Pentamethine sulfobenzoindocyanine dyes with low net charge states and high photostability†
Damien E.
Dobson
,
Emily R.
Mahoney
,
Toan P.
Mach
,
Ryan J.
LeTourneau
and
Hans F.
Schmitthenner
 *
*
School of Chemistry and Materials Science, Rochester Institute of Technology, Rochester, NY, USA 14623. E-mail: hfssch@rit.edu
First published on 4th December 2019
Abstract
A series of Cy5.5 dye analogs and targeted probes with net charges varied from −3 to 0 were synthesized by an optimized method, followed by comparing their spectral and photostability properties in saturated solutions of air, oxygen, and argon. The Cy5.5 analogs with reduced charge were relatively stable when irridated at their excitation maxima, with a trend of higher stability with lower net charge states. The photostability of dyes was markedly lower in pure oxygen and higher in inert argon relative to ambient atmospheric conditions. The stability of c(RGDyK) conjugates as models of targeted molecular imaging agents mirrored these results and demonstrated the practical utility of the new family of Cy5.5 fluorophores.
Introduction
With the rapid growth of research in molecular imaging there is a pressing need for near infrared (NIR) dye-based imaging agents.1,2 The synthesis of targeted molecular imaging agents (TMIAs), or probes, for fluorescence or photoacoustic based imaging requires access to biocompatible NIR dyes that are affordable, or that are available through practical and robust synthetic methods.3,4 Equally important is access to dyes that are photostable at the excitation wavelengths used for irradiation during imaging protocols.5,6Among the most popular fluorescent labels in molecular imaging and cell biology are the family of indocyanine (Cy) dyes.2,6 In particular, since the discovery of Wagonner,7,8 sulfoindocyanine dyes have been widely used in optical molecular imaging (OMI) due to their high absorbance in the near infrared (NIR) spectrum (650–1100 nm) and the water solubility induced by their sulfonate groups.2,9,10 Moreover, for use in targeted molecular imaging, a critical feature was the ability to add acid functionality to the side chains of these dyes. Converting the acids to active functional groups such as succinimidyl esters facilitates conjugation to biomarker targeting groups including small molecules, inhibitors, peptides, proteins, antibodies, RNA, and DNA.11–13
The strong absorbance of indocyanine dyes makes them useful for fluorescent imaging,14 but it is also conducive for their use in photoacoustic imaging (PAI), also known as multispectral optoacoustic imaging (MSOT).15,16 PAI does not rely on high quantum yields, but takes advantage of the high molar absorptivity of the indocyanine dyes and has greater depth of penetration than fluorescence imaging.17,18
The tetra-sulfonated sulfobenzoindocyanine dye Cy5.5, (Cy5.5-4S, (1))8 and closely related Alexa Fluor 680 (AF 680, (5)),19 shown in Fig. 1, are examples of commercial dyes that are useful in wide field microscopy, confocal fluorescence microscopy (CFM), flow cytometry, and related imaging techniques.2,19–23 This is primarily due to the exquisitely matched excitation spectrum of Cy5.5 and AF 680 with the widely employed HeNe laser at 633 nm utilized in CFM, as shown in Fig. 2.8
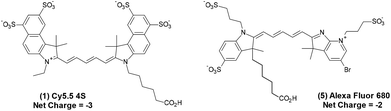 | ||
| Fig. 1 Commercially available Cy5.5 (1) with net charge −3 and AF 680 (5),19 net charge −2, marketed as their triethylamine or sodium salts. | ||
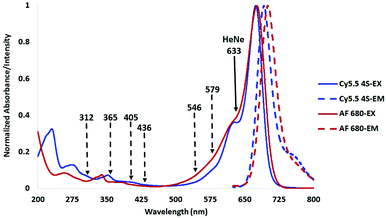 | ||
| Fig. 2 Excitation and emission spectra of compounds (1) and (5) in H2O showing the HeNe laser line at 633 nm (solid), and Hg arc lamp lines (dashed) used in prior stability studies. | ||
In addition to the Alexa Fluor dyes, other analogs with structures closely resembling the chromophore in this class of Cy 5.5 dyes (named by Wagonner7,8) are commercially available as DyLight, HyLight, Seta10 and LiCor IRdyes.24
As shown in Fig. 2, Cy5.5 has a strong absorbance band from 620–720 nm, with a vibrational shoulder between 620 and 640, which is an ideal excitation wavelength region for irradiation in order to collect the entire emitted signal from 650 to 710 nm using a diode array or bandpass filter.14
Other widely-used longer wavelength dyes in this class that similarly feature activatable functional groups to enable bioconjugation include Cy7, Cy7.5, Alexa Fluor 750, Alexa Fluor 790, IRDye800CW, IRDye800RS, and others.12 Although these dyes have considerable emission intensity around 800 nm, their excitation bands are shifted well above the spectral overlap of the HeNe laser and they require excitation by light sources at wavelengths higher than 633 nm.
In order to achieve irradiation above 633 nm, and collection of signal data above 750 nm, additional lasers, filter sets, and diode array detectors are required. These increase the costs of instrumentation excessively and are therefore not as prevalent in microscopy. For this reason, Cy5.5 and AF 680 are often the current dyes of choice in microscopy such as CFM and other in vitro OMI techniques.20
Two major concerns arise when sulfoindocyanine dyes are used. The first is the limited availability and high cost of commercial dyes, often marketed as their active esters or other active functional groups used for bioconjugation reactions. With a limited number of available chromophores, structural variation is also limited which hinders the ability to tailor physical–chemical properties in strategies to maximize bioavailability and clearance.
The second concern is photo-degradation (photo-bleaching, or (dye-fade) that occurs in dyes of this class. It is highly desirable for a dye to maintain its full performance throughout experiments with time courses from several minutes to several hours or, in some cases, multiple days. These may involve repeated scans in both fluorescent and photoacoustic studies. In PAI, where sound is produced by absorption of light, fluorescence is not utilized, the loss of acoustic signal due to photobleaching is a larger concern than quantum yield and brightness.25
As part of a broader research program on targeted molecular imaging agents (TMIAs), we needed to address both of these concerns. The availability of stable NIR dyes was central to our modular approach to targeted molecular imaging agents (TMIAs) for OMI and PAI.26 We addressed this by optimizing the synthesis of Cy5.5 to provide analogs with features for enhancing bioavailability, tissue and cell penetration, clearance and photostability.
