more heating, the polymers dissolve, if the concentration of LiNTf2 is low enough, signaling a UCST type transition. The need for a threshold ionic strength so that a polycation is be able to have an UCST transition has been observed also for other systems as well.49,50,63 NaCl was used to increase the ionic strength without increasing concentration of LiNTf2.
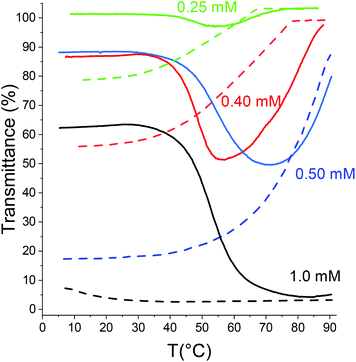 | ||
| Fig. 2 Heating (solid lines) and cooling (dashed lines) for 1 mg mL−1 solutions of PC2N in 500 mM NaCl solution with varying LiNTf2 concentration written next to the lines. | ||
In cooling curves, the UCST transition is reversible, but the LCST like transition is not. The process that appears to be an LCST transition may be a product of non-equilibrium state as the particles form below their UCST cloud points (TcU). Thus, the apparent transition would be only a product of particles merging when the temperature increases, which is supported by the observation that the temperature at which the transmittance starts to decrease is essentially independent of the LiNTf2 concentration. This may happen because the NTf2 ions enrich into the cores of the formed particles in order to minimize the contact with water. When the solution temperature exceeds the glass transition temperature (Tg) of the core, the particles start to merge. It has been shown that polycations with NTf2 as their counterion often have Tgs below 100 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64–67 and the presence of water may plasticize the polyelectrolyte further. Other possibility is that the LCST transition is slow to reverse and the UCST reverses fast, a situation that has been observed for cationic copolymers of PNIPAm in the presence of LiNTf2 and NaCl.50 Regardless of the nature or existence of the LCST type cloud point (TcL): this is the first time when a UCST has been observed for PC2N.
64–67 and the presence of water may plasticize the polyelectrolyte further. Other possibility is that the LCST transition is slow to reverse and the UCST reverses fast, a situation that has been observed for cationic copolymers of PNIPAm in the presence of LiNTf2 and NaCl.50 Regardless of the nature or existence of the LCST type cloud point (TcL): this is the first time when a UCST has been observed for PC2N.
When comparing TcU values of PC2N in LiNTf2 solution with 500 mM with previously reported polycations under same conditions (Table S1†), less LiNTf2 is required to achieve a given value of TcU than is the case with the previously studied polycations. This indicates that PC2N has higher affinity to NTf2 ions or the interactions of the cation with water are less favorable than with the polycations studied earlier. The latter is the more probable explanation since the alkyl content of PC2N is higher than for the other polymers reported in Table S1.†
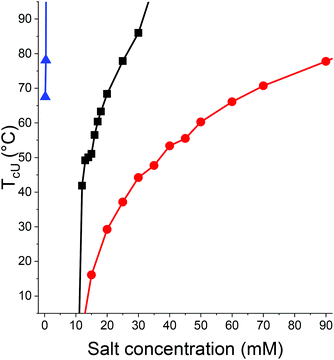
As seen in Fig. 2, the concentration range between complete solubility and complete insolubility for PC2N with LiNTf2 and NaCl is rather narrow. In addition, the range of obtained values of TcU is not large. The picture changes when LiOTf and NaSCN are used as the added salts (Fig. 3). Both salts are able to induce a UCST for PC2N without additional NaCl due to higher required salt concentrations, which in turn provide enough ionic strength with the single salt alone. The range of obtainable TcU values is much wider. The possibility to use LiOTf as the only added salt in systems where LiNTf2 alone cannot induce a UCST has been observed before, so the results of this study are consistent with previous observations.49 NaNO3 can be used to induce TcU for PC2N, but the concentrations required for the phase transition are higher than 2 M (Fig. S9†).
Even with 500 mM NaCl, the effect of LiNTf2 on the solubility of PC2N is the strongest of the studied salts. LiNTf2 is followed first by LiOTf, then by NaSCN, and finally by NaNO3. Therefore, it can be deduced that either the strength of interaction between PC2N with the ions or the hydrophobicity of the ion pairs follows the same order. It has been already observed before that considerably lower concentration of LiNTf2 is required to turn a polycation insoluble in water than is the case with LiOTf, which is in line with the results in Fig. 3.49
T cU of PC2N with a constant concentration of NaSCN can be tuned with high concentrations of urea (Fig. S10†). An analogous situation has been observed for poly(N,N-diethylacrylamide) (PDEA), an LCST polymer, which experiences an increase in cloud point with increasing concentration of urea while the cloud point of PNIPAm decreases.68 The main difference between PDEA and PNIPAm is that the former can only accept hydrogen bonds whereas the latter is capable of hydrogen bonding with itself. In this sense, PC2N resembles more PDEA. It is possible that in concentrated aqueous urea acts like a surfactant for water-insoluble polymers, thus reducing hydrophobic interactions.69 Such an effect is a plausible explanation for the solubility-enhancing effect of urea for PC2N as well as it making the phase separation less favorable and thus reduces the temperature of the UCST transition.
In addition, NaCl (up to a concentration of 4.5 M), Na2SO4 (up to 1.8 M), and NaH2PO4 (up to 4.5 M) were tested for PC2N, but no transition was observed for the samples. The anions of the salts are before NO3− in the Hofmeister series. Thus, the insolubility-inducing effect of the anions does not follow similar trends for PC2N as it does for PNIPAm. A 0.45 M solution of NaPS did not induce any phase transition for PC2N either. Therefore, the salting-out strength of the salts for PC2N can be written in the following order: NaH2PO4, NaCl, NaPS, (Na2SO4) < NaNO3 < NaSCN < LiOTf < LiNTf2 with 500 mM NaCl < LiNTf2. The unordered salts in left produce no detectable phase transition. The order of NaNO3 and Na2SO4 cannot be determined as the latter is saturated at lower concentration than is needed to induce a UCST with NaNO3. Therefore, it is not possible to study whether similar concentrations of the sulfate would cause phase transition to appear as well.
The rate of decrease of TcU with concentration for strong polycations of the anion has been observed to follow reversed Hofmeister series by other investigators.44,53 Biswas and Mandal noted for SCN−, ClO4−, I−, BF4− that more polarizable the anion, the lower the concentration needed to achieve insolubility in water at a given temperature.44 This observation is in line with Fig. 3, since the order of the ionic radii of the anions follows the order NTf2−(3.25–3.72 Å)70–72 > OTf−(2.67–3.07 Å)70,73 > SCN−(2.15–2.20 Å)74 > NaNO3(1.79 Å).75 The polarizability of an anion is proportional to its size: larger anions are more polarizable.74,75 However, the reversed Hofmeister series is not completely followed for PC2N as it has been shown that for PNIPAm that LiNTf2 acts like a strong kosmotrope for PNIPAm.50 Nevertheless, LiNTf2 has the strongest effect for PC2N (Fig. 3). Lysozyme, a positive charged protein also follows a reversed Hofmeister series with low salt concentrations, but the behavior reverts to the classical case with higher concentrations.76
To study the effect of the same ions from the Hofmeister series for a more hydrophobic polycation PC3N was studied as well. Behavior of PC3N in 8 mM NaSCN (Fig. S11†) was similar as what was observed for PC2N in LiNTf2 solution with NaCl (Fig. 3). There was clearly some particle formation at low temperatures, which had a transition resembling an LCST. No clear TcU transition could be observed in the studied range, although adding urea caused the solution to become clearer at high temperatures. The range between complete solubility and insolubility/particle formation is quite narrow as in 7 mM NaSCN the polymer was completely soluble. A similar observation was made by Biswas et al., who observed no UCST type transition, only turbidity up to 90 °C for poly(triphenyl-4-vinylbenzylphosphonium chloride) in 5 mM SCN−.52 The same polymer had clear TcU in halide solutions with significantly higher concentrations.
