Raf-like kinases CBC1 and CBC2 negatively regulate stomatal opening by negatively regulating plasma membrane H+-ATPase phosphorylation in Arabidopsis†
Maki
Hayashi‡
 a,
Hodaka
Sugimoto
a,
Hirotaka
Takahashi
b,
Motoaki
Seki
a,
Hodaka
Sugimoto
a,
Hirotaka
Takahashi
b,
Motoaki
Seki
 cd,
Kazuo
Shinozaki
cd,
Kazuo
Shinozaki
 e,
Tatsuya
Sawasaki
e,
Tatsuya
Sawasaki
 b,
Toshinori
Kinoshita
f and
Shin-ichiro
Inoue
*a
b,
Toshinori
Kinoshita
f and
Shin-ichiro
Inoue
*a
aDivision of Biological Science, Graduate School of Science, Nagoya University, Chikusa, 464-8602, Nagoya, Japan. E-mail: shin@bio.nagoya-u.ac.jp; Fax: +81-52-789-4780; Tel: +81-52-789-4780
bProteo-Science Center (PROS), Ehime University, 3 Bunkyo-cho, Matsuyama, Ehime 790-8577, Japan
cRIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
dRIKEN Center for Sustainable Resource Science, 1-7-22, Suehiro, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan
eGene Discovery Research Group, RIKEN Center for Sustainable Resource Science, 3-1-1 Koyadai, Tsukuba, Ibaraki 305-0074, Japan
fInstitute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Chikusa, Nagoya 464-8602, Japan
First published on 23rd December 2019
Abstract
Stomatal pores, which are surrounded by pairs of guard cells in the plant epidermis, regulate gas exchange between plants and the atmosphere, thereby controlling photosynthesis and transpiration. Blue light works as a signal to guard cells, to induce intracellular signaling and open stomata. Blue light receptor phototropins (phots) are activated by blue light; phot-mediated signals promote plasma membrane (PM) H+-ATPase activity via C-terminal Thr phosphorylation, serving as the driving force for stomatal opening in guard cells. However, the details of this signaling process are not fully understood. In this study, through an in vitro screening of phot-interacting protein kinases, we obtained the CBC1 and CBC2 that had been reported as signal transducers in stomatal opening. Promoter activities of CBC1 and CBC2 indicated that both genes were expressed in guard cells. Single and double knockout mutants of CBC1 and CBC2 showed no lesions in the context of phot-mediated phototropism, chloroplast movement, or leaf flattening. In contrast, the cbc1cbc2 double mutant showed larger stomatal opening under both dark and blue light conditions. Interestingly, the level of phosphorylation of C-terminal Thr of PM H+-ATPase was higher in double mutant guard cells. The larger stomatal openings of the double mutant were effectively suppressed by the phytohormone abscisic acid (ABA). CBC1 and CBC2 interacted with BLUS1 and PM H+-ATPase in vitro. From these results, we conclude that CBC1 and CBC2 act as negative regulators of stomatal opening, probably via inhibition of PM H+-ATPase activity.
Introduction
Vascular plants have numerous stomata in the epidermis; stomatal pores open in response to blue light components of sunlight. Stomata act as plant gas vents for CO2 uptake and the release of O2 and water (transpiration), and are therefore crucial for photosynthesis regulation and plant growth. A pair of guard cells constitutes a stomatal unit; a single guard cell contains all signaling components for blue light-dependent stomatal opening, which is achieved through an increase in guard cell volume.1–3 Blue light is perceived by blue light receptor phototropins (phot1 and phot2) and activates plasma membrane (PM) H+-ATPase through phosphorylation of C-terminal penultimate Thr in guard cells.4–6 Activated PM H+-ATPase then pumps out H+ to the extracellular space, causing hyperpolarization of the PM, which induces K+ influx and subsequent water uptake into guard cells. Thus, guard cell volume increases and the stomata open. In addition to the activation of PM H+-ATPase, blue light also inhibits activity of the S-type anion channels and anion release from guard cells in a phot-dependent manner.7 It is thought that inhibition of the anion channels contributes to hyperpolarization of guard cell PM and stomatal opening under light conditions.Recent studies have demonstrated that some signaling components act as positive regulators for blue light-dependent PM H+-ATPase activation in guard cells, including Blue Light Signaling 1 (BLUS1),8 Blue Light-dependent H+-ATPase Phosphorylation (BHP),9 and type 1 protein phosphatase (PP1).10 BLUS1 is a protein kinase that is a substrate for phots.8 BHP is another protein kinase, which has been shown to interact with BLUS1 in vivo and in vitro, and with PP1 in vitro.9 Based on the results of protein–protein interactions and pharmacological experiments, the phot signal is thought to be transmitted to BLUS1, BHP, PP1, and PM H+-ATPase, in this order.3,9 However, the mechanisms regulating these components are not fully understood, and an unidentified protein kinase that phosphorylates and activates PM H+-ATPase is believed to participate in this signaling. Guard cells of blus1 mutant showed slight stomatal closure and medium alkalization in response to blue light.8 The results suggest that there is a BLUS1-independent negative signaling in blue light-dependent stomatal opening. However, the signaling components involved in this process remain completely unknown.
Raf-like kinases Convergence of Blue Light and CO2 (CBC) 1 and CBC2 were identified as positive regulators in both blue light- and low CO2-induced stomatal opening.11 CBC1 is a substrate of phototropins in guard cells.11 CBC1 and CBC2 are involved in blue light-dependent inhibition of S-type anion channels without regulation of blue light-dependent PM H+-ATPase activation in guard cells.11
In addition to stomatal opening, phots mediate blue light responses including phototropism, chloroplast movement, leaf positioning, and leaf flattening, to promote photosynthesis and plant growth especially in weak light environments.8,12–14 To date, several interactors for phots have been reported;15 among these, three proteins have been characterized as phot substrates in addition to CBC1: ATP-Binding Cassette B19 (ABCB19),16 Phytochrome Kinase Substrate 4 (PKS4),17 and BLUS1. ABCB19 regulates phototropism; however, the involvement of this protein in other phot-mediated responses has not been determined. PKS1, 2, and 4 regulate phototropism, leaf flattening, and leaf positioning, but not chloroplast movement or stomatal opening.18,19 BLUS1 is specifically expressed in guard cells and regulates only stomatal opening in phot-mediated responses.8 ABCB19 is not a positive regulator of phototropism,16 and phosphorylation of PKS4 by phot1 negatively regulates phototropism.17 Therefore, there may be other phot substrates that act as positive regulators in phototropism and/or chloroplast movements and as regulators in the BLUS1-independent negative signaling for stomatal opening.
In this study, we screened Arabidopsis protein kinases physically interacting with phots, and obtained the CBC1. We then performed functional analyses of CBC1 and its closest homolog, CBC2, to determine their roles in phot-mediated responses. We showed that both kinases are not involved in the phot-mediated responses except for stomatal opening. Unlike the results of a previous report,11 we showed that CBC1 and CBC2 function as negative regulators of the guard cell PM H+-ATPase phosphorylation in the blue light-dependent stomatal opening.