While the importance of dye stability and the destructive effect of oxygen on activated dye species is known in reports involving dye chemistry and photo sciences,27,28 these concerns are less common in literature involving molecular imaging, and in vitro or in vivo studies.29
To address the multiple concerns above, a strategy was devised to synthesize pentamethine sulfobenzoindocyanine (Cy 5.5 type) dyes with varied charge states. Upon testing photostability with a fluorimeter, it was discovered unexpectedly that these displayed greater photostability than the parent commercially available dyes.
Results and discussion
Background on photostability measurements of Cy dyes
In our research, we noticed the high stability of the deep blue solutions for many months of a tri-sulfonated analog of Cy5.5, (Cy5.5 3S, (2)) when exposed to ambient light and temperature. High stability for several hours was also observed in extended fluorimetry experiments and in repeated CFM imaging experiments.In addition to the investigation of the photostability of indocyanine green (ICG),28 there are reports in which related indocyanine dyes used for bioconjugation were methodically measured using a light source at or near the wavelength of irradiation.27,29–33 However, in additional studies which methodically compared the photostability of dyes within Cy5, Cy5.5, Cy7 and related IRDye800CW families, the excitation leading to dye fade was carried out by employing lamps with spectral distributions that were quite distant from the excitation band of the analyte.34,35
In a key study involving the comparison of Cy3, Cy5, Cy5.5, and Cy7 to the corresponding Alexa Fluor dyes 555, 633, 647, 660, 680, 700, and 750, a 100 W mercury arc lamp was utilized as an excitation source.34 While the report concluded that Alexa Fluor 555 and 647 were more photostable than the corresponding Cy3 and Cy5 dyes, results comparing Alexa Fluor 680 and Cy5.5 were notably absent. Yet, a conclusion was made in this report that Alexa Fluor dyes, as a class, were more stable than Cy and other dye classes. This statement clearly needed further analysis.
Mercury arc lamps have strong signature emissions at 312, 365, 405, 436, 546, and 579 nm. Upon close analysis of the excitation spectra of Cy5.5 and its analogs as shown in Fig. 2, a secondary absorbance at 325–380 nm is observed, while AF 680 possesses a secondary absorbance at 315–350 nm, outside the spectral distribution of lines from a mercury arc lamp as shown in Fig. 2.
Photostability results derived from the use of mercury lamps may thus not be comparable or relevant to indocyanine dyes if the chosen light source does not match the region utilized for excitation of the dye in question. In addition, if two dyes absorb differently at an irrelevant shorter wavelength, testing results by irradiation at those wavelengths will render the results non-comparable.
In a separate report, the high photostability of zwitterionic analogs of IRDye800CW was reported by Su.35 In this study, stability was similarly measured with a 100 W mercury arc lamp at 365 nm, distant from the wavelengths of excitation that are in the range of 750–760 nm for these NIR dyes.
While these methods of testing stability may be relevant to long term exposure to ambient room light or use in unfiltered mercury lamps, we propose that it is more important to compare the stability of compounds by irradiating them at or near their maximum absorption wavelength to emulate the conditions in which they will be employed as imaging agents. In the case of CFM, the HeNe laser line at 633 nm, shown in Fig. 2, is often used for Cy5 and Cy5.5 type dyes. Measurement of photostability stability at 633 or a wavelength near the absorption maximum would be more useful than an Hg arc lamp line at 365 nm.8,13
Synthesis of Cy5.5 analogs with lower net charge states
As our CFM instrument was limited to a maximum excitation wavelength of 633 nm, the NIR fluorophore of choice was Cy5.5. Our initial studies involved bio-conjugates of Cy5.5 4S (1) purchased from GE Healthcare and the closely matching chromophore Alexa Fluor 680 (AF 680), purchased from Invitrogen, as shown in Fig. 2.In order to provide necessary quantities for synthesis of targeted molecular imaging agents (TMIAs), we initially developed the synthesis of an analog of Cy5.5 that contained two sulfonates on the aromatic rings and one on the indole side chain, (Cy5.5 3S, (2)), as shown in Fig. 3. This dye was water soluble and it imparted solubility to lipophilic peptides upon conjugation. In addition, it appeared to be remarkably stable in solution during handling and CFM experimentation. This dye became our work-horse for the synthesis of targeted imaging agents leading us to explore related dyes.
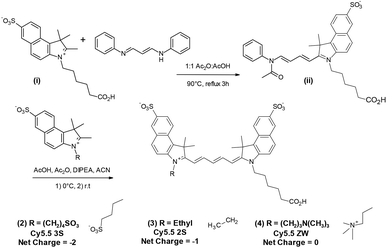 | ||
| Fig. 3 Synthesis of Cy5.5 analogs (2–4) with net charge −2 to 0 from a common half dye precursor (ii). | ||
In a search for dyes with further improved photostability, a plan was devised to synthesize a family of dyes differing in their substitution of sulfonates on the aromatic rings, and on the indole side chains. Enlightened by a report from Choi36 which described advantages of a charge balanced, zwitterionic analog of IRDye800CW for improved bioavailability, rapid renal clearance, and low nonspecific tissue uptake, combined with the report from Su,35 we strove to complete the span of net charges by synthesizing a similarly charge balanced form of Cy5.5, Cy5.5 ZW (4), net charge 0.
A set of four Cy5.5 analogs was thus envisioned in which the net charge was methodically varied from −3 to 0, as shown in Fig. 3. The net charge was based on charge after bioconjugation as in the manner described by Choi, thus not including the original carboxylic acid.36
The strategy for synthesizing the dye set with net charge varied from −2 to 0 in the class of Cy5.5 dyes is shown in Fig. 3. The key step for optimization was the conjugation of a sulfobenzoindole half dye (i) containing a C-6 acid side chain, common to each subsequent analog, with sulfobenzoindoles substituted with side chains varying from a C4 sulfonate, an ethyl group, and a C-4 trimethylamine functionality to yield members (2)–(4) of the Cy5.5 dye family.