Like PC2N, PC3N did have a TcU in NaNO3 solution (Fig. S12†). The observed behavior resembles that of PC2N in LiNTf2–NaCl solution (Fig. 2) with the difference that the LCST like transition at lower temperatures is clearly reversible. The transitions are generally broad, and it is not clear if they are complete, so the exact values for the TcU cannot be determined accurately. However, much more NaNO3 is clearly needed to turn the polymer insoluble compared to NaSCN, which indicates, analogously to PC2N, that the thiocyanate anion interacts more strongly than nitrate with PC3N.
When NaCl is introduced in a PC3N solution, the picture changes. A clear TcL appears for the polymer (Fig. S13†). The transitions are rather sharp, but high salt concentrations are needed for the phase transition to appear. It is possible that the phase transition is a two-stage process as several samples display a slight decrease in transmittance at approximately 30 °C, which is sometimes accompanied by a mild downward slope. But this may be an artifact as well. The sharp transition is interpreted to be the TcL in the discussion and the values are reported in Fig. 4.
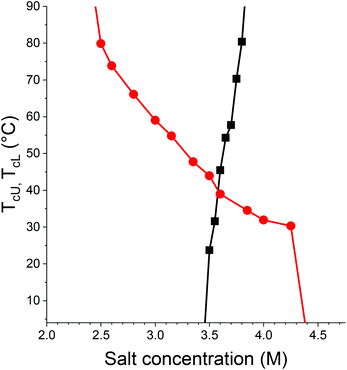 | ||
Fig. 4
T
cU for 1 mg mL−1 PC3N in aqueous NaH2PO4 (■) and TcL for 1 mg mL−1 PC3N in NaCl solution ( ) as a function salt concentration. ) as a function salt concentration. | ||
PC3N becomes insoluble in NaCl solution between 4.25 M and 4.4 M (Fig. 4 and S13†), although the trend seen in Fig. 4 would suggest that there should be only a minor decrease in TcL between the two concentrations. This is explainable by very strong hysteresis between the heating and cooling runs. The hysteresis for PC3N in 3.5 M NaCl is observed to be more than 35 °C, if the transition is reversible at all (Fig. S13†). The solutions with high concentrations of NaCl are made near or above their cloud points and the strong hysteresis or irreversibility of the transition causes the apparent disappearance of the phase transition. Behavior like this has been observed for LCST type transitions for polymers with cationic units.50 Although the samples are stored refrigerated overnight, it is evidently not enough for complete redissolution.
In addition to TcL in NaCl solutions, PC3N has a TcU in NaH2PO4 solutions (Fig. 4). It is somewhat problematic to compare the effects of the salts because of the opposite thermoresponsive behaviors, but since less NaCl is required for a transition temperature to appear for the polymer in the first place, the interaction between Cl− and the polymer is considered to be stronger. Opposite interpretation is also possible as the polymer tolerates more NaCl than NaH2PO4 without turning completely insoluble. A similar situation can be observed for TcLs of PC4N (please see discussion below), although with considerably less overlap. Similar reasoning is used to order the ions in that case as well.
Although polarizability of SCN− (6.74 Å3)77 is the highest for the anions studied with PC3N and it has the strongest effect as well, more polarizable anions are not always more effective in salting out PC3N. This is clearly visible from the fact that H2PO4− (5.79 Å3) is more polarizable than either nitrate (4.05–4.13 Å3) or chloride (3.25–3.42 Å3) yet PC3N can tolerate more H2PO4− than either of the latter ions.75,77 Thus, anion polarizability cannot be the only determining factor for the strength of interaction between PC3N and the anion in all cases.
Na2CO3 (up to 2.0 M) and Na2SO4 (up to 1.8 M) were also tested with PC3N, but no transition was observed, and it is possible that the reversed Hofmeister behavior is not followed for these two salts. Based on the results above, a similar series for the salting-out strength that was written for PC2N can be written for PC3N as well: (Na2CO3, Na2SO4) < NaH2PO4 < NaCl < NaNO3 < NaSCN. The position of Na2SO4 and Na2CO3 is uncertain, since no transitions could be observed. As the obtainable concentrations were also lower than those of NaCl and NaH2PO4 needed for any phase transition to appear, the salts cannot be ordered.
However, if one considers the fact that the ionic strengths of 1.8 M Na2SO4 and 2.0 M Na2CO3 solutions are 5.4 M and 6.0 M, respectively, a series based on ionic strength for can be written as Na2CO3, Na2SO4 < NaH2PO4 < NaCl < NaNO3 < NaSCN. The respective order of carbonate and sulfate is still unclear, but both have weaker effect at higher ionic strength than chloride or dihydrogen phosphate. Based on ionic strength, the reversed Hofmeister series is being followed by PC3N as well.
PC4N with LiNTf2 in 500 mM NaCl (Fig. S14†) forms particles not unlike the ones formed by PC2N under similar conditions (Fig. 2). The transmittance decreases as more LiNTf2 is introduced, which is natural since polycations with NTf2 as their counterion are generally insoluble in water.33,35,61,62 Addition of SDS without NaCl yielded qualitatively similar results as LiNTf2 and NaCl (Fig. S15†). The peculiar shape of some of the curves is most probably due to non-equilibrium nature of the systems as the solutions are made by quickly mixing the stock solutions. Analysis of the particles formed in the presence of LiNTf2 or SDS using several samples was attempted with dynamic light scattering (DLS). Measurements were conducted over the same temperature range as was used in the transmittance measurements. Multimodal distributions with particle sizes exceeding 1 μm were observed in all cases, so DLS is an ill-suited method for analyzing these particular samples.
