Palladium(ii)-catalyzed stereoselective synthesis of C-glycosides from glycals with diaryliodonium salts†
Abstract
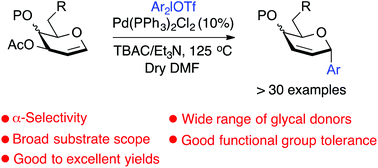
An efficient palladium(II) mediated C-glycosylation of glycals with diaryliodonium salts is described, providing a new strategy for the synthesis of 2,3-dideoxy C-aryl glycosides with excellent stereoselectivity. The C-glycosylation of a diverse range of glycals, including D-glucal, D-galactal, D-allal, L-rhamnal, L-fucal, L-arabinal, D-maltal, and D-lactal, occurred effectively and the corresponding C-glycosides were obtained in moderate to good yields. This protocol is commended as a significant addition to the field of carbohydrate chemistry due to the rich functional group compatibility, broad range of substrate scope and exceptional α-stereoselectivity.
- This article is part of the themed collections: Glycosylation: New methodologies and applications and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...