A variety of methods reported for the dye forming step in the synthesis of indocyanine dyes were tested. These were hampered by the known formation of symmetrical dyes, as well as hydrolyzed “half dye” precursors.7,8
From the analysis of a procedure described in a patent application,37 and through further experimentation, we discovered that, while the addition of diisopropylethyl amine (DIPEA) was important, maintaining acidic to neutral conditions during this entire step prevented the formation of the symmetric full dye. This method was then applied to the synthesis of Cy5.5 analogs 2–4 shown in Fig. 3.
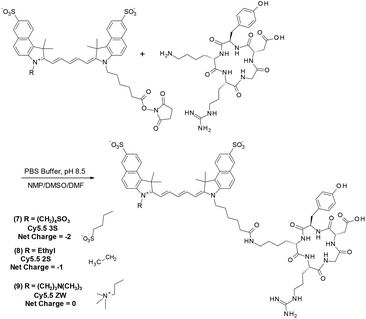
To determine the photostability of analogs relevant to TMIAs used in research, each of the five Cy5.5 dyes were conjugated to the well-known, cyclic peptide, c(RGDyK) which is known to bind preferentially to the αvβ3 integrin receptor, a biomarker in angiogenesis and many cancer types.23,38,39 Thus, c(RGDyK) was coupled to the NHS esters of fluorophores (1–5) to produce probes (6–10) as shown in Fig. 4 for analogs 7–9.
As the αvβ3 integrin receptor is well established biomarker in angiogenesis and in many cancer types, c(RGDyK) targeting strategies have been widely used for tumor cells or vasculature studying cancer therapy and diagnosis.38,39 The TMIA derived from commercial Cy5.5 (6), for example, is identical to that first reported by Chen et al.40 and similar to a c(KRGDf) analog reported by Gurfinkel.23
Photophysical measurements
The photophysical properties of all five dyes including measurements of λEX, λEM, extinction coefficients (ε), relative quantum yields (RQY, ΦR), were determined and are shown in Table 1. Extinction coefficients were measured in water and methanol (Table S1 in the ESI†), while RQYs were determined only in PBS as this is the biologically relevant solvent, with AF 680 used as a standard for the RQY experiments utilizing the reported quantum yield.41 Calibration plots, along with excitation and emission spectra are found in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for the five fluorophores and their c(RGDyK) conjugates
P values for the five fluorophores and their c(RGDyK) conjugates
| Compound | Φ R | ε (L mol−1 cm−1) | CLog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pc Pc |
λ EX (nm) | λ EM (nm) |
|---|---|---|---|---|---|
a Alexa Fluor 680 (AF 680) was used as a standard for RQY. All samples were analysed in PBS buffer.
b Extinction coefficients reported are in H2O.
c CLog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values were calculated using ChemDraw Professional 17 at physiological pH (pH = 7.4).
d Maximum excitation and emission wavelengths are in H2O. P values were calculated using ChemDraw Professional 17 at physiological pH (pH = 7.4).
d Maximum excitation and emission wavelengths are in H2O.
|
|||||
| (1) Cy5.5 4S | 0.18 | 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−10.38 | 674 | 692 |
| (2) Cy5.5 3S | 0.12 | 218![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−9.11 | 681 | 700 |
| (3) Cy5.5 2S | 0.12 | 197![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.53 | 680 | 700 |
| (4) Cy5.5 ZW | 0.13 | 185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−8.77 | 678 | 698 |
| (5) AF 680 | 0.36 | 153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−14.36 | 677 | 701 |
| (6) Cy5.5 4S-c(RGDyK) | — | — | −11.67 | 676 | 693 |
| (7) Cy5.5 3S-c(RGDyK) | — | — | −10.40 | 682 | 701 |
| (8) Cy5.5 2S-c(RGDyK) | — | — | −0.77 | 681 | 702 |
| (9) Cy5.5 ZW-c(RGDyK) | — | — | −8.86 | 681 | 701 |
| (10) AF 680-c(RGDyK) | — | — | −15.66 | 677 | 700 |
In order to compare the effect net ionic charge of the five related fluorophores on their pharmacokinetic properties, the CLog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P was also calculated and as reported in Table 1. It is further useful to note that the CLog
P was also calculated and as reported in Table 1. It is further useful to note that the CLog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of Cy5.5 2S (3) and Cy5.52S-c(RGDyK) (8) satisfy the Log
P of Cy5.5 2S (3) and Cy5.52S-c(RGDyK) (8) satisfy the Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P requirement for Lipinski's Rule of 5 which should improve their performance in bioavailability, cell and tissue penetration, and pharmacokinetic properties.42
P requirement for Lipinski's Rule of 5 which should improve their performance in bioavailability, cell and tissue penetration, and pharmacokinetic properties.42
Photostability measurements
In order to effectively measure photostability of flourophores at each wavelength of excitation, we rationalized that excitation using a fluorimeter would closely emulate the excitation modes in most laser and tuneable diode fluorescent microscopes and other in vitro and in vivo imaging systems. Photostability measurements were therefore acquired with a Shimadzu RF6000 Spectro Fluorophotometer equipped with a 150 W xenon arc lamp.For all five dyes and their bio-conjugates, each photostability measurement was acquired in 18 MΩ water, pH 7, with temperature control (22.0 °C) and continuous stirring of the sample while in the fluorimeter. Each sample was placed under constant irradiation, tuned for the wavelength of their maximum excitation. While under these irradiation conditions, the fluorescence intensity at the maximum emission wavelength was plotted as a function of time over the time course of 2 h.
In reviewing early literature, it was apparent that oxygen impacts the photostability of NIR dyes.14 One report describes improving stability by designing a “self-healing” chromophore by the addition of a p-nitro benzyl group (NPA). Interestingly, NPA quenches the triplet state generated during excitation by intramolecular electron transfer between the dye and NPA.30
Few sources were found which compare photobleaching experiments on Cy dyes under inert conditions, to conditions in which the analyte is purged with Ar or He gas to eliminate fluorescence quenching by dissolved O2. Likewise, few studies were found in which the solutions were methodically enriched with O2 to promote photobleaching.