It has been observed previously that a polymer with the same polycation as PC4N, but with 1-pentanesulfonate (PS) as its counterion undergoes a LCST type transition at 64 °C with polymer concentration of 3.0 wt%.28 To get a more quantitative image of the phenomenon, the amount of NaPS in 1 mg mL−1 solution of PC4N was varied, with or without adding NaCl (Fig. 5). At low concentrations, the addition of the organic anion lowers the cloud point and this effect can be weakened by addition of a competing electrolyte NaCl, as observed already in this study and in previous publications.49,50 When used together with other salts, the amounts of NaCl were always kept below the values needed to induce observable cloud points when NaCl is used as the only salt (Fig. 5). TcL measured for the aforementioned 3.0 wt% solution by Kohno et al. is well in line with the result obtained by adding NaPS in the solution with a constant polymer concentration.28 Therefore, it seems not to matter for this particular polycation–counterion pair whether or not the counterion is introduced within the polymer structure or added to the solution afterwards. This indicates that the anion concentration is more significant in deciding the cloud point and the concentration of the polycation is only of secondary importance. Similar observations have been made for other polycations with counterion induced UCST type phase transitions.49
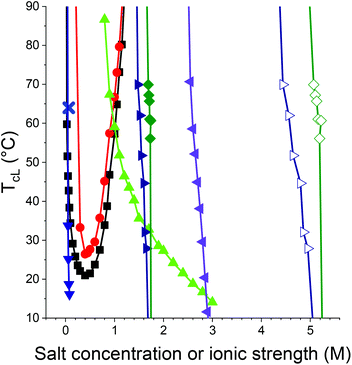 | ||
Fig. 5 LCST type cloud point temperature as a function of salt concentration for 1 mg mL−1 solution of PC4N in NaPS solution (■), in NaPS with 250 mM NaCl ( ), in NaCl ( ), in NaCl ( ), in NaNO3 with 250 mM NaCl ( ), in NaNO3 with 250 mM NaCl ( ), in Na2HPO4 ( ), in Na2HPO4 ( ), in Na2SO4 ( ), in Na2SO4 ( ), and in Na2CO3 ( ), and in Na2CO3 ( ). The ionic strengths for Na2SO4 and Na2CO3 are given as corresponding empty symbols. The value obtained by Kohno et al. for a 3.0 wt% polymer solution of PC4N with the PS anion, assuming that the density of the solution was 1 kg L−1, is marked in the graph with an ). The ionic strengths for Na2SO4 and Na2CO3 are given as corresponding empty symbols. The value obtained by Kohno et al. for a 3.0 wt% polymer solution of PC4N with the PS anion, assuming that the density of the solution was 1 kg L−1, is marked in the graph with an  .27 NaCl added to NaPS and NaNO3 is not taken into account in the salt concentration. .27 NaCl added to NaPS and NaNO3 is not taken into account in the salt concentration. | ||
However, as the amount of NaPS increases, the cloud point starts to increase again. A similar observation was already made for the UCST behavior of poly(2-methacryloyloxyethyltrimethylammonium iodide) in presence of LiOTf or the potassium salt of the same anion, KOTf.49 Also, the highest insolubility is reached at an approximate concentration of 0.5 M in both cases. The reason behind the increase in solubility could be that the PS ion bounds on the formed aggregate and thus solubilizing it, analogously to the case with PC2N, NaSCN, and urea. Alternatively, since the mass concentration of NaPS is already 87 mg mL−1 or 8.7 wt% (assuming density of 1 kg L−1) when the molar concentration is 0.5 M, the solvent has significant organic content. This will then lead to better solubility of the polymer and thus to increasing cloud point. In other words, the situation is analogous to adding organic solvent into the mixture.
When NaCl was added into the solution together with NaPS, the interaction between the pentanesulfonate ion and PC4N weakens due to the presence of competing anions, but qualitatively the behavior stays the same as with NaPS as the only salt.
Also shown in Fig. 5 is the salt concentration dependencies of cloud points for PC4N in NaNO3 with 250 mM NaCl. NaNO3 without added NaCl showed formation of particles with a TcL without actual dissolution at low temperatures (Fig. S16†), but again addition of NaCl yielded clear transitions. Presence of higher concentrations of NaCl as the only salt yielded TcLs in a similar fashion as was observed for PC3N already above. Lower concentrations of NaCl were required for a given value of TcLs, which is natural due to the longer alkyl chains connected to the ammonium group. An important difference between PC3N and PC4N is that NaH2PO4 induces an LCST for PC4N (Fig. 5) instead of the UCST observed for PC3N (Fig. 4).
Contrary to PC3N, Na2SO4 and Na2CO3 induce a TcL for PC4N at achievable concentrations (Fig. 5). Lower concentrations of Na2SO4 and Na2CO3 are required for a given value of TcL than is for NaH2PO4 and some overlap with NaCl is observed as well. When ionic strength is taken into account, the situation changes and the ionic strengths required to induce TcLs with Na2SO4 and Na2CO3 are considerably higher than with other salts.
Based on the results described above, the phase transition-inducing effect of different salts can be ordered into a series: NaH2PO4 < Na2CO3 < Na2SO4 < NaCl < NaPS with 250 mM < NaPS < NaNO3 with 250 mM NaCl < NaNO3, LiNTf2 with 500 mM NaCl, SDS. The order of the salts on the right-hand side of the series cannot be determined, since no clear solutions with a phase transition could be achieved for PCN4N in the presence of these salts; only turbid solutions were obtained. The order of NaH2PO4, NaCl, and NaNO3 is the same as was already observed for PC3N. Additionally, the more organic repeating unit of PC4N made it possible to observe phase transitions for Na2SO4 and Na2CO3 solutions as well at concentrations well below saturation.
As was the case with PC3N, when taking ionic strength into account, the relative order of Na2CO3, Na2SO4, and NaH2PO4 changes for PC4N as well. With ionic strength, the series becomes Na2CO3 < Na2SO4 < NaH2PO4 < NaCl < NaPS with 250 mM < NaPS < NaNO3 with 250 mM NaCl < NaNO3, LiNTf2 with 500 mM NaCl, SDS. The order fits the reversed Hofmeister series, but this may be only coincidental for the divalent ions. However, it seems likely that the monovalent ones obey the inverse series.
PC4N experienced cononsolvency in water–DMF mixtures (Fig. S17†), a behavior which been observed e.g. for PNIPAm in water–solvent mixtures.78–80 However, the behavior of PC4N is fundamentally different from PNIPAm since the transmittance stays constant over the studied temperature range (Fig. S18†).
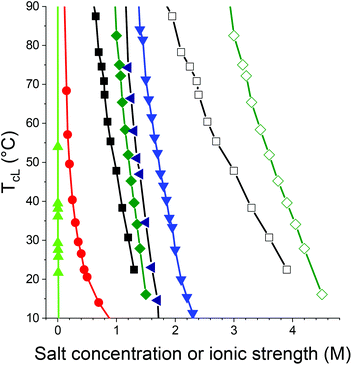
Apart from the water insoluble PC6N, the polymer with the longest alkyl chains in the series is PC5N. As can be expected from the earlier polymers in the series, the concentration of a given salt that is needed to induce a TcL for the polymer is lower than for PC4N. This is natural, since the longer alkyl chain makes it less favorable for the polymer to dissolve in water. This is also seen in the observation that mixtures of PC5N and NaPS yield associates, which turn the solutions turbid, as has been observed for many polymer–ion combinations already. However, when 0.1 M of NaCl is added into the solution together with NaPS, a clear LCST type behavior is observed (Fig. 6). The concentration of NaCl was kept low due to the observation that the concentration of NaCl is enough to induce a phase transition for PC5N is not high. Otherwise, a similar LCST behavior than for PC4N was observed for PC5N as well with the same salts. The effects of salts on TcLs of PC4N and PC5N are compared in Fig. S19.†
Based on the data in Fig. 6, the strength of the phase transition inducing effect of different sodium salts for PC5N can be arranged to the following order: NaH2PO4 < Na2CO3 < Na2SO4 < NaC l < NaPS with 100 mM NaCl < NaPS. The order is the same as already observed for PC4N i.e. the monovalent anions follow reversed Hofmeister series, but NaH2PO4, Na2CO3 and Na2SO4 are in different order than they are in the classical series. However, when ionic strength is used as a measure, the series resembles again reversed Hofmeister series: Na2CO3 < Na2SO4 < NaH2PO4 < NaCl < NaPS with 100 mM NaCl < NaPS.