Materials and methods
Plant material and growth conditions
Wild-type (WT) Columbia-0 and T-DNA insertion mutants of cbc1 (SALK_005187) and cbc2-2 (SAIL_63_F05), all of which are Columbia-0 background, were obtained from the Arabidopsis Biological Resource Center (ABRC; https://abrc.osu.edu). Seeds were treated at 4 °C for 3 days, and then planted on soil and grown under a photoperiod of 16 h light and 8 h darkness under white fluorescent lights (50 μmol m−2 s−1) at 22–25 °C and relative humidity of 55–75% in a temperature-controlled growth room. We used 4–5-week-old plants for phenotypic analyses, including chloroplast movement, leaf flattening, stomatal opening, and preparation of total RNA. Etiolated seedlings were grown on half-strength Murashige and Skoog (MS) media containing 0.8% agar in the dark for 4 days in the same growth room and used for phototropic assays. Prior to β-glucuronidase (GUS) staining, plants were grown on half-strength MS media for 3 weeks in the same growth room.Phototropin-interacting protein kinase screening
All proteins used in this analysis were expressed using a wheat germ cell-free protein synthesis system.20–22 Full-length cDNAs of PHOT1 and PHOT2 were amplified by reverse-transcription polymerase chain reaction (RT-PCR) from WT cDNAs using specific primers (Table S1†). Amplified PHOT1 and PHOT2 fragments were cloned into the pFLAG-MAC vector (Sigma-Aldrich) via the HindIII and SmaI sites, respectively. The coding regions of FLAG-tagged phots were amplified by PCR from these vectors using specific primers (Table S1†). DNA fragments were cloned into the KpnI site of the pEU3-NII vector, and the resulting plasmids were used for protein synthesis of FLAG-phot1 and FLAG-phot2 as baits for protein–protein interaction screening. The full-length cDNA of each Arabidopsis protein kinase (562 members) was amplified from the RIKEN Arabidopsis full-length (RAFL) cDNA library and added to the DNA regions of the SP6 promoter and N-terminal biotin ligation site using split-primer PCR as described previously.20–22In vitro transcription, translation, and biotin labelling were performed according to methods described previously.23,24 FLAG-tagged phot1 and phot2 were synthesized in the presence of 100 μM flavin mononucleotide as a chromophore.21To detect interactions between FLAG-tagged phots and protein kinases, 5 μL of each protein-synthesized mixture was mixed and reacted in a 20 μL reaction mixture containing 50 mM Tris-HCl (pH 7.6), 100 mM potassium acetate, 10 mM MgCl2, 0.1 mM DTT, 5 μg mL−1 anti-FLAG antibody (Sigma-Aldrich), 1 mg mL−1 bovine serum albumin (BSA), 0.1 μL streptavidin-coated donor beads, and 0.1 μL anti-IgG acceptor beads at 23 °C for 1 h. Luminescent signals were analyzed using the Amplified Luminescent Proximity Homogeneous Assay Screen (AlphaScreen) detection program (PerkinElmer Life and Analytical Sciences).24
In vitro pull-down assay
All proteins for pull-down assays were expressed using a wheat germ cell-free protein synthesis system. Full-length cDNAs of CBC1 and CBC2 were amplified by PCR using specific primers (Table S1†) and cloned into the SmaI site of the pEU6G vector using the In-Fusion cloning system (Clontech). Full-length cDNAs of PHOT1, PHOT2, BLUS1, and AHA1 were amplified by PCR using specific primers (Table S1†) and cloned into the SmaI site of the pEU6H vector. The resulting plasmids (2 μg each) were used for protein synthesis of glutathione S-transferase (GST)-CBC1, GST-CBC2, 6× histidine (His)-phot1, His-phot2, His-BLUS1, and His-AHA1 using the TNT SP6 High-Yield Wheat Germ Protein Expression System (Promega) according to the manufacturer's instructions.Following in vitro protein synthesis, a reaction mixture containing a GST-tagged protein and one containing a His-tagged protein were mixed in equal amounts and added to 0.5% TritonX-100. The mixtures were incubated with 30 μL glutathione-Sepharose 4B (GE Healthcare) for 30 min at 4 °C. GST-tagged proteins were then purified with glutathione-Sepharose 4B from the mixture and washed three times with Tris-buffered saline (TBS); boundary matrices were then solubilized by adding 20 μL sodium dodecyl sulfate (SDS) sample buffer [5% (w/v) SDS, 30 mM Tris-HCl pH 8.0, 3 mM EDTA, 30% (w/v) sucrose, 0.012% (w/v) Coomassie Brilliant Blue, 15% (v/v) 2-mercaptoethanol]. Solubilized samples were subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) on 10% acrylamide gel and immunoblotted using anti-GST or His-antibody (Sigma-Aldrich).
