Synthesis of artemisinic acid derived glycoconjugates and their anticancer studies†
Abstract
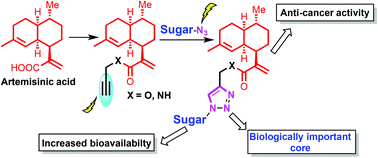
Glycoconjugates, due to their diverse functions, are widely regarded as biologically important molecules. Artemisinic acid 1 occurs naturally in the plant Artemisia annua and is considered to be the biogenetic precursor of the antimalarial drug, artemisinin 2. We report herein the design and synthesis of diverse artemisinic acid derived glycoconjugates. We have synthesized 12-O-artemisinic acid-glycoconjugates (7a–k) and 12-N-artemisinic acid-glycoconjugates (8a–k) by utilizing Cu(I)-catalyzed azide-alkyne cycloaddition reactions (Click chemistry) with various synthesized sugar azides (6a–k) in good to excellent yields along with two fluorescently labeled compounds, 12-O-artemisinic acid-glycoconjugate 11 and 12-N-artemisinic acid-glycoconjugate 12, to investigate the mode of action of these compounds in biological systems. All the synthesized artemisinic acid glycoconjugates were assayed for their efficacy against the MCF7 cell line. Our anticancer studies indicated that all the synthesized compounds inhibited the growth of MCF7 cells in a dose dependent manner, barring compounds 4 and 7d. However, these compounds exhibit moderate cytotoxicity, as is evident from their IC50 values.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...