To study whether concentration or ionic strength is the right measure in ordering the anions into series, in addition to Na2SO4, MgSO4 was used together with PC5N as well. The rationale behind choosing this salt is that the divalent cation increases the ionic strength significantly without increasing the amount of the anion in the solution. As it was hypothesized that the LCST increases only from the interactions between the polycation and the anion. Quite surprisingly, when using magnesium instead of sodium as the cation the cloud points increase for approximately 30–40 °C when keeping the sulfate salt concentration constant. Therefore, the effect from the cation in the salt is quite significant and – in contrast to the anion effect – it does not follow reversed Hofmeister series as Mg2+ is more chaotropic than Na+.81 But direct Hofmeister series is not followed by all cations either, since it was noticed in an earlier study that LiOTf has stronger UCST inducing effect than KOTf,49 although Li+ is stronger chaotrope than K+.81 The strong cation effect prevents the study of effect of ionic strength without changing the anion concentration. Thus, it cannot be known if the ionic strength based reversed Hofmeister series is generalizable or is it followed only by the monovalent anions. While writing this, further studies that will shed light on the matter are underway.
The orders of strength of phase separation inducing effect for the polymer series is summarized in Table 2. The results for all studied polycations can be combined to the following series: NaH2PO4 < Na2CO3 < Na2SO4 < NaCl < NaPS < NaNO3 < NaSCN < LiOTf < LiNTf2, which matches the reversed Hofmeister series with the addition of LiNTf2, LiOTf, and NaPS, except in the case of Na2CO3 and Na2SO4, which have divalent anions. If one uses ionic strength instead of concentration, series changes to Na2CO3 < Na2SO4 < NaH2PO4 < NaCl < NaPS < NaNO3 < NaSCN < LiOTf < LiNTf2 and follows the reversed Hofmeister series.
| Polymer | Type of transition | Strength of interaction with the polycation |
|---|---|---|
| PC2N | UCST | NaH2PO4, Na2SO4, NaCl < NaNO3 < NaSCN < LiOTf < LiNTf2 with 500 mM NaCl < LiNTf2 |
| PC3N | LCST/UCST | NaH2PO4 < NaCl < NaNO3 < NaSCN |
| PC4N | LCST | NaH2PO4 < Na2CO3 < Na2SO4 < NaCl < NaPS with 250 mM < NaPS < NaNO3 with 250 mM NaCl < NaNO3, LiNTf2 with 500 mM NaCl, SDS |
| PC5N | LCST | NaH2PO4 < Na2CO3 < Na2SO4 < NaCl < NaPS with 100 mM NaCl < NaPS |
Similar results were obtained by Jana et al., who studied the effect of ions on cationic polypeptides.53 They found out that SO42− behaves unlike the monovalent ions – which induce a UCST for the polymers – but causes precipitation in concentrations between those required for SCN− and ClO4−. However, if one assumes that the authors used SO42− as a salt with a monovalent cation and calculates the required ionic strength for the precipitation to take place, then the ionic strength required for SO42− is the highest of the studied series. Thus, the findings are in line with the ones in this paper.
Table S2† summarizes findings in other studies regarding the salting out effect of different anions for polycations. It is often reported that the insolubility inducing effect follows a reversed for Hofmeister series for monovalent ions or the same order as observed in this study.27,28,30,32,33,35,37,41,43,48–50,52,53 An exception of this rule was reported by Biswas and Mandal for a series of carboxylate-containing polyelectrolytes with imidazolium groups at low pH where the acids are completely protonated.44 However, this was the only conflicting case found from the literature and it did follow the series to some extent. Thus, the comparison strongly suggests that the findings of the article are generalizable for many other polycation–counterion systems as well. As stated above, the series found in this paper does not always act like reversed Hofmeister series for PNIPAm as NTf2− was found to act like a strong kosmotrope, although it salts polycations out more efficiently than the strongest chaotrope, SCN−.50
It has been shown that the effect of Hofmeister anions on thermoresponsive behavior for poly(ethylene oxide)-b-poly(propylene oxide)-b-poly-(ethylene oxide) (PEO-PPO-PEO) triblock copolymers is markedly different for weakly hydrated anions right from Cl− than it is for the more strongly hydrated ones.77 This is the same point where the behavior changes in for the PNpC series and the polarizability of the anion does not predict the effect of the anion on the solubility anymore, although the very large NTf2− does not fit to this rationalization (please see discussion above). The mechanism governing the strength of interaction between the polycation and the anion is evidently complex.
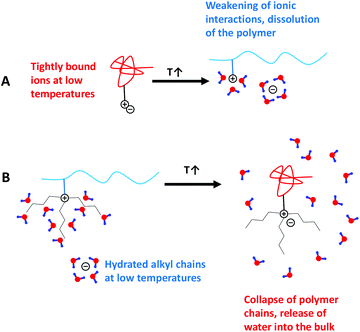
The fact that PC4N and PC5N possess an LCST behavior can be rationalized to arise from the hydration of the alkyl chains connected to the ammonium group. Hydration of alkyl chains is entropically unfavorable due to the formation of an ordered water cage around the hydrophobic groups.82–86 The unfavorable entropy of hydration then leads in to phase separation when temperature is increased. Similar effects have been used to explain the LCST behavior of PNIPAm87–90 and polysulfobetaines with long alkyl chains.91 The negative enthalpy of dissolution keeps the polymers in solution at low temperatures and this is modulated by anion–polycation interactions, which may also make the overall entropy of dissolution less negative.
The effect of salt concentration on the cloud point can be explained by ion pairing with the cationic ammonium group present in the repeating units. The pairing weakens the interactions between water and the cation, which in turn reduces the enthalpy of dissolution and thus lowers the temperature in which it is favorable for the polymer to dehydrate.
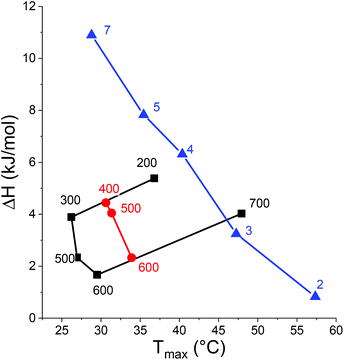
A consequence of the alkyl hydration was observed when determining the enthalpies of the phase transition for PC4N and PC5N in NaPS solutions by DSC. The relatively high enthalpy associated with the phase transition of PNIPAm has been attributed largely to the release of water in the water cages into the bulk.89,92 The hydrogen bonding in water cages is stronger than in bulk water and this difference is then observed as an endothermic transition. Similar rationalization is reasonable with the polymers from this work as well. As PC5N has longer alkyl chains than PC4N, PC5N also needs more coordinated water to accommodate its longer alkyl chains in water compared to PC4N and more water molecules are released to the bulk per repeating unit. Thus, the enthalpy per repeating unit associated with the phase transition is significantly higher at a given transition temperature (Fig. 7). Outside the extremes for PC4N with NaPS as the only salt, the enthalpy decreases linearly with the transition temperature. Similar behavior has been observed for PNIPAm and its copolymers as well.92,93 No distinct peaks could be seen outside the range shown in Fig. 6 for PC4N.