We therefore concluded it was important to establish the dependence of photostability on dissolved O2 in solution for sulfo benzoindocyanine dyes. To test the effects, each dye and conjugate were tested in 2 h photobleaching experiments under three conditions: (A) the solution purged with Ar gas, (B) the solution at ambient atmosphere, and (C) the solution saturated with O2 gas.
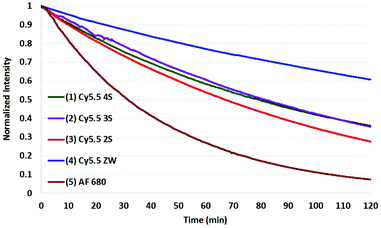
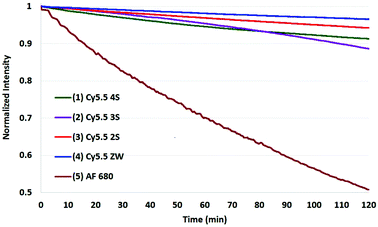
The results of photobleaching experiments for each of the four Cy5.5 and Alexa Fluor 680 dyes and conjugates to c(RGDyK), measured under ambient atmospheric conditions matching most biological experimentation, are shown in Fig. 5 and 6. The concentrations for all solutions were 0.55 μM in H2O, with pH 7. The 0.55 μM was chosen to keep the dyes close but less than 0.1 Abs units. Concentrations over this can introduce inter-filter effects that may affect RQY and fluorescence measurements. The concentrations for different dyes were kept uniform rather than attempt to standardize concentrations for absorption. Instead, in each plot the Y axis was normalized and scaled to the lowest signal.
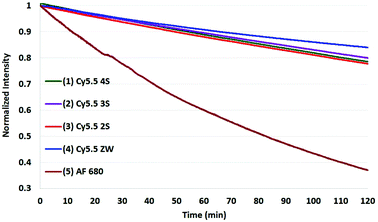 | ||
| Fig. 5 Photobleaching of the four Cy5.5 dyes (0.55 μM) with net charges ranging from −3 to 0 and AF 680 (5) at ambient atmosphere. The Y axis was normalized and scaled to the lowest signal. | ||
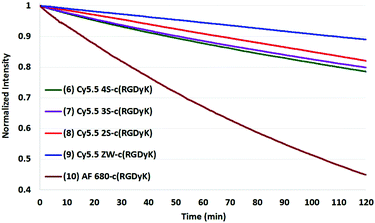 | ||
| Fig. 6 Photobleaching of four Cy5.5-c (RGDyK) conjugates (0.55 μM) and AF 680 (10) with net charges −3 to 0 and at ambient atmosphere. The Y axis was normalized, and scaled to the lowest signal. | ||
The photobleaching experiments for the same set of dyes with the solutions saturated with oxygen gas are show in Fig. 7. Measurements of the corresponding dyes with the solutions purged with argon gas are shown in Fig. 8.
The corresponding data on the c(RGDyK) conjugates are reported along with the full set of data for the dyes and conjugates at the three different conditions in the ESI† and summarized in Table 2.
| Compound | % Degradation degasseda | % Degradation atmospherea | % Degradation O2 enricheda |
|---|---|---|---|
| a All photobleaching experiments were carried out in H2O, pH 7.0, at a concentration of 0.55 μM. | |||
| (1) Cy5.5 4S | 9 | 22 | 64 |
| (2) Cy5.5 3S | 13 | 20 | 36 |
| (3) Cy5.5 2S | 6 | 34 | 72 |
| (4) Cy5.5 ZW | 3 | 16 | 39 |
| (5) AF 680 | 49 | 63 | 93 |
| (6) Cy5.5 4S-c(RGDyK) | 5 | 21 | 54 |
| (7) Cy5.5 3S-c(RGDyK) | 6 | 20 | 54 |
| (8) Cy5.5 2S-c(RGDyK) | 5 | 18 | 64 |
| (9) Cy5.5 ZW-c(RGDyK) | 0 | 11 | 41 |
| (10) AF 680-c(RGDyK) | 25 | 55 | 88 |
When excited at their maximum wavelengths, all four Cy5.5 dyes were relatively similar under inert argon conditions, degrading less than 10% over two hours in all but one experiment. In atmospheric conditions, this increased to 20–30% for Cy5.5 dyes and their c(RGDyK) conjugates. In saturated oxygen solution, the degradation increased to a range of 36–64% for the four Cy5.5 dyes showing a clear dependence of photo-bleaching on oxygen.
The zwitterionic dye, Cy5.5 ZW had the highest stability in all three conditions. The stability of the other Cy5.5 were ordered as a function of net charge with 0 being the most stable, followed by −1, −2, then −3 charges in argon and in atmospheric conditions. However, in saturated oxygen, Cy5.5 2S, suffered the largest fade amongst the Cy5.5 dyes.
The AF 680 exhibited poorer photostability, degrading 49% under argon, 63% in atmospheric conditions, and 93% dye-fade in saturated oxygen. The c(RGDyK) conjugates of AF 680 showed 25, 55, and 88% photodegradation, respectively.
In comparing the c(RGDyK) conjugates to the Cy5.5 dyes in their free acid forms as shown in Table 2, the conjugates show slightly better stability than the respective dyes, with only 5–6% degradation for the −3, −2, and −1 net charge states. The stability the Cy5.5 ZW-conjugate, with a net charge of 0, was remarkable compared to all dye conjugates with 0% degradation after two hours in argon, and 11% in atmospheric conditions (plots shown in the ESI†).
The results show that while AF 680 has a superior quantum yield, it may not be the dye of choice in an experimentation involving excitation at or near its peak absorption wavelength at 680 nm, particularly over an extended time course, or in repeated scan experiments in CFM, OMI, or PAI.