Expression of CBC1 and CBC2 mRNAs by RT-PCR
Total RNAs were isolated from rosette leaves of 5-week-old WT plants using NucleoSpin RNA Plant (TaKaRa). First-strand cDNA was synthesized from the RNAs using the PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa). The CBC1, CBC2 and TUB2 fragments were amplified from cDNAs by PCR using specific primers (Table S1†). The TUB2 fragment was used as an internal standard.Functional analyses of phot-mediated responses
Measurements of phototropic curvature, chloroplast accumulation, avoidance responses, and leaf flattening were performed according to methods described previously.25Measurement of stomatal aperture
Stomatal apertures in the leaf abaxial epidermis were measured according to methods described previously,25,26 with some modification (Fig. 3A, B and Fig. S3†). To induce blue light-dependent stomatal opening, epidermal fragments were isolated using a blender at the end of the dark period and incubated in basal reaction buffer including 5 mM MES/bistrispropane (pH 6.5), 50 mM KCl, and 0.1 mM CaCl2 in the dark or light (red light: 50 μmol m−2 s−1; blue light: 10 μmol m−2 s−1) for 3 h at room temperature. To observe abscisic acid (ABA)-induced inhibition of stomatal opening (Fig. 4A), epidermal fragments were isolated from dark-adapted plants as described above and treated with 20 μM ABA in the basal reaction buffer. The epidermis was incubated for 3 h under light. To observe ABA-induced stomatal closure (Fig. 4B), epidermal fragments were isolated and treated with light in the basal reaction buffer for 3 h, and then 20 μM ABA was added to the buffer and the mixture was further incubated for 3 h under light. Stomatal apertures in the abaxial epidermis were then measured microscopically.Immunohistochemical staining
To detect phosphorylation of C-terminal penultimate Thr of PM H+-ATPase and total PM H+-ATPase in guard cells, immunohistochemical staining was performed using epidermal fragments following the method described in our previous studies.9,26 Fluorescence signal intensity was quantified using ImageJ software (http://imagej.nih.gov/ij/) following the method described in our previous studies.9,26Expression patterns of CBC1 and CBC2 determined by promoter-GUS assays
We constructed plasmid vectors for the promoter-GUS assay by amplifying promoter regions of CBC1 (2187 bps) and CBC2 (2197 bps) via PCR using WT genomic DNA and specific primers (Table S1†). The PCR products were cloned into the PstI/NcoI and EcoRI/NcoI sites of the pCAMBIA1303 vector (CAMBIA), respectively. The resulting vectors were transformed into the Agrobacterium tumefaciens GV3101 strain. Arabidopsis WT plants were transformed using an Agrobacterium-mediated method.27 Transgenic plants were selected according to hygromycin resistance and used for histochemical GUS staining experiments. GUS staining was performed using 3-week-old transgenic plants and observed as described previously.9Transient expression of CBC1 and CBC2 genes using particle bombardment
Intracellular localization of green fluorescent protein (GFP), CBC1-GFP, and CBC2-GFP proteins was analyzed by transient expression using particle bombardment in Vicia faba guard cells. Full-length cDNAs of CBC1 and CBC2 were amplified by PCR using specific primers (Table S1†), and the PCR products were inserted into the NcoI site of the CaMV35S-sGFP(S65T)-NOS3′ vector using the In-Fusion cloning system. Plasmid DNAs were attached to the surface of gold particles (1.0 μm Gold Microcarriers; Promega) and then transfected to the abaxial side of V. faba leaves via particle bombardment (IDERA GIE-III; Tanaka). Transfected V. faba leaves were maintained in the dark for 12–14 h at room temperature to induce protein expression. Epidermal strips were isolated from the abaxial sides of leaves, and GFP fluorescence was observed in guard cells using a confocal laser-scanning microscope (FluoView FV10i; Olympus).Results
CBC1 and CBC2 interact with phototropins
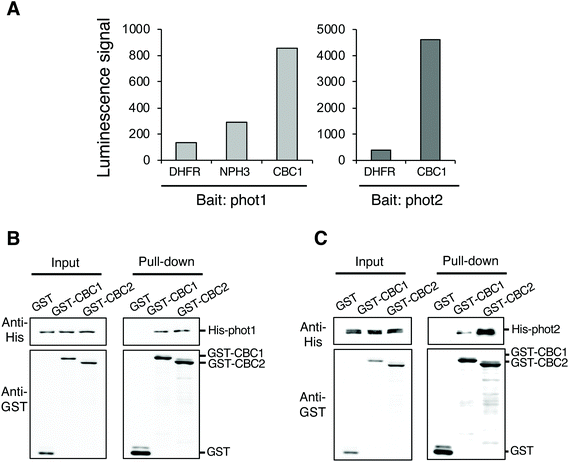
To identify protein kinases interacting with phots, we performed in vitro protein–protein interaction screening using the AlphaScreen program in combination with a wheat germ cell-free protein synthesis system. We used RAFL cDNA clones to construct an Arabidopsis protein kinase library; these were expressed individually using an in vitro transcription and translation system.20–22 We then screened phot-interacting protein kinases from among the 562 protein kinases in the library using the AlphaScreen system, finally obtaining a Raf-like kinase CBC1 (Fig. 1A). In the in vitro system, a luminescent signal is generated when donor and acceptor beads are brought into close proximity. Bead proximity depends on interactions between proteins that have been conjugated onto each bead. Phot1 or phot2 and each protein kinase from the library were conjugated on donor and acceptor beads as bait and prey proteins, respectively. Dihydrofolate reductase (DHFR) was used as a negative control protein in our analysis and showed a slight interaction with phot1 and phot2 in this system, expressed as a luminescence signal in the AlphaScreen (Fig. 1A). In contrast, CBC1 showed a high level of interaction with both phot1 and phot2. The luminescence signal indicating CBC1 binding to phot1 was stronger than that of a phot-interactive protein Nonphototropic Hypocotyl 3 (NPH3) to phot1, which served as a positive control in our analysis.19,28CBC1 (At3g01490) and its closest homolog CBC2 (At5g50000) belong to the Raf-like kinase subfamily of the mitogen-activated protein kinase kinase kinase (MAPKKK) family.29,30 The Raf-like kinase subfamily of the MAPKKK family contains the B and C clades. The C clade contains seven groups (C1–C7) and both CBCs belong to group C7, which contains five members.29 To confirm the interaction between phots and either CBC1 or CBC2, we performed in vitro pull-down assays (Fig. 1B and C). All proteins in the assays were found to be similarly expressed using the wheat germ cell-free protein synthesis system. GST, GST-tagged CBCs, and His-tagged phots were individually expressed using an in vitro translation system, and a mixture containing GST, GST-CBC1, or GST-CBC2 was mixed with one containing His-phot1 or His-phot2. GST or GST-tagged CBC protein was then purified from the mixture using glutathione Sepharose 4B beads. When GST, GST-CBC1, and GST-CBC2 were purified, His-phot1 or His-phot2 was co-purified with only GST-CBC1 and GST-CBC2 (Fig. 1B and C). These results indicate that CBC1 and CBC2 interact with both phot1 and phot2 in vitro. Binding of CBC proteins with phot1 was similarly detected in in vitro pull-down and BiFC assays of a previous study.11 Our results showed that phot2 also interacts with both CBC proteins in vitro. Therefore, these CBC kinases may have the same functions in phot-mediated signaling.
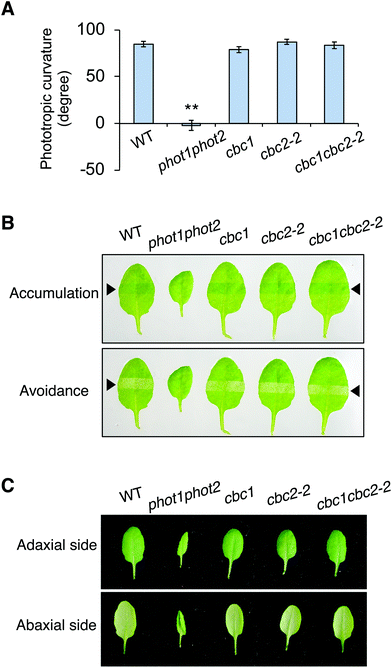
Neither CBC1 nor CBC2 regulate phot-mediated phototropism, chloroplast movement, or leaf flattening
CBC1 and CBC2 positively regulate blue light-dependent stomatal opening11 but the involvement of these kinases in other phot-mediated responses has not been determined. To investigate the functions of CBC1 and CBC2 in phot-mediated blue light responses, we obtained T-DNA insertion mutants of cbc1 (SALK_005187) and cbc2-2 (SAIL_63_F05) from the ABRC. The same cbc1 mutant and a different cbc2 mutant (SAIL_740_G01) were used in the previous study.11 T-DNA was inserted into the first exon of the CBC1 gene in cbc111 and the first intron of the CBC2 gene in cbc2-2 (Fig. S1A†). After isolation of each homozygous mutant, the mutants were crossed with each other, and the cbc1cbc2-2 double mutant was isolated. We performed RT-PCR and detected no transcripts of CBC1 or CBC2 in the double mutant (Fig. S1B†), indicating that cbc1, cbc2-2, and cbc1cbc2-2 are all null mutants. These mutants showed no obvious phenotype under our growth conditions, in contrast to that of the phot1-5 phot2-1 (phot1phot2) double mutant (Fig. S1C†).We then investigated phot-mediated responses in the single and double mutants of cbc1 and cbc2-2. First, we measured phototropic curvature in the hypocotyls of etiolated seedlings (Fig. 2A). Phototropic bending was induced in cbc1, cbc2-2, and cbc1cbc2-2 by unilateral blue light (0.1 μmol m−2 s−1) to an extent similar to that of the WT, unlike the phot1phot2 mutant.