PC3N is an interesting borderline case since it can have either an LCST or a UCST type behavior, depending on the anion. This is in a stark contrast with the other polycations of the series as PC2N has only TcU whereas PC4N and PC5N both have only TcL. A similar change in behavior has been observed for a series poly(sulfobetaine methacrylate)s.91 The polymers were shown to have either an LCST or a UCST, depending on the length of the alkyl substituent in the quaternary ammonium.
As PC2N has no long alkyl substituents, its solution behavior is governed only by competition between ionic attraction (enthalpy gain) and ion pair dissociation/polymer dissolution (entropy gain). This will lead to UCST behavior, since the dissociation becomes more favorable at higher temperatures. The situation is analogous to the case of zwitterionic polymers, where the ion pairing takes place intramolecularly.9 How much entropy is gained is modulated by the amount of the ions present in the solution. The need for the threshold ionic strength for the UCST transition to appear, which has been observed for other polycations as well,49,50,63 can be rationalized in two different ways. Either it is caused by electrostatic repulsions, which will prevent the polymer from collapsing or other anions are needed to be present in order to substitute the hydrophobic ones in the ion clouds surrounding the polymer chains so that the polymer is able to dissolve molecularly. The suggested reason behind the differences in the thermal response of polycations with longer and shorter alkyl chains are schematically shown in Scheme 2.
Conclusions
The effect of a various anions on the phase transition of a series of poly[trialkyl(4-vinylbenzyl)ammonium chlorides] was studied with the alkyl chain length ranging from ethyl to pentyl. Poly[triethyl(4-vinylbenzyl)ammonium chloride] developed a UCST behavior in the presence of LiNTf2 and NaCl, NaSCN, and LiOTf. Poly[tripropyl(4-vinylbenzyl)ammonium chloride] also had a UCST in NaH2PO4 solution. All the other polymers had LCST type transitions in presence of various salts. No thermosensitive behavior has been reported for most of the studied polycation–counterion pairs. The switch from UCST to LCST can be attributed to the entropy penalty of the alkyl hydration becoming more significant when the alkyl chain becomes longer. With a short alkyl chain, the governing factor is the strength of interaction between the polycation and the anion.It was found during the study that by increasing the alkyl chains, different anions can be used to induce thermoresponsive behavior for the polycations. By combining the partially overlapping results, a following series for polycation–anion interaction can be constructed: H2PO4− < CO32− < SO42− < Cl− < PS− < NO3− < SCN− < OTf− < NTf2−.
For salts with monovalent anions, it was found out that the strength of the insolubility inducing effect follows reversed Hofmeister series, but the reasons behind this will require more studies. Salts with divalent anions do not fall into the reversed series, as its effect is stronger than its position in the Hofmeister series would indicate but do follow the series when ionic strength is used as the measure. Also, the cation effect is significant.
This study introduces a systematic way of designing a polycation based thermosensitive system by matching an anion from reversed Hofmeister series with cation hydrophobicity. In addition, four new UCST systems based on PC2N (with LiNTf2-NaCl, LiOTf, NaSCN, and NaNO3), one based on PC3N (NaH2PO4), a new LCST system based on PC3N (NaCl), five new LCST systems based on PC4N (NaCl, NaH2PO4, NaNO3–NaCl, Na2SO4, Na2CO3), and five new LCST systems based on PC5N (NaCl, Na2SO4, NaH2PO4, NaPS–NaCl, Na2CO3) were observed for the first time. These are also the first reported thermoresponsive systems with PC2N, PC3N, or PC5N as polycation and with NO3−, SO42−, or H2PO4− as the anion together with any polycation. Therefore, this study considerably broadens the range of available polycation-counterion combinations with LCST or UCST in aqueous solutions.
The findings increase the number of available tools for the polymer scientist in designing new thermoresponsive systems. Practically unlimited number of polycation–anion pairs can be designed this way. It is reasonable to assume that a suitable salt can be found for a vast majority of water-soluble polycations in order to induce a thermal phase transition for them. This is the first time when the series is studied in this scale and more studies are required to understand its extent and nature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Fincancial support from the Academy of Finland (no. 307475) and from the Emil Aaltonen foundation is gratefully acknowledged.Notes and references
- J. Seuring and S. Agarwal, Polymers with Upper Critical Solution Temperature in Aqueous Solution, Macromol. Rapid Commun., 2012, 33, 1898–1920 CrossRef CAS PubMed.
- V. Aseyev, H. Tenhu and F. M. Winnik, Non-ionic thermoresponsive polymers in water, Adv. Polym. Sci., 2011, 242, 29–89 CrossRef CAS.
- J. Seuring and S. Agarwal, Polymers with Upper Critical Solution Temperature in Aqueous Solution: Unexpected Properties from Known Building Blocks, ACS Macro Lett., 2013, 2, 597–600 CrossRef CAS.
- J. Niskanen and H. Tenhu, How to manipulate the upper critical solution temperature (UCST)?, Polym. Chem., 2017, 8, 220–232 RSC.
- W. Sun and P. Wu, A molecular level study of the phase transition process of hydrogen-bonding UCST polymers, Phys. Chem. Chem. Phys., 2018, 20, 20849–20855 RSC.
- J. Seuring and S. Agarwal, Non-ionic homo- and copolymers with H-donor and H-acceptor units with an UCST in water, Macromol. Chem. Phys., 2010, 211, 2109–2117 CrossRef CAS.
- J. Seuring and S. Agarwal, First Example of a Universal and Cost-Effective Approach: Polymers with Tunable Upper Critical Solution Temperature in Water and Electrolyte Solution, Macromolecules, 2012, 45, 3910–3918 CrossRef CAS.
- S. Glatzel, A. Laschewsky and J. Lutz, Well-Defined Uncharged Polymers with a Sharp UCST in Water and in Physiological Milieu, Macromolecules, 2011, 44, 413–415 CrossRef CAS.
- P. Mary, D. D. Bendejacq, M. Labeau and P. Dupuis, Reconciling Low- and High-Salt Solution Behavior of Sulfobetaine Polyzwitterions, J. Phys. Chem. B, 2007, 111, 7767–7777 CrossRef CAS PubMed.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Thermoresponsive polyelectrolytes derived from ionic liquids, Polym. Chem., 2015, 6, 2163–2178 RSC.
- Y. Kohno and H. Ohno, Key Factors to Prepare Polyelectrolytes Showing Temperature-Sensitive Lower Critical Solution Temperature-type Phase Transitions in Water, Aust. J. Chem., 2012, 65, 91–94 CrossRef CAS.