In reported structure activity relationships pertaining to dye stability of indocyanine dyes, it was suggested that the nucleophilicity at the indole C2, which is dependent on aromatic substitution,27,31 and substitution on the indole nitrogen29 play a significant role. These reports and others in which the photodegradation of indocyanine Green (ICG) was examined, indicate that the excitation of oxygen by the dye from triplet to single oxygen is the primary cause of photobleaching.28,43
While these studies focus on the indocyanine dye class, there appear to be no reports that describe the improved relative stability of benzoindocyanine dye structures (Cy5.5 dyes) versus the indole cyanine class (Cy5 or Cy7 dyes). It is likely that the additional fused aromatic ring provides delocalization, and markedly reduces the nucleophilicity of the indole C2, imparting increased photostability in Cy5.5 analogs.
As AF 680 is a hybrid structure with an indole at one end of the pentamethine chain, and a 5-bromo-7-azaindole on the other, it is plausible that it does receive the advantage of aromaticity that is beneficial to Cy 5.5 and related dyes (2–4), though, in light of earlier studies involving the effect of electron withdrawing groups on C2 nucleophilicity, the aromatic bromine substituent likely adds to its stability.27,31
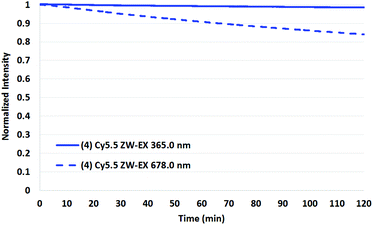
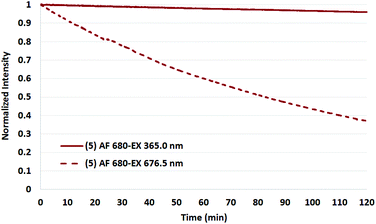
To test the validity of the prior reports measured at 365 nm with a mercury lamp,34 a representative set of assays were conducted in which the four Cy5.5 dyes and AF 680 were irradiated in the fluorimeter at 365 nm.
As shown in Fig. 9 and 10 for two representative dyes, a minor degree of degradation was observed for all dyes at 365 nm compared to irradiation at the peak excitation wavelengths (≈680 nm). In particular, a greater degree of photobleaching was observed for AF 680 when irradiated at its excitation maxima. The details of these experiments and the plots of excitation wavelength dependence on photostability are reported in the Photostability section of the ESI.†
Experimental
Materials and methods
The details of chemicals, buffers, solvents, HPLC instrumentation, and preparative HPLC, TLC-MS, and HPLC-MS is described in the ESI.†Liquid Chromatograph-Mass Spectrometry LC-MS was carried on a Waters 2695 Alliance HPLC with a Waters 2998 Diode Array Detector and a Waters 3100 SQ Mass Spectrometer was used.
Thin-layer chromatography-mass spectrometry (TLC-MS) and flow injection-mass spectral analysis (FIA) was carried out on an Advion Expression Compact Mass Spectrometer, model L (CMS-L) equipped with a Plate Express module for TLC.
High resolution mass spectra (HRMS) were obtained on a Waters Synapt G2Si (School of Chemical Sciences, University of Illinois at Urbana-Champaign) using the following parameters: Flow injection at flow rate of 0.1 ml min−1, H2O/ACN/0.1% formic acid, positive and negative mode ESI, cone voltage = 25, capillary voltage = 3.0, ion source temperature = 100 °C, desolvation temperature = 180 °C, nebulizing gas (N2) flow = 200 L h−1, cone gas (N2) flow = 5 L h−1.
The NMR (1H, 13C, 2D correlation) data were obtained using a Bruker Avance III 500 MHz NMR spectrometer using TopSpin 3.2 software. Chemical shifts (δ) are reported in ppm relative to TMS.
UV-Vis data was obtained using a Shimadzu UV-2600 UV-Vis Spectrophotometer with LabSolutions UV-VIS (version 1.03) software. Sample temperature was kept constant at 22.0 ± 0.1 °C with the Shimadzu CPS-100 Cell Positioner. All UV-Vis data was collected under the spectrum tab with parameters as follows: Spectrum type: absorbance, Start WL: 800 nm, End WL: 200 nm, Data interval: 0.2 nm, Scan speed: medium, Slit width: 1.0 nm. All samples and references were analyzed in capped 6Q quartz cuvettes with a 1 cm path length.
Emission spectra and dye fade data was obtained using a Shimadzu RF6000 Spectro Fluorophotometer with LabSolutions RF (version 1.12) software. Sample temperature was kept constant at 22.0 ± 0.1 °C in the instrument with a VWR 1160S Heated Refrigerated Circulating Bath. Samples were stirred in the instrument using a Scinics Co. Multistirrer CC-301. All emission spectra were collected under the spectrum tab with parameters as follows: Spectrum type: emission, Excitation WL: 600 nm, Emission WL start: 625 nm, Emission WL end: 800 nm, Data interval: 0.2 nm, Scan speed: 60 nm min−1, Excitation slit width: 3.0 nm, Emission slit width: 5.0 nm, Sensitivity: auto.
Dye fade data was collected under the time course tab with parameters as follows: Timing mode: manual, Timing unit: second, Cycle time: 1 s. Number of readings: 7200, Excitation slit width: 15.0 nm, Emission slit width: 5.0 nm, Sensitivity: low, Accumulation time: 5 s. Excitation and emission wavelengths in the parameters were set to the compound's respective values where absorption and emission were at maximum. All samples were analyzed in capped 6Q quartz cuvettes with a 1 cm path length and stirred with a small magnetic stir bar during each entire experiment. The concentrations for all solutions were 0.55 μM in H2O, pH 7.