Next, chloroplast relocation movements were determined in the cbc mutants using a slit-band assay (Fig. 2B).25,31,32 To induce chloroplast accumulation and avoidance responses, rosette leaves were locally irradiated by weak blue light (1 μmol m−2 s−1) and strong blue light (90 μmol m−2 s−1), respectively, through a 2 mm slit. Chloroplast accumulation and avoidance responses change the color of the local irradiated leaf area to darker and lighter green, respectively. As shown in Fig. 2B, leaves of all cbc mutants showed chloroplast accumulation and avoidance responses similar to those of WT. In contrast, phot1phot2 leaves showed no color changes in response to weak or strong blue light.
Rosette leaves of phot1phot2 curled downward, whereas those of WT were flat under our growth conditions (Fig. 2C). Leaves of single and double mutants of cbc1 and cbc2-2 were similarly flat, similar to those of WT under the same conditions.
Together, these results indicate that cbc1, cbc2-2, and cbc1cbc2-2 mutants have no lesions in the context of phot-mediated phototropism, chloroplast movement, or leaf flattening.
CBC1 and CBC2 redundantly and negatively regulate stomatal opening
In order to confirm findings of the CBC functions in stomatal opening,11 we also examined phot-mediated stomatal opening in cbc1, cbc2-2, and cbc1cbc2-2 mutants. To observe blue light- and phot-dependent stomatal opening, we irradiated rosette leaf epidermises in basal reaction buffer using mixed light (red light: 50 μmol m−2 s−1; blue light: 10 μmol m−2 s−1) for 3 h.25,33,34 Similar experiments were performed in the study of Hiyama et al. using cbc1, cbc2, and cbc1cbc2 mutants, and the results showed that blue light-dependent stomatal opening was slightly impaired in cbc2 epidermis and severely in cbc1cbc2 epidermis.11 They also showed that stomatal apertures were smaller in cbc2 and cbc1cbc2 epidermis than in WT epidermis even under darkness.11 However, we obtained unexpected results of the stomatal aperture measurements. Stomata in WT, cbc1, and cbc2-2 epidermises closed similarly in the dark and opened in response to light (Fig. 3A and B). In contrast, stomata in cbc1cbc2-2 epidermises opened partially in the dark and opened further in response to light. Our results indicate that CBC1 and CBC2 have a redundant function of negatively regulating stomatal opening in both dark and blue light conditions.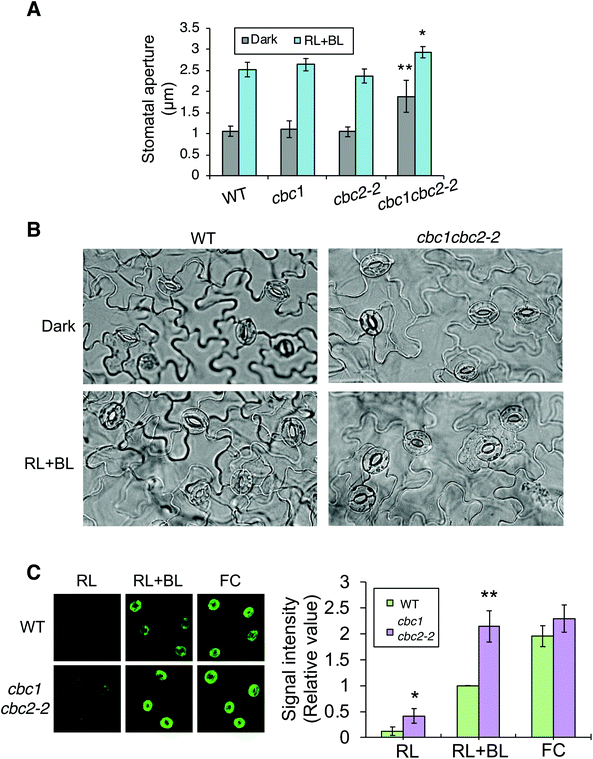 | ||
| Fig. 3 Effects of CBC1 and CBC2 mutations on blue light-dependent stomatal responses. (A) Blue light-dependent stomatal opening in epidermises of WT, phot1phot2, cbc1, cbc2-2, and cbc1cbc2-2 mutant plants. Epidermal fragments were isolated from dark-adapted plants and treated with or without light (RL + BL) (red light, 50 μmol m−2 s−1; blue light, 10 μmol m−2 s−1) for 3 h. Values are means ± standard deviation (SD) (n = 3); 30 stomata were measured in each experiment. Differences from WT were evaluated using Student's t test (**P < 0.01, *P < 0.05). (B) Photographs of leaf epidermises of WT and cbc1cbc2-2. Leaf epidermal fragments were treated as described above and photographed. (C) Immunohistochemical detection of plasma membrane (PM) H+-ATPase phosphorylation in guard cells of WT and cbc1cbc2-2. Blue light- or fusicoccin (FC)-dependent phosphorylation of guard cell PM H+-ATPase was performed as described previously.9,26 Anti-phosphorylated penultimate Thr residue antibodies were used as primary antibodies in immunohistochemical staining. Epidermal fragments were isolated as described above and incubated under red light (50 μmol m−2 s−1) for 20 min (RL), and then under blue light (10 μmol m−2 s−1) for 2.5 min, superimposed over red light (RL + BL). Subsequently, we applied 10 μM FC to dark-adapted epidermis for 5 min (FC). Typical fluorescence images are shown. Signal intensity was obtained by quantifying fluorescent images as described previously.26 Values are means ± SD (n = 3); 30 stomata were measured in each experiment. Data are expressed relative to Blue WT values. Differences from WT were evaluated using Student's t test (**P < 0.01, *P < 0.05). | ||
Phot signaling activates guard cell PM H+-ATPase through C-terminal penultimate Thr phosphorylation in a blue light-dependent manner.6,26,35 The previous study indicated that blue light-dependent phosphorylation of PM H+-ATPase was not impaired in cbc1cbc2 guard cell protoplasts.11 Since stomata in cbc1cbc2-2 epidermis opened larger than those in WT under both dark and light conditions (Fig. 3A and B), we next examined blue light-dependent phosphorylation of PM H+-ATPase in WT and cbc1cbc2-2 guard cells using an immunohistochemical technique (Fig. 3C).9,26 Guard cell PM H+-ATPase was barely phosphorylated in WT epidermis by red light (Fig. 3C: RL) and was phosphorylated by blue light superimposed on red light (Fig. 3C: RL + BL). Interestingly, guard cell PM H+-ATPase in the cbc1cbc2-2 epidermis was slightly phosphorylated by red light and strongly phosphorylated by blue light. The amount of guard cell PM H+-ATPase was similar between WT and cbc1cbc2-2 guard cells (Fig. S2†). Phosphorylation levels of PM H+-ATPase in cbc1cbc2-2 guard cells were approximately double those in WT guard cells under both red and blue light conditions (Fig. 3C: graph).