- Y. Men, X. Li, M. Antonietti and J. Yuan, Poly(tetrabutylphosphonium 4-styrenesulfonate): a poly(ionic liquid) stabilizer for graphene being multi-responsive, Polym. Chem., 2012, 3, 871–873 RSC.
- B. Ziółkowski and D. Diamond, Thermoresponsive poly(ionic liquid) hydrogels, Chem. Commun., 2013, 49, 10308–10310 RSC.
- W. Li and P. Wu, Unusual thermal phase transition behavior of an ionic liquid and poly(ionic liquid) in water with significantly different LCST and dynamic mechanism, Polym. Chem., 2014, 5, 5578–5590 RSC.
- S. Chen, Y. Peng, Q. Wu, A. Chang, A. Qu, J. Shen, J. Xie, Z. H. Farooqi and W. Wu, Synthesis and characterization of responsive poly(anionic liquid) microgels, Polym. Chem., 2016, 7, 5463–5473 RSC.
- Y. Kohno, Y. Deguchi and H. Ohno, Ionic liquid-derived charged polymers to show highly thermoresponsive LCST-type transition with water at desired temperatures, Chem. Commun., 2012, 48, 11883–11885 RSC.
- Y. Men, H. Schlaad, A. Voelkel and J. Yuan, Thermoresponsive polymerized gemini dicationic ionic liquid, Polym. Chem., 2014, 5, 3719–3724 RSC.
- Y. Deguchi, Y. Kohno and H. Ohno, Design of Ionic Liquid-Derived Polyelectrolyte Gels Toward Reversible Water Absorption/Desorption System Driven by Small Temperature Change, Aust. J. Chem., 2014, 67, 1666–1670 CrossRef CAS.
- Y. Deguchi, Y. Kohno and H. Ohno, A fine tuning of LCST-type phase transition of poly(ionic liquid)s in water, Chem. Lett., 2015, 44, 238–240 CrossRef CAS.
- G. Wang and P. Wu, In-depth study of the phase separation behaviour of a thermoresponsive ionic liquid and a poly(ionic liquid) in concentrated aqueous solution, Soft Matter, 2015, 11, 5253–5264 RSC.
- Y. Zhang, H. Tang and P. Wu, Insights into the thermal phase transition behavior of a gemini dicationic polyelectrolyte in aqueous solution, Soft Matter, 2018, 14, 4380–4387 RSC.
- P. J. Flory and J. E. Osterheld, Intrinsic viscosities of polyelectrolytes. Polyacrylic acid, J. Phys. Chem., 1954, 58, 653–661 CrossRef CAS.
- R. Buscall and T. Corner, The phase separation behavior of aqueous solutions of poly(acrylic acid) and its partial sodium salts in the presence of sodium chloride, Eur. Polym. J., 1982, 18, 967–974 CrossRef CAS.
- X. Cai, L. Zhong, Y. Su, S. Lin and X. He, Novel pH-tunable thermoresponsive polymers displaying lower and upper critical solution temperatures, Polym. Chem., 2015, 6, 3875–3884 RSC.
- T. Wolf, T. Rheinberger, J. Simon and F. R. Wurm, Reversible Self-Assembly of Degradable Polymersomes with Upper Critical Solution Temperature in Water, J. Am. Chem. Soc., 2017, 139, 11064–11072 CrossRef CAS PubMed.
- C. Xing, Z. Shi, J. Tian, J. Sun and Z. Li, Charge-determined LCST/UCST behavior in ionic polypeptoids, Biomacromolecules, 2018, 19, 2109–2116 CrossRef CAS PubMed.
- Y. Men, H. Schlaad and J. Yuan, Cationic Poly(ionic liquid) with Tunable Lower Critical Solution Temperature-Type Phase Transition, ACS Macro Lett., 2013, 2, 456–459 CrossRef CAS.
- Y. Kohno, Y. Deguchi, N. Inoue and H. Ohno, Temperature-Driven and Reversible Assembly of Homopolyelectrolytes Derived from Suitably Designed Ionic Liquids in Water, Aust. J. Chem., 2013, 66, 1393–1398 CrossRef CAS.
- A. Okafuji, Y. Kohno and H. Ohno, Thermoresponsive Poly(Ionic Liquid)s in Aqueous Salt Solutions: Salting-Out Effect on Their Phase Behavior and Water Absorption/Desorption Properties, Macromol. Rapid Commun., 2016, 37, 1130–1134 CrossRef CAS PubMed.
- M. Li, X. He, Y. Ling and H. Tang, Dual thermoresponsive homopolypeptide with LCST-type linkages and UCST-type pendants: synthesis, characterization, and thermoresponsive properties, Polymer, 2017, 132, 264–272 CrossRef CAS.
- T. Thavanesan, C. Herbert and F. A. Plamper, Insight in the Phase Separation Peculiarities of Poly(dialkylaminoethyl methacrylate)s, Langmuir, 2014, 30, 5609–5619 CrossRef CAS PubMed.
- H. Yoshimitsu, A. Kanazawa, S. Kanaoka and S. Aoshima, Well-Defined Polymeric Ionic Liquids with an Upper Critical Solution Temperature in Water, Macromolecules, 2012, 45, 9427–9434 CrossRef CAS.
- R. Marcilla, J. A. Blazquez, J. Rodriguez, J. A. Pomposo and D. Mecerreyes, Tuning the solubility of polymerized ionic liquids by simple anion-exchange reactions, J. Polym. Sci., Part A: Polym. Chem., 2003, 42, 208–212 CrossRef.
- E. Karjalainen, N. Chenna, P. Laurinmäki, S. J. Butcher and H. Tenhu, Diblock copolymers consisting of a polymerized ionic liquid and poly(N-isopropylacrylamide). Effects of PNIPAM block length and counter ion on self-assembling and thermal properties, Polym. Chem., 2013, 4, 1014–1024 RSC.
- R. Marcilla, J. A. Blazquez, R. Fernandez, H. Grande, J. A. Pomposo and D. Mecerreyes, Synthesis of novel polycations using the chemistry of ionic liquids, Macromol. Chem. Phys., 2005, 206, 299–304 CrossRef CAS.
- E. I. Privalova, E. Karjalainen, M. Nurmi, P. Mäki-Arvela, K. Eränen, H. Tenhu, D. Y. Murzin and J. Mikkola, Imidazolium-Based Poly(ionic liquid)s as New Alternatives for CO2 Capture, ChemSusChem, 2013, 6, 1500–1509 CrossRef CAS PubMed.
- Y. Deng, Y. Xu, X. Wang, Q. Yuan, Y. Ling and H. Tang, Water-Soluble Thermoresponsive α-Helical Polypeptide with an Upper Critical Solution Temperature: Synthesis, Characterization, and Thermoresponsive Phase Transition Behaviors, Macromol. Rapid Commun., 2015, 36, 453–458 CrossRef CAS PubMed.
- X. Cao and Z. An, RAFT Synthesis in Water of Cationic Polyelectrolytes with Tunable UCST, Macromol. Rapid Commun., 2015, 36, 2107–2110 CrossRef CAS PubMed.