Relative quantum yields (RQY, ΦR) were obtained using a Shimadzu RF6000 Spectro Fluorophotometer with LabSolutions RF (version 1.12) software. Sample temperature was kept constant at 22.0 ± 0.1 °C in the instrument while stirring. Relative quantum yields were determined using the Quantum Yield tab with the following parameters: Excitation WL: 645.0 nm, Emission WL start: 660.0 nm, Emission WL end: 800 nm, Scan speed: 200 nm min−1, Excitation slit width: 5.0 nm, Emission slit width: 5.0 nm, Sensitivity: auto, Refractive index (H2O): 1.3333. The absorbance of the standard and unknowns were also entered. All samples for RQY measurement were in PBS Buffer (50 mM potassium phosphate, 150 mM NaCl, pH 7.2) and purged with Ar before acquisition. Alexa Fluor 680 (Invitrogen) with its published RQY was used as the reference sample.
UV-Vis samples were not purged with Ar gas, or enriched with O2 gas. They were placed in the instrument and allowed to equilibrate for 10 minutes to temperature before acquisition. For emission spectra and RQY, samples were purged with Ar for 10 minutes, sealed, and placed in the spectro-fluorophotometer while stirring to equilibrate to temperature for an additional 10 minutes. Data acquisition was started following the 10 minutes equilibration. For dye fade, samples were purged with Ar gas or enriched with O2 gas in the same manner for emission spectra and RQY. The concentration used for dye fade and emission spectra was 0.55 μM in all experiments.
Experimental procedures
The synthesis of all intermediates (i–v), one exemplary Cy5.5 dye (4), one NHS ester (4a) and one exemplary c(RGDyK) conjugate (9) are described below. The remainder of dyes (2, 3) and conjugates (6, 7, 8, and 10) are described, along with all structures in the experimental procedures section of the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ac2O
1 Ac2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glacial AcOH (5 mL) was added to the flask and used as the solvent. The flask was stirred and heated to 90 °C for 3 h and monitored every 0.5 h via LC-MS (method MeOH: 20, 8 min). Upon completion, the reaction was triturated in triplicate with 2 5 mL additions of t-butanol. The product was collected by vacuum filtration and dried under high vacuum. The yield was 0.660 g (93%). LC-MS (LR, ESI) = calcd for C32H34N2O6S: 574.21 (m/z), found: 573.32 [M − H]−.
glacial AcOH (5 mL) was added to the flask and used as the solvent. The flask was stirred and heated to 90 °C for 3 h and monitored every 0.5 h via LC-MS (method MeOH: 20, 8 min). Upon completion, the reaction was triturated in triplicate with 2 5 mL additions of t-butanol. The product was collected by vacuum filtration and dried under high vacuum. The yield was 0.660 g (93%). LC-MS (LR, ESI) = calcd for C32H34N2O6S: 574.21 (m/z), found: 573.32 [M − H]−.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH
1 EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA. The product was collected by vacuum filtration and dried under high vacuum. The yield was 3.05 g (69%). LC-MS (LR, ESI) = calcd for C19H25NO6S2: 427.10 (m/z), found: 428.38 [M + H]+.
IPA. The product was collected by vacuum filtration and dried under high vacuum. The yield was 3.05 g (69%). LC-MS (LR, ESI) = calcd for C19H25NO6S2: 427.10 (m/z), found: 428.38 [M + H]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc
1 EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2O to precipitate the product. The wash was decanted, and the pure product was dried under high vacuum. The yield was 15.50 mg (98%). LC-MS (LR, ESI) of the butyl amide (structure not shown) = calcd for C49H64N4O7S2: 884.42 (m/z), found: 885.46 [M + H]+.
Et2O to precipitate the product. The wash was decanted, and the pure product was dried under high vacuum. The yield was 15.50 mg (98%). LC-MS (LR, ESI) of the butyl amide (structure not shown) = calcd for C49H64N4O7S2: 884.42 (m/z), found: 885.46 [M + H]+.
Details of the data and results are described under the following headings: Photostability: Dye fade data; UV-VIS/Emission/Spectral Data; HPLC Data, HRMS Data, NMR Spectra in the ESI.
Conclusions
A family of pentamethine sulfobenzoindocyanine dyes containing the Cy 5.5 fluorophore, with net charge states from −2 to 0 has been introduced and compared to commercial Cy 5.5 (net charge −3) and Alexa Fluor 680 (net charge −2). These have been found to have advantaged photostability properties which are useful for molecular imaging and, in particular, studies which require extended time frames or repeated scans.A practical, robust method for the synthesis of this family of dyes has been developed which makes use of a mono-substituted sulfonate on each benzoindole rather than di-substitution as in the commercial Cy5.5 NIR dye. Additionally, methods used for bioconjugation to bio-active peptides, exemplified by the integrin seeking peptide c(RGDyK), are presented.
A method for testing photostability with a fluorimeter has been described along with data that demonstrates the importance of irradiating the NIR dyes at or near the wavelength where they will be excited during imaging experiments, for the purpose of assessing relative photostability.
Cy5.5 analogs and their c(RGDyK) conjugates were relatively stable when irradiated at their excitation maxima, with a trend showing higher stability with lower net charge states. In contrast, Alexa Fluor 680, while exhibiting a higher relative quantum yield, was less stable in each set of conditions including the ambient atmospheric conditions used in most imaging studies.
The new Cy5.5 dyes (2–4) feature a range of charge states and physical–chemical properties which are now accessible to chemists, biologists, and molecular imaging scientists. Among these, a new zwitterionic dye, Cy5.5 ZW (4), shows remarkable photostability under argon purged conditions, and in ambient atmospheric conditions which emulates most biological studies involving molecular imaging.
The importance of molecular oxygen in dye fade studies in choosing optimal agents for optical and photoacoustic imaging is brought to light for imaging scientists utilizing NIR dyes who may not be aware of this. Combined with the availability of stable dyes for such imaging motifs as confocal fluorescence microscopy (CFM) and photoacoustic imaging (PAI), this information, and the development of photostable forms of sulfobenzoindocyanine dyes for practical applications should be broadly useful and valuable to those involved with molecular imaging research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by NIH–NCI grants: 1-R15CA219915-01 (H.S.) and 1-R15CA192148-01 (H.S.), RIT Honors Program (D.D., E.M., R.L., T.M.), Emerson Foundation (E.M.), and Daniel J. Pasto Award (D.D.) for summer funding.We thank Mr Furong Sun, Director of Mass Spectrometry, University of Illinois (U–C) for the high-resolution mass spectra and Mr Ken Hellstern, Senior Sales Engineer, Shimadzu Scientific Instruments, Inc. for advice on fluorimetry, and Dr Joan Potenza, formerly of Eastman Kodak Company, for her valuable insights into cyanine dye chemistry.