We determined the effect of the PM H+-ATPase activator fusicoccin (FC) on C-terminal Thr phosphorylation of PM H+-ATPase in guard cells. FC activates guard cell PM H+-ATPase and induces stomatal opening at saturation, without blue light irradiation.33–35 FC indirectly inhibits the dephosphorylation of phosphorylated PM H+-ATPase and accumulates the phosphorylated form of PM H+-ATPase.4,36,37 In guard cells of the WT epidermis, PM H+-ATPase was strongly phosphorylated by FC, to a greater extent than that seen under blue light (Fig. 3C: RL + BL and FC). In contrast to these results, guard cell PM H+-ATPase in the cbc1cbc2-2 double mutant was strongly phosphorylated by blue light, to an extent similar to that of FC-treated WT guard cells. Therefore, phosphorylation levels of PM H+-ATPase were similar between blue light and FC treatments in double mutant guard cells (Fig. 3C: RL + BL and FC). FC-dependent PM H+-ATPase phosphorylation was similar between WT and double mutant guard cells. Our results indicate that CBC1 and CBC2 negatively regulate PM H+-ATPase phosphorylation in guard cells.
To clarify whether the difference of stomatal phenotypes between two studies of Hiyama et al. and ours was derived from the difference of cbc2 mutant alleles, we compared blue light-dependent stomatal opening between cbc1cbc2 and cbc1cbc2-2 mutants (Fig. S3†). We found that cbc1cbc2 and cbc1cbc2-2 mutants similarly showed promotion of stomatal opening under both dark and light conditions in our experiments.
CBC1 and CBC2 negatively regulate blue light-dependent stomatal opening in an ABA-independent manner
ABA is produced in plants in response to drought stress, and closes stomata under light to prevent water loss.38 Since cbc1cbc2-2 stomata promoted stomatal opening under both dark and light conditions (Fig. 3A and B), we next tested whether ABA suppresses the mutant stomatal phenotype.First, we observed ABA-inhibited stomatal opening. Epidermal fragments were isolated from leaves of dark-adapted plants and treated with ABA in the dark. Epidermal samples were then irradiated for 3 h. Stomata in the WT epidermis opened in response to light (RL + BL) in the absence of ABA; however, stomatal opening was strongly inhibited under light in the presence of ABA (Fig. 4A). The inhibitory effect of ABA was similarly observed in the cbc1cbc2-2 epidermis. Next, we observed ABA-induced stomatal closure under light conditions. Epidermal fragments were isolated from leaves and irradiated for 3 h to open stomata. The epidermis was then treated with ABA and incubated under light for 3 h. WT stomata effectively closed in response to ABA under light (Fig. 4B). Stomatal closure was similarly induced by ABA application in the cbc1cbc2-2 epidermis. Together, these results indicate that cbc1cbc2-2 stomata open to a greater extent than those of WT but responded to ABA by closing normally.
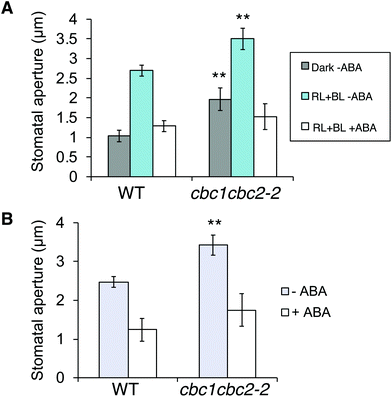 | ||
| Fig. 4 Effects of CBC1 and CBC2 mutations on abscisic acid (ABA)-inhibited stomatal opening and ABA-induced stomatal closure. (A) Inhibition of stomatal opening by ABA in WT and cbc1cbc2-2 epidermises. Epidermal fragments were isolated from dark-adapted plants and kept in the dark (Dark–ABA), or irradiated with light (RL + BL–ABA) as described in Fig. 3A. Light conditions were as described in Fig. 3A. ABA (20 μM) was applied to the epidermis for 3 h under light (RL + BL + ABA). As an ABA solvent control, the same amount of dimethyl sulfoxide (DMSO) was added to the –ABA samples. (B) ABA-induced stomatal closure in WT and cbc1cbc2-2. Epidermal fragments were illuminated with light for 3 h to open stomata, and then ABA (20 μM) or DMSO was applied under light for 3 h. Values are means ± SD (n = 3); 30 stomata were measured in each experiment. Differences from WT were evaluated using Student's t test (**P < 0.01). | ||
CBC1 and CBC2 are expressed and localized in guard cell cytoplasm
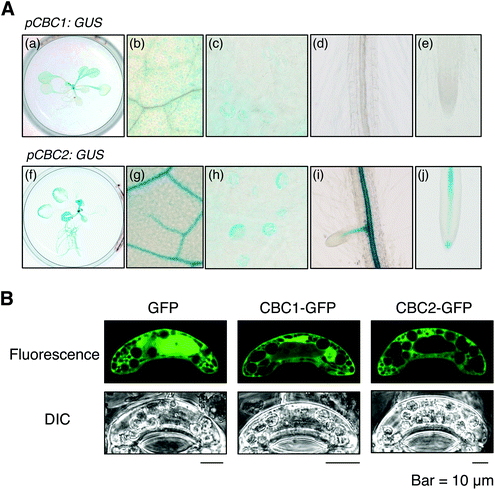
We determined the expression patterns of CBC1 and CBC2 using transgenic plants expressing the GUS reporter gene under the control of each native promoter (Fig. 5A). GUS staining of the CBC1 promoter was only observed in the aerial part, and the signals were specific in guard cells (Fig. 5A, a–e). In contrast, GUS staining of the CBC2 promoter was observed in all plant organs. The stain was particularly strongly observed in vascular tissues and guard cells (Fig. 5A, f–j). Guard cell expression of CBC1 and CBC2 genes was similarly detected by RT-PCR in the previous study.11We next determined the subcellular localization of the CBC1 and CBC2 proteins by transient expression of GFP-fused proteins. GFP, CBC1-GFP, and CBC2-GFP proteins were expressed in V. faba guard cells via particle bombardment under the control of the 35S promoter (Fig. 5B). The fluorescence signal from GFP alone was observed in both the cytoplasm and nucleus. However, fluorescence signals from CBC1-GFP and CBC2-GFP were observed only in the cytoplasm. The results agree with those of the previous study using Arabidopsis transgenic plants.11 Together, these results indicate that both CBC proteins are expressed in guard cell cytoplasm as reported previously.11
CBC1 and CBC2 interact with signaling components involved in phot-mediated stomatal opening
To clarify the relationship between CBC1 and CBC2 in signaling for blue light-dependent stomatal opening, we investigated whether CBCs interact with known signaling components BLUS1 and PM H+-ATPase using in vitro pull-down assays. All proteins were expressed using the wheat germ cell-free protein synthesis system and used for in vitro pull-down assays, as shown in Fig. 1B and C. His-tagged BLUS1 was coprecipitated with GST-tagged CBC1 and GST-CBC2, but not with GST alone (Fig. 6A). Similarly, His-tagged AHA1, a major isoform of PM H+-ATPase in stomatal opening,39 specifically interacted with GST-tagged CBC1 and GST-CBC2 (Fig. 6B). Therefore, the two CBCs commonly interacted with phots, BLUS1, and PM H+-ATPase in vitro.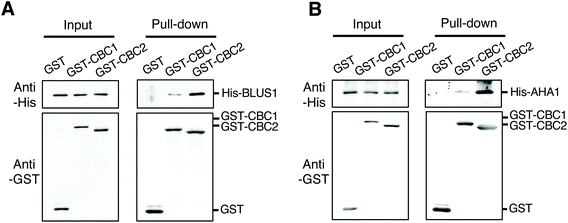 | ||