- Y. Wu, X. Wang, Y. Ling and H. Tang, Preparation and thermoresponsive properties of helical polypeptides bearing pyridinium salts, RSC Adv., 2015, 5, 40772–40778 RSC.
- H. Yoshimitsu, A. Kanazawa, S. Kanaoka and S. Aoshima, Cationic polymerization of vinyl ethers with alkyl or ionic side groups in ionic liquids, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 1774–1784 CrossRef CAS.
- C. Ge, S. Liu, C. Liang, Y. Ling and H. Tang, Synthesis and UCST-type phase behavior of α-helical polypeptides with Y-shaped and imidazolium pendants, Polym. Chem., 2016, 7, 5978–5987 RSC.
- S. Liu, C. Ge, Y. Ling and H. Tang, Preparation and UCST-Type Phase Behaviours of Poly(γ-4-methylbenzyl-l-glutamate) Pyridinium Tetrafluoroborate Conjugates in Methanol or Water, Aust. J. Chem., 2017, 70, 245–251 CrossRef CAS.
- J. Xiao, M. Li, W. Liu, Y. Li, Y. Ling and H. Tang, Synthesis and thermoresponsive properties of poly(L-cysteine)s bearing imidazolium salts, Eur. Polym. J., 2017, 88, 340–348 CrossRef CAS.
- Y. Biswas and T. K. Mandal, Structural Variation in Homopolymers Bearing Zwitterionic and Ionic Liquid Pendants for Achieving Tunable Multi-Stimuli Responsiveness and Hierarchical Nanoaggregates, Macromolecules, 2017, 50, 9807–9820 CrossRef CAS.
- C. Ge, L. Zhao, Y. Ling and H. Tang, Thermo and pH dual responsive polypeptides derived from “clickable” poly(γ-3-methylthiopropyl-L-glutamate), Polym. Chem., 2017, 8, 1895–1905 RSC.
- M. Li, Z. Zheng, L. Sun, Y. Ling, S. Luan and H. Tang, SO2, temperature, and oxidation multi-responsive homopolypeptide: Synthesis, characterization, and exploration of their potential applications, Eur. Polym. J., 2018, 109, 523–531 CrossRef CAS.
- J. Huang, X. Chen, H. Qin, H. Liang and J. Lu, A new thermoresponsive polymer with reactive aldehyde groups for post-modification to tune the solubility and phase transition temperature, Polymer, 2019, 160, 99–106 CrossRef CAS.
- C. Liang, X. Wang, R. Zhou, H. Shi, S. Yan, Y. Ling, S. Luan and H. Tang, Thermo- and oxidation-responsive homopolypeptide: synthesis, stimuli-responsive property and antimicrobial activity, Polym. Chem., 2019, 10, 2190–2202 RSC.
- E. Karjalainen, V. Aseyev and H. Tenhu, Counterion-Induced UCST for Polycations, Macromolecules, 2014, 47, 7581–7587 CrossRef CAS.
- E. Karjalainen, V. Aseyev and H. Tenhu, Upper or lower critical solution temperature, or both? Studies on cationic copolymers of N-isopropylacrylamide, Polym. Chem., 2015, 6, 3074–3082 RSC.
- V. Baddam, V. Aseyev, S. Hietala, E. Karjalainen and H. Tenhu, Polycation-PEG Block Copolymer Undergoes Stepwise Phase Separation in Aqueous Triflate Solution, Macromolecules, 2018, 51, 9681–9691 CrossRef CAS.
- Y. Biswas, T. Maji, M. Dule and T. K. Mandal, Tunable doubly responsive UCST-type phosphonium poly(ionic liquid): a thermosensitive dispersant for carbon nanotubes, Polym. Chem., 2016, 7, 867–877 RSC.
- S. Jana, Y. Biswas and T. K. Mandal, Methionine-based cationic polypeptide/polypeptide block copolymer with triple-stimuli responsiveness: DNA polyplexation and phototriggered release, Polym. Chem., 2018, 9, 1869–1884 RSC.
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS.
- E. V. Korchagina, X. Qiu and F. M. Winnik, Effect of Heating Rate on the Pathway for Vesicle Formation in Salt-Free Aqueous Solutions of Thermosensitive Cationic Diblock Copolymers, Macromolecules, 2013, 46, 2341–2351 CrossRef CAS.
- H. He, M. Zhong, B. Adzima, D. Luebke, H. Nulwala and K. Matyjaszewski, A Simple and Universal Gel Permeation Chromatography Technique for Precise Molecular Weight Characterization of Well-Defined Poly(ionic liquid)s, J. Am. Chem. Soc., 2013, 135, 4227–4230 CrossRef CAS PubMed.
- K. Skrabania, A. Miasnikova, A. M. Bivigou-Koumba, D. Zehm and A. Laschewsky, Examining the UV-vis absorption of RAFT chain transfer agents and their use for polymer analysis, Polym. Chem., 2011, 2, 2074–2083 RSC.
- Y. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, Specific Ion Effects on the Water Solubility of Macromolecules: PNIPAM and the Hofmeister Series, J. Am. Chem. Soc., 2005, 127, 14505–14510 CrossRef CAS PubMed.
- Y. Zhang and P. S. Cremer, Interactions between macromolecules and ions: the Hofmeister series, Curr. Opin. Chem. Biol., 2006, 10, 658–663 CrossRef CAS PubMed.
- Y. Zhang, S. Furyk, L. B. Sagle, Y. Cho, D. E. Bergbreiter and P. S. Cremer, Effects of Hofmeister Anions on the LCST of PNIPAM as a Function of Molecular Weight, J. Phys. Chem. C, 2007, 111, 8916–8924 CrossRef CAS PubMed.
- K. Vijayakrishna, S. K. Jewrajka, A. Ruiz, R. Marcilla, J. A. Pomposo, D. Mecerreyes, D. Taton and Y. Gnanou, Synthesis by RAFT and Ionic Responsiveness of Double Hydrophilic Block Copolymers Based on Ionic Liquid Monomer Units, Macromolecules, 2008, 41, 6299–6308 CrossRef CAS.
- K. Vijayakrishna, D. Mecerreyes, Y. Gnanou and D. Taton, Polymeric vesicles and micelles obtained by self-assembly of ionic liquid-based block copolymers triggered by anion or solvent exchange, Macromolecules, 2009, 42, 5167–5174 CrossRef CAS.
- E. Karjalainen, V. Aseyev and H. Tenhu, Influence of Hydrophobic Anion on Solution Properties of PDMAEMA, Macromolecules, 2014, 47, 2103–2111 CrossRef CAS.
- K. Nakamura, T. Saiwaki, K. Fukao and T. Inoue, Viscoelastic Behavior of the Polymerized Ionic Liquid Poly(1-ethyl-3-vinylimidazolium bis(trifluoromethanesulfonylimide)), Macromolecules, 2011, 44, 7719–7726 CrossRef CAS.
- K. Nakamura, K. Fukao and T. Inoue, Dielectric Relaxation and Viscoelastic Behavior of Polymerized Ionic Liquids with Various Counteranions, Macromolecules, 2012, 45, 3850–3858 CrossRef CAS.