References
- C.-H. Tung, Fluorescent peptide probes for in vivo diagnostic imaging, Pept. Sci., 2004, 76, 391–403 CrossRef CAS PubMed.
- S. Luo, E. Zhang, Y. Su, T. Cheng and C. Shi, A review of NIR dyes in cancer targeting and imaging, Biomaterials, 2011, 32, 7127–7138 CrossRef CAS PubMed.
- R. Weissleder, Molecular imaging in cancer, Science, 2006, 312, 1168–1171 CrossRef CAS PubMed.
- S. Achilefu, Lighting up Tumors with Receptor-Specific Optical Molecular Probes, Technol. Cancer Res. Treat., 2004, 3, 393–409 CrossRef CAS PubMed.
- C. D. Geddes, Reviews in Fluorescence 2009, Springer Science & Business Media, 2011 Search PubMed.
- C. Shi, J. B. Wu and D. Pan, Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy, J. Biomed. Opt., 2016, 21, 050901 CrossRef PubMed.
- R. B. Mujumdar, L. A. Ernst, S. R. Mujumdar, C. J. Lewis and A. S. Waggoner, Cyanine dye labeling reagents: Sulfoindocyanine succinimidyl esters, Bioconjugate Chem., 1993, 4, 105–111 CrossRef CAS PubMed.
- S. R. Mujumdar, R. B. Mujumdar, C. M. Grant and A. S. Waggoner, Cyanine-Labeling Reagents: Sulfobenzindocyanine Succinimidyl Esters, Bioconjugate Chem., 1996, 7, 356–362 CrossRef CAS PubMed.
- C. Bouteiller, G. Clavé, A. Bernardin, B. Chipon, M. Massonneau, P.-Y. Renard and A. Romieu, Novel water-soluble near-infrared cyanine dyes: synthesis, spectral properties, and use in the preparation of internally quenched fluorescent probes, Bioconjugate Chem., 2007, 18, 1303–1317 CrossRef CAS PubMed.
- L. D. Patsenker, A. L. Tatarets and E. A. Terpetschnig, in Advanced Fluorescence Reporters in Chemistry and Biology I: Fundamentals and Molecular Design, ed. A. P. Demchenko, Springer, Berlin, Heidelberg, 2010, pp. 65–104 Search PubMed.
- S. Zhu, R. Tian, A. L. Antaris, X. Chen and H. Dai, Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery, Adv. Mater., 2019, 31, 1900321 CrossRef PubMed.
- S. A. Hilderbrand, K. A. Kelly, R. Weissleder and C.-H. Tung, Monofunctional Near-Infrared Fluorochromes for Imaging Applications, Bioconjugate Chem., 2005, 16, 1275–1281 CrossRef CAS PubMed.
- M. Veiseh, P. Gabikian, S.-B. Bahrami, O. Veiseh, M. Zhang, R. C. Hackman, A. C. Ravanpay, M. R. Stroud, Y. Kusuma, S. J. Hansen, D. Kwok, N. M. Munoz, R. W. Sze, W. M. Grady, N. M. Greenberg, R. G. Ellenbogen and J. M. Olson, Tumor paint: a chlorotoxin: Cy5.5 bioconjugate for intraoperative visualization of cancer foci, Cancer Res., 2007, 67, 6882–6888 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer Science & Business Media, 2013 Search PubMed.
- A. Taruttis, S. Morscher, N. C. Burton, D. Razansky and V. Ntziachristos, Fast Multispectral Optoacoustic Tomography (MSOT) for Dynamic Imaging of Pharmacokinetics and Biodistribution in Multiple Organs, PLoS One, 2012, 7, e30491 CrossRef CAS PubMed.
- M. D. Laramie, M. K. Smith, F. Marmarchi, L. R. McNally and M. Henary, Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging, Molecules, 2018, 23(11), 2766–2788 CrossRef PubMed.
- S. Wang, J. Lin, T. Wang, X. Chen and P. Huang, Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications, Theranostics, 2016, 6, 2394–2413 CrossRef CAS PubMed.
- Z. Chen, X. L. Deán-Ben, S. Gottschalk and D. Razansky, Performance of optoacoustic and fluorescence imaging in detecting deep-seated fluorescent agents, Biomed. Opt. Express, 2018, 9, 2229–2239 CrossRef CAS PubMed.
- T. E. McCann, N. Kosaka, Y. Koide, M. Mitsunaga, P. L. Choyke, T. Nagano, Y. Urano and H. Kobayashi, Activatable optical imaging with a silica-rhodamine based near infrared (SiR700) fluorophore: a comparison with cyanine based dyes, Bioconjugate Chem., 2011, 22, 2531–2538 CrossRef CAS PubMed.
- G. T. Dempsey, J. C. Vaughan, K. H. Chen, M. Bates and X. Zhuang, Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging, Nat. Methods, 2011, 8, 1027–1036 CrossRef CAS PubMed.
- Y. Dai, X. Chen, J. Yin, X. Kang, G. Wang, X. Zhang, Y. Nie, K. Wu and J. Liang, Investigation of injection dose and camera integration time on quantifying pharmacokinetics of a Cy5.5-GX1 probe with dynamic fluorescence imaging in vivo, J. Biomed. Opt., 2016, 21, 86001 CrossRef PubMed.
- Y. Dai, J. Yin, Y. Huang, X. Chen, G. Wang, Y. Liu, X. Zhang, Y. Nie, K. Wu and J. Liang, In vivo quantifying molecular specificity of Cy5.5-labeled cyclic 9-mer peptide probe with dynamic fluorescence imaging, Biomed. Opt. Express, 2016, 7, 1149–1159 CrossRef CAS PubMed.