| Fig. 6 Interactions of CBC1 and CBC2 with Blue Light Signaling1 (BLUS1) and PM H+-ATPase in in vitro pull-down assays. (A) In vitro pull-down assay of GST-CBC1/CBC2 and His-BLUS1. (B) In vitro pull-down assay of GST-CBC1/CBC2 and His-AHA1. Experiments were performed as described and shown in Fig. 1B and C. | ||
Discussion
Stomatal aperture and phosphorylation of guard cell PM H+-ATPase were promoted in the cbc1cbc2-2 epidermis compared to WT under both dark and light conditions (Fig. 3), suggesting that CBC1 and CBC2 act as negative regulators in stomatal opening. The promotion of stomatal aperture in the cbc1cbc2-2 epidermis may be at least partially due to enhancement of PM H+-ATPase phosphorylation in guard cells. In addition, the negative regulation of stomatal opening by CBC1 and CBC2 was independent of ABA, because cbc1cbc2-2 stomata responded normally to ABA (Fig. 4). Recently, it was demonstrated that negative signaling was also transmitted in parallel with positive signaling in blue light- and phot-dependent manners for stomatal opening.8 Thus, CBC1 and CBC2 may similarly participate in the phot-mediated negative signaling in guard cells. Blue light-dependent medium alkalization observed in blus1 guard cells may be induced by the CBCs-dependent negative regulation of PM H+-ATPase phosphorylation. Phosphorylation of the C-terminal Thr of PM H+-ATPase is suppressed by phosphorylation of the upstream site of Ser-931.40,41 Since both CBC1 and CBC2 interacted with PM H+-ATPase in vitro (Fig. 6B), these CBCs may phosphorylate Ser-931 of PM H+-ATPase to suppress C-terminal Thr phosphorylation in guard cells. Further analysis will be needed to clarify the biological and biochemical functions of these two CBCs. Negative regulation of blue light-dependent stomatal opening may tightly modulate the stomatal aperture to prevent excessive stomatal opening under light conditions in the absence of strong drought stress.In contrast to our conclusion, Hiyama et al. concluded that CBC1 and CBC2 redundantly function as positive regulators of blue light-dependent stomatal opening from the results of stomatal aperture measurements with leaf epidermises and stomatal conductance assays with intact leaves using cbc1cbc2 mutant.11 To address the difference in stomatal apertures in the previous and this studies, we compared blue light-dependent stomatal opening between cbc1cbc2 and cbc1cbc2-2 epidermises (Fig. S3†). The results showed that stomata in both double mutant epidermises opened larger than those in WT under both dark and blue light conditions, indicating that the phenotypic difference is not derived from difference in the cbc2 mutant alleles. In Hiyama et al., stomata in WT epidermis opened relatively large (about 2 μm) even in the dark.11 We speculate that it was difficult to evaluate the opened stomata phenotype of cbc1cbc2 epidermis in their experiments. These differences in stomatal aperture may be brought about by difference in the growth conditions of plants. However, we did clearly observe stomatal opening in response to blue light in cbc1cbc2-2 and cbc1cbc2 mutant epidermises in our experiments (Fig. 3A, B and Fig. S3†); therefore, we believe that functions of CBC1 and CBC2 as positive regulators are not so strong in the blue light-dependent stomatal opening. In both Hiyama et al. and our studies, difference of stomatal aperture between dark and light was less in the cbc1cbc2 double mutants than in WT. In this point, both studies show similar stomatal phenotypes. Thus, Hiyama et al. might conclude that CBC1 and CBC2 function as a positive regulator in blue light-dependent stomatal opening. Hiyama et al. also showed that cbc1cbc2 mutant leaves exhibited strong impairment of stomatal opening in response to a low CO2 concentration in stomatal conductance assays, and concluded that CBC1 and CBC2 function as positive regulators of both blue light-dependent and low CO2-dependent stomatal opening.11 More recently, Ando and Kinoshita (2018) also measured stomatal apertures using whole leaves of cbc1cbc2 mutant and they showed strong impairment of blue light-dependent stomatal opening compared to WT plants.42 Measurements of blue light-dependent stomatal opening using intact leaves made it impossible to separate out the effects of low CO2-dependent stomatal opening, because CO2 is consumed by photosynthesis during light irradiation on the inner side of the leaf. High Leaf Temperature 1 (HT1) specifically mediates CO2-dependent stomatal movement; however, attenuation of blue light-dependent stomatal opening was observed in a stomatal conductance assay using ht1 leaves.43 The results of that study suggest that blue light-dependent stomatal opening is masked by impairment of the CO2-dependent stomatal responses. Therefore, it may be difficult to evaluate blue light-dependent stomatal opening using stomatal conductance assays with intact leaves of the cbc1cbc2 mutant.
Under red light irradiation, the stomatal responses of cbc1cbc2 mutants were different from those of WT (Fig. 3C).11 These results suggest that CBC1 and CBC2 function not only in blue light signaling, but also in other signaling for regulation of stomatal aperture. We can not exclude the possibility that stomatal blue light phenotypes in cbc1cbc2 mutants are derived from alteration of a more general regulatory mechanism, such as stomatal CO2 signaling.