- M. D. Green, D. Salas-de la Cruz, Y. Ye, J. M. Layman, Y. A. Elabd, K. I. Winey and T. E. Long, Alkyl-Substituted N-Vinylimidazolium Polymerized Ionic Liquids: Thermal Properties and Ionic Conductivities, Macromol. Chem. Phys., 2011, 212, 2522–2528 CrossRef CAS.
- A. S. Shaplov, E. I. Lozinskaya, D. O. Ponkratov, I. A. Malyshkina, F. Vidal, P. Aubert, O. V. Okatova, G. M. Pavlov, L. I. Komarova, C. Wandrey and Y. S. Vygodskii, Bis(trifluoromethylsulfonyl)amide based “polymeric ionic liquids”: Synthesis, purification and peculiarities of structure-properties relationships, Electrochim. Acta, 2011, 57, 74–90 CrossRef CAS.
- J. Wang, B. Liu, G. Ru, J. Bai and J. Feng, Effect of Urea on Phase Transition of Poly(N-isopropylacrylamide) and Poly(N,N-diethylacrylamide) Hydrogels: A Clue for Urea-Induced Denaturation, Macromolecules, 2016, 49, 234–243 CrossRef CAS.
- R. Zangi, R. Zhou and B. J. Berne, Urea's Action on Hydrophobic Interactions, J. Am. Chem. Soc., 2009, 131, 1535–1541 CrossRef CAS PubMed.
- M. Ue, Mobility and ionic association of lithium and quaternary ammonium salts in propylene carbonate and Î3-butyrolactone, J. Electrochem. Soc., 1994, 141, 3336–3342 CrossRef CAS.
- A. P. Abbott, Model for the conductivity of ionic liquids based on an infinite dilution of holes, ChemPhysChem, 2005, 6, 2502–2505 CrossRef CAS PubMed.
- D. R. MacFarlane, M. Forsyth, E. I. Izgorodina, A. P. Abbott, G. Annat and K. Fraser, On the concept of ionicity in ionic liquids, Phys. Chem. Chem. Phys., 2009, 11, 4962–4967 RSC.
- S. E. Okan and D. C. Champeney, Molar conductance of aqueous solutions of sodium, potassium, and nickel trifluoromethanesulfonate at 25 °C, J. Solution Chem., 1997, 26, 405–414 CrossRef CAS.
- Y. Iwadate, K. Kawamura, K. Igarashi and J. Mochinaga, Effective ionic radii of nitrite and thiocyanate estimated in terms of the Boettcher equation and the Lorentz-Lorenz equation, J. Phys. Chem., 1982, 86, 5205–5208 CrossRef CAS.
- M. Li, B. Zhuang, Y. Lu, Z. Wang and L. An, Accurate Determination of Ion Polarizabilities in Aqueous Solutions, J. Phys. Chem. B, 2017, 121, 6416–6424 CrossRef CAS PubMed.
- Y. Zhang and P. S. Cremer, The inverse and direct Hofmeister series for lysozyme, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15249–15253 CrossRef CAS PubMed.
- B. A. Deyerle and Y. Zhang, Effects of Hofmeister Anions on the Aggregation Behavior of PEO-PPO-PEO Triblock Copolymers, Langmuir, 2011, 27, 9203–9210 CrossRef CAS PubMed.
- F. M. Winnik, H. Ringsdorf and J. Venzmer, Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide), Macromolecules, 1990, 23, 2415–2416 CrossRef CAS.
- H. G. Schild, M. Muthukumar and D. A. Tirrell, Cononsolvency in mixed aqueous solutions of poly(N-isopropylacrylamide), Macromolecules, 1991, 24, 948–952 CrossRef CAS.
- R. O. R. Costa and R. F. S. Freitas, Phase behavior of poly(N-isopropylacrylamide) in binary aqueous solutions, Polymer, 2002, 43, 5879–5885 CrossRef CAS.
- H. I. Okur, J. Hladilkova, K. B. Rembert, Y. Cho, J. Heyda, J. Dzubiella, P. S. Cremer and P. Jungwirth, Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions, J. Phys. Chem. B, 2017, 121, 1997–2014 CrossRef CAS PubMed.
- F. H. Stillinger, Water revisited, Science, 1980, 209, 451–457 CrossRef CAS.
- R. L. Baldwin, Temperature dependence of the hydrophobic interaction in protein folding, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 8069–8072 CrossRef CAS.
- G. Hummer, S. Garde, A. E. Garcia, M. E. Paulaitis and L. R. Pratt, Hydrophobic Effects on a Molecular Scale, J. Phys. Chem. B, 1998, 102, 10469–10482 CrossRef CAS.
- L. F. Scatena, M. G. Brown and G. L. Richmond, Water at hydrophobic surfaces: Weak hydrogen bonding and strong orientation effects, Science, 2001, 292, 908–912 CrossRef CAS PubMed.
- N. T. Southall, K. A. Dill and A. D. J. Haymet, A View of the Hydrophobic Effect, J. Phys. Chem. B, 2002, 106, 521–533 CrossRef CAS.
- K. Otake, H. Inomata, M. Konno and S. Saito, Thermal analysis of the volume phase transition with N-isopropylacrylamide gels, Macromolecules, 1990, 23, 283–289 CrossRef CAS.
- H. Inomata, S. Goto and S. Saito, Phase transition of N-substituted acrylamide gels, Macromolecules, 1990, 23, 4887–4888 CrossRef CAS.
- E. C. Cho, J. Lee and K. Cho, Role of bound water and hydrophobic interaction in phase transition of poly(N-isopropylacrylamide) aqueous solution, Macromolecules, 2003, 36, 9929–9934 CrossRef CAS.
- S. A. Deshmukh, S. K. R. S. Sankaranarayanan, K. Suthar and D. C. Mancini, Role of Solvation Dynamics and Local Ordering of Water in Inducing Conformational Transitions in Poly(N-isopropylacrylamide) Oligomers through the LCST, J. Phys. Chem. B, 2012, 116, 2651–2663 CrossRef CAS PubMed.
- N. Wang, B. T. Seymour, E. M. Lewoczko, E. W. Kent, M. Chen, J. Wang and B. Zhao, Zwitterionic poly(sulfobetaine methacrylate)s in water: from upper critical solution temperature (UCST) to lower critical solution temperature (LCST) with increasing length of one alkyl substituent on the nitrogen atom, Polym. Chem., 2018, 9, 5257–5261 RSC.
- Y. Maeda, T. Higuchi and I. Ikeda, FTIR Spectroscopic and Calorimetric Studies of the Phase Transitions of N-Isopropylacrylamide Copolymers in Water, Langmuir, 2001, 17, 7535–7539 CrossRef CAS.
- H. Feil, Y. H. Bae, J. Feijen and S. W. Kim, Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers, Macromolecules, 1993, 26, 2496–2500 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The experimental details of the study. Also, Fig. S1–S20 and Tables S1 and S2. See DOI: 10.1039/d0py00917b |
| ‡ Present address: Karlsruher Institut für Technologie (KIT), Institut für Bio-und Lebensmitteltechnik, Teilinstitut IV: Molekulare Aufarbeitung von Bioprodukten, Fritz-Haber-Weg 2, 76131 Karlsruhe, Germany. |
| This journal is © The Royal Society of Chemistry 2020 |