- M. Gurfinkel, S. Ke, W. Wang, C. Li and E. M. Sevick-Muraca, Quantifying molecular specificity of alphavbeta3 integrin-targeted optical contrast agents with dynamic optical imaging, J. Biomed. Opt., 2005, 10, 034019 CrossRef PubMed.
- H. L. Osterman and A. Schutz-Geschwender, Seeing Beyond the Visible with IRDye Infrared Dyes, 2012, p. 18 Search PubMed.
- T. Stahl, T. W. Allen and P. Béard, in Photonics West – Biomedical Optics, 2014 Search PubMed.
- H. Schmitthenner, S. Beach, C. Weidman and T. Barrett, Modular Imaging Agents Containing Amino Acids and Peptides, United States, US20150038672A1, 2015.
- C. Schwechheimer, F. Rönicke, U. Schepers and H.-A. Wagenknecht, A new structure–activity relationship for cyanine dyes to improve photostability and fluorescence properties for live cell imaging ESI available: Synthetic procedures, experimental procedures, DNA preparation, optical measurements, cell images and complete analytical data, Chem. Sci., 2018, 9, 6557–6563, 10.1039/c8sc01574k.
- E. Engel, R. Schraml, T. Maisch, K. Kobuch, B. König, R.-M. Szeimies, J. Hillenkamp, W. Bäumler and R. Vasold, Light-Induced Decomposition of Indocyanine Green, Invest. Ophthalmol. Visual Sci., 2008, 49, 1777–1783 CrossRef PubMed.
- X. Chen, X. Peng, A. Cui, B. Wang, L. Wang and R. Zhang, Photostabilities of novel heptamethine 3H-indolenine cyanine dyes with different N-substituents, J. Photochem. Photobiol., A, 2006, 181, 79–85 CrossRef CAS.
- T. Li, L. Liu, T. Jing, Z. Ruan, P. Yuan and L. Yan, Self-Healing Organic Fluorophore of Cyanine-Conjugated Amphiphilic Polypeptide for Near-Infrared Photostable Bioimaging, ACS Appl. Mater. Interfaces, 2018, 10, 14517–14530 CrossRef CAS PubMed.
- B. R. Renikuntla, H. C. Rose, J. Eldo, A. S. Waggoner and B. A. Armitage, Improved Photostability and Fluorescence Properties through Polyfluorination of a Cyanine Dye, Org. Lett., 2004, 6, 909–912 CrossRef CAS PubMed.
- O. Mader, K. Reiner, H.-J. Egelhaaf, R. Fischer and R. Brock, Structure Property Analysis of Pentamethine Indocyanine Dyes: Identification of a New Dye for Life Science Applications, Bioconjugate Chem., 2004, 15, 70–78 CrossRef CAS PubMed.
- R. B. Altman, Q. Zheng, Z. Zhou, D. S. Terry, J. D. Warren and S. C. Blanchard, Enhanced photostability of cyanine fluorophores across the visible spectrum, Nat. Methods, 2012, 9, 428–429 CrossRef CAS PubMed.
- J. E. Berlier, A. Rothe, G. Buller, J. Bradford, D. R. Gray, B. J. Filanoski, W. G. Telford, S. Yue, J. Liu, C.-Y. Cheung, W. Chang, J. D. Hirsch, J. M. Beechem, R. P. Haugland and R. P. Haugland, Quantitative comparison of long-wavelength Alexa Fluor dyes to Cy dyes: fluorescence of the dyes and their bioconjugates, J. Histochem. Cytochem., 2003, 51, 1699–1712 CrossRef CAS PubMed.
- D. Su, C. L. Teoh, A. Samanta, N.-Y. Kang, S.-J. Park and Y.-T. Chang, The development of a highly photostable and chemically stable zwitterionic near-infrared dye for imaging applications, Chem. Commun., 2015, 51, 3989–3992 RSC.
- H. S. Choi, K. Nasr, S. Alyabyev, D. Feith, J. H. Lee, S. H. Kim, Y. Ashitate, H. Hyun, G. Patonay, L. Strekowski, M. Henary and J. V. Frangioni, Synthesis and In Vivo Fate of Zwitterionic Near-Infrared Fluorophores, Angew. Chem., Int. Ed., 2011, 50, 6258–6263 CrossRef CAS PubMed.
- R. Achanath, S. Balaji and J. H. Johansen, Dye compositions and dye syntheses, WO2012001050A1, 2012.
- F. Danhier, A. Le Breton and V. Préat, RGD-Based Strategies To Target Alpha(v) Beta(3) Integrin in Cancer Therapy and Diagnosis, Mol. Pharm., 2012, 9, 2961–2973 CrossRef CAS PubMed.
- F. Debordeaux, L. Chansel-Debordeaux, J.-B. Pinaquy, P. Fernandez and J. Schulz, What about αvβ3 integrins in molecular imaging in oncology?, Nucl. Med. Biol., 2018, 62–63, 31–46 CrossRef CAS PubMed.
- Z. Cheng, Y. Wu, Z. Xiong, S. S. Gambhir and X. Chen, Near-Infrared Fluorescent RGD Peptides for Optical Imaging of Integrin αvβ3 Expression in Living Mice, Bioconjugate Chem., 2005, 16, 1433–1441 CrossRef CAS PubMed.
- Fluorescence quantum yields (QY) and lifetimes (τ) for Alexa Fluor dyes—Table 1.5—US, https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook/tables/fluorescence-quantum-yields-and-lifetimes-for-alexa-fluor-dyes.html, (accessed 12 August 2019).
- L. Z. Benet, C. M. Hosey, O. Ursu and T. I. Oprea, BDDCS, the Rule of 5 and Drugability, Adv. Drug Delivery Rev., 2016, 101, 89–98 CrossRef CAS PubMed.
- C.-Y. Tang, F.-Y. Wu, M.-K. Yang, Y.-M. Guo, G.-H. Lu and Y.-H. Yang, A Classic Near-Infrared Probe Indocyanine Green for Detecting Singlet Oxygen, Int. J. Mol. Sci., 2016, 17(2), 219–227 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00445a |
| This journal is © The Royal Society of Chemistry and Owner Societies 2020 |