CBC1 and CBC2 are involved in blue light-dependent inhibition of S-type anion channel activity in guard cells, and the inhibition is thought to contribute to stomatal opening under light conditions.11 However, in our stomatal aperture measurement, stomata in cbc1cbc2 and cbc1cbc2-2 epidermises showed clear opening in response to blue light (Fig. 3A, B and Fig. S3†). Actually, anion channel activity was hardly detected without high CO2 or bicarbonate application.7,11 These results suggest that blue light-inhibited S-type anion channel activity may not strongly contribute to blue light-dependent stomatal opening in the aperture measurement using epidermis under ambient CO2 conditions.
CBC1 was expressed in guard cells, whereas CBC2 was expressed in both guard cells and vascular tissues (Fig. 5A). Guard cells are the only cells in which CBC1 and CBC2 co-occur. Consistent with the expression results, phot-mediated blue light responses were induced normally, except for stomatal opening in the cbc1cbc2-2 mutant (Fig. 2 and 3). Both CBCs are likely to function in the guard cell cytoplasm to regulate stomatal opening. CBC2 may regulate other physiological responses in vascular tissues in addition to stomatal responses. Further investigation is needed to clarify the physiological roles of CBC2 in other tissues.
In plants, the Raf-like kinase subfamily belongs to the MAPKKK family, and comprises clade B and C members, which are thought to function differently from typical MAPKKKs involved in the MAPK cascade.44 Plants have a large MAPKKK family compared to mammals, including a particularly large Raf-like kinase subfamily.45 However, the functions of plant Raf-like kinases are largely unknown, except for the involvement of some B and C clade members in biotic and abiotic stress signaling in plants.44 Constitutive Triple Response1 functions as a negative regulator of ethylene response and Enhanced Disease Resistance1 is involved in disease resistance.46,47 In Physcomitrella patens, ABA and Abiotic Stress-responsive RAF-like Kinase is involved in the activation of the SnRK2s, which are core protein kinases in ABA signaling, and also mediates ABA and hyperosmotic signals.48 Raf-like kinases in the C subgroup have been shown to regulate stomatal responses to CO2 and blue light.3 HT1 is a member of the C5 subgroup and a key component of stomatal responses to both low and high CO2 concentrations.43 Recently, we identified BHP in the C1 subgroup as a positive regulator of blue light-dependent stomatal opening between phots and PM H+-ATPase in guard cells.9 In addition to BHP, we showed that another C1 member, At1g4000/VH1-INTERACTING KINASE (VIK) also positively regulates blue light-dependent stomatal opening, in a manner different from that of BHP.9 VIK may regulate signaling for stomatal opening after the action of PM H+-ATPase in guard cells. The C7 members CBC1 and CBC2 are involved in blue light-dependent stomatal opening and CO2-dependent stomatal movement, as demonstrated by our results and those of a previous study.11 Clade C Raf-like kinases are thought to have spread uniquely during the process of plant evolution, since they are likely to mediate plant-specific responses including stomatal movement. HT1 has been shown to phosphorylate CBC1 and CBC2 in vitro,11 suggesting that these two signals for stomatal opening in response to CO2 and blue light availability converge at the CBCs. There may be other crosstalk points among the Raf-like kinases in guard cells; such signal convergence is important for characterizing the regulation of stomatal aperture in changing environments.
Conclusion
We showed that phot-interacting protein kinases CBC1 and CBC2 redundantly function as negative regulators of stomatal opening in Arabidopsis thaliana. CBC1 and CBC2 likely form a signaling complex with phots, BLUS1, and PM H+-ATPase in guard cells to suppress stomatal opening through inhibition of blue light-dependent PM H+-ATPase phosphorylation. We therefore propose that involvement of CBC1 and CBC2 in the PM H+-ATPase regulation in addition to S-type anion channel regulation in guard cells. Future studies of the relationships between these two CBCs and key stomatal regulators, including BLUS1, BHP, and PM H+-ATPase, may further elucidate the molecular mechanisms and physiological significance of CBC-mediated negative regulation of stomatal opening.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
We thank K. Shimazaki (Kyushu Univ., Japan) for providing seeds of the cbc1cbc2 mutant and A. Kawajiri for technical assistance. This work was supported, in part, by Japan Society for the Promotion of Science (JSPS) KAKENHI grants (no. JP15K07101 and JP25840105 to S.I.), Grants-in-Aid for Scientific Research in Innovative Areas (no. JP15H05956 to T.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), and The Hori Sciences And Arts Foundation (to S.I.).References
- K. Shimazaki, M. Doi, S. M. Assmann and T. Kinoshita, Annu. Rev. Plant Biol., 2007, 58, 219–247 CrossRef CAS PubMed.
- S. Inoue, A. Takemiya and K. Shimazaki, Curr. Opin. Plant Biol., 2010, 13, 587–593 CrossRef CAS.
- S. Inoue and T. Kinoshita, Plant Physiol., 2017, 174, 531–538 CrossRef CAS PubMed.
- T. Kinoshita and K. Shimazaki, Plant Cell Physiol., 2001, 42, 424–432 CrossRef CAS PubMed.
- M. Doi, A. Shigenaga, T. Emi, T. Kinoshita and K. Shimazaki, J. Exp. Bot., 2004, 55, 517–523 CrossRef CAS PubMed.
- K. Ueno, T. Kinoshita, S. Inoue, T. Emi and K. Shimazaki, Plant Cell Physiol., 2005, 46, 955–963 CrossRef CAS PubMed.
- H. Marten, R. Hedrich and M. R. Roelfsema, Plant J., 2007, 1, 29–39 CrossRef PubMed.
- A. Takemiya, N. Sugiyama, H. Fujimoto, T. Tsutsumi, S. Yamauchi, A. Hiyama, Y. Tada, J. M. Christie and K. Shimazaki, Nat. Commun., 2013 DOI:10.1038/ncomms3094.
- M. Hayashi, S. Inoue, Y. Ueno and T. Kinoshita, Sci. Rep., 2017 DOI:10.1038/srep45586.
- A. Takemiya, T. Kinoshita, M. Asanuma and K. Shimazaki, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13549–13554 CrossRef CAS.
- A. Hiyama, A. Takemiya, S. Munemasa, E. Okuma, N. Sugiyama, Y. Tada, Y. Murata and K. Shimazaki, Nat. Commun., 2017 DOI:10.1038/s41467-017-01237-5.
- A. Takemiya, S. Inoue, M. Doi, T. Kinoshita and K. Shimazaki, Plant Cell, 2005, 17, 1120–1127 CrossRef CAS.
- J. M. Christie, Annu. Rev. Plant Biol., 2007, 58, 21–45 CrossRef CAS PubMed.
- S. Inoue, T. Kinoshita, A. Takemiya, M. Doi and K. Shimazaki, Mol. Plant, 2008, 1, 15–26 CrossRef CAS PubMed.
