Synthesis of multisegmented block copolymer by Friedel–Crafts hydroxyalkylation polymerization†
Abstract
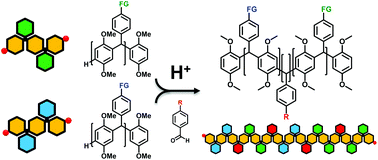
Multisegmented block copolymers (MSBCPs) were synthesized by acid-catalyzed Friedel–Crafts (F–C) hydroxyalkylation polymerization in a two-step process from a feedstock of commercially available or easily synthesized benzaldehyde (BA) derivatives and 1,4-dimethoxy benzene (DMB). Polymerizations of DMB with various BA monomers afforded a library of triarylmethane-backboned oligomers with nitro, fluoro, alkyl ester, dimethylamino and tert-butyl pendant functional groups. Using these oligomers as macromonomers in a subsequent F–C hydroxyalkylation polymerization under the same setup produced MSBCPs with tunable compositions of segments and high number-average molar mass ∼105 g mol−1. Glass transition temperatures (Tg) measured for the oligomers and MSBCP became tunable between 121.3 °C and 227.7 °C. Meanwhile, water contact angle measurements of the spin-coated membranes were compared and a pH-responsive MSBCP was synthesized to demonstrate switchable surface wettability and polarity.


 Please wait while we load your content...
Please wait while we load your content...