- S. Inoue, A. Takemiya and K. Shimazaki, Curr. Opin. Plant Biol., 2010, 13, 587–593 CrossRef CAS PubMed.
- J. M. Christie, H. Yang, G. L. Richter, S. Sullivan, C. E. Thomson, J. Lin, B. Titapiwatanakun, M. Ennis, E. Kaiserli, O. R. Lee, J. Adamec, W. A. Peer and A. S. Murphy, PLoS Biol., 2011 DOI:10.1371/journal.pbio.1001076.
- E. Demarsy, I. Schepens, K. Okajima, M. Hersch, S. Bergmann, J. Christie, K. Shimazaki, S. Tokutomi and C. Fankhauser, EMBO J., 2012, 31, 3457–3467 CrossRef CAS PubMed.
- P. Lariguet and C. Fankhauser, Plant J., 2004, 40, 826–834 CrossRef CAS.
- M. de Carbonnel, P. Davis, M. R. Roelfsema, S. Inoue, I. Schepens, P. Lariguet, M. Geisler, K. Shimazaki, R. Hangarter and C. Fankhauser, Plant Physiol., 2010, 152, 1391–1405 CrossRef CAS.
- T. Sawasaki, T. Ogasawara, R. Morishita and Y. Endo, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 14652–14657 CrossRef CAS.
- T. Sawasaki, Y. Hasegawa, R. Morishita, M. Seki, K. Shinozaki and Y. Endo, Phytochemistry, 2004, 65, 1549–1555 CrossRef CAS.
- K. Nemoto, T. Seto, H. Takahashi, A. Nozawa, M. Seki, K. Shinozaki, Y. Endo and T. Sawasaki, Phytochemistry, 2011, 72, 1136–1144 CrossRef CAS.
- T. Sawasaki, M. D. Gouda, T. Kawasaki, T. Tsuboi, Y. Tozawa, K. Takai and Y. Endo, Methods Mol. Biol., 2005, 310, 131–144 CrossRef CAS.
- H. Takahashi, A. Nozawa, M. Seki, K. Shinozaki, Y. Endo and T. Sawasaki, BMC Plant Biol., 2009 DOI:10.1186/1471-2229-9-39.
- S. Inoue, T. Kinoshita, M. Matsumoto, K. I. Nakayama, M. Doi and K. Shimazaki, Proc. Natl. Acad. Sci. U. S. A., 2008b, 105, 5626–5631 Search PubMed.
- M. Hayashi, K. Takahashi and T. Kinoshita, Plant Cell Physiol., 2011, 52, 1238–1248 CrossRef CAS PubMed.
- S. J. Clough and A. F. Bent, Plant J., 1998, 16, 735–743 CrossRef CAS PubMed.
- A. Motchoulski and E. Liscum, Science, 1999, 286, 961–964 CrossRef CAS.
- K. Ichimura, et al., Mitogen-activated protein kinase cascades in plants: a new nomenclature, Trends Plant Sci., 2002, 7, 301–308 CrossRef CAS.
- C. Jonak, L. Okresz, L. Bogre and H. Hirt, Curr. Opin. Plant Biol., 2002, 5, 415–424 CrossRef CAS.
- S. Inoue, T. Matsushita, Y. Tomokiyo, M. Matsumoto, K. I. Nakayama, T. Kinoshita and K. Shimazaki, Plant Physiol., 2011, 156, 117–128 CrossRef CAS PubMed.
- N. Suetsugu, T. Kagawa and M. Wada, Plant Physiol., 2005, 139, 151–162 CrossRef CAS PubMed.
- T. Kinoshita, M. Doi, N. Suetsugu, T. Kagawa, M. Wada and K. Shimazaki, Nature, 2001, 414, 656–660 CrossRef CAS PubMed.
- S. Inoue, N. Iwashita, Y. Takahashi, E. Gotoh, E. Okuma, M. Hayashi, R. Tabata, A. Takemiya, Y. Murata, M. Doi, T. Kinoshita and K. Shimazaki, Plant Cell Physiol., 2017, 58, 1048–1058 CrossRef CAS PubMed.
- T. Kinoshita and K. Shimazaki, EMBO J., 1999, 18, 5548–5558 CrossRef CAS.
- L. Baunsgaard, A. T. Fuglsang, T. Jahn, H. A. Korthout, A. H. de Boer and M. G. Palmgren, Plant J., 1998, 13, 661–671 CrossRef CAS.
- M. R. Fullone, S. Visconti, M. Marra, V. Fogliano and P. Aducci, J. Biol. Chem., 1998, 273, 7698–7670 CrossRef CAS PubMed.
- J. I. Schroeder, G. J. Allen, V. Hugouvieux, J. M. Kwak and D. Waner, Annu. Rev. Plant Physiol. Plant Mol. Biol., 2001, 52, 627–658 CrossRef CAS PubMed.
- S. Yamauchi, A. Takemiya, T. Sakamoto, T. Kurata, T. Tsutsumi, T. Kinoshita and K. Shimazaki, Plant Physiol., 2016, 4, 2731–2743 Search PubMed.
- A. T. Fuglsang, Y. Guo, T. A. Cuin, Q. Qiu, C. Song, K. A. Kristiansen, K. Bych, A. Schulz, S. Shabala, K. S. Schumaker, M. G. Palmgren and J. K. Zhu, Plant Cell, 2007, 19, 1617–1634 CrossRef CAS PubMed.
- J. Falhof, J. T. Pedersen, A. T. Fuglsang and M. Palmgren, Mol. Plant, 2016, 9, 323–337 CrossRef CAS PubMed.
- E. Ando and T. Kinoshita, Plant Physiol., 2018, 178, 838–849 CrossRef CAS.
- M. Hashimoto, J. Negi, J. Young, M. Israelsson, J. I. Schroeder and K. Iba, Nat. Cell Biol., 2006, 8, 391–397 CrossRef CAS PubMed.
- M. Menges, R. Dóczi, L. Okrész, P. Morandini, L. Mizzi, M. Soloviev, J. A. Murray and L. Bögre, New Phytol., 2008, 179, 643–662 CrossRef CAS.
- A. Champion, A. Picaud and Y. Henry, Trends Plant Sci., 2004, 9, 123–129 CrossRef CAS PubMed.
- J. J. Kieber, M. Rothenberg, G. Roman, K. A. Feldmann and J. R. Ecker, Cell, 1993, 72, 427–441 CrossRef CAS.
- C. A. Frye, D. Tang and R. W. Innes, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 373–378 CrossRef CAS.
- M. Saruhashi, T. K. Ghosh, K. Arai, Y. Ishizaki, K. Hagiwara, K. Komatsu, Y. Shiwa, K. Izumikawa, H. Yoshikawa, T. Umezawa, Y. Sakata and D. Takezawa, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 6388–6396 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00329k |
| ‡ Present address: Department for Plant and Environmental Sciences, University of Copenhagen, 1871 Frederiksberg, Denmark. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2020 |