Open Access Article
Open Access ArticleRuthenium-catalysed cyclisation reactions of 1,11-dien-6-ynes leading to biindenes†
Takanori
Matsuda
 * and
Naoto
Yonekubo
* and
Naoto
Yonekubo
Department of Applied Chemistry, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: mtd@rs.tus.ac.jp
First published on 17th February 2020
Abstract
1,2-Bis(2-allylphenyl)ethynes undergo cycloisomerisation reactions in the presence of Cp*Ru(II) catalysts to produce 2,2′-dimethyl-3H,3′H-1,1′-biindenes. On the other hand, tandem ring-closing metathesis of 1,2-bis(2-allylphenyl)ethynes using the Hoveyda–Grubbs 2nd generation catalyst led to the formation of 2,2′-unsubstituted biindenes. Various symmetrical and unsymmetrical bicyclic dienes were prepared by these ruthenium-based cyclisation methods.
Introduction
3H,3′H-1,1′-Biindenes have previously been prepared by the oxidative homocouplings of (1H-inden-1-yl)lithiums to yield diastereomeric mixtures of 1H,1′H-1,1′-biindenes, followed by base-promoted double bond isomerisation.1 There are fewer than fifty known biindenes, and some of them have been used as ligands for transition metals,2 while a biindene-derived diol has been used as a chiral ligand in the titanium(IV)-catalysed enantioselective additions of diethylzinc to aldehydes.3Transition-metal-catalysed cycloisomerisation reactions of enynes are powerful tools for the synthesis of various carbo- and heterocyclic compounds.4 This method allows for the rapid atom-economical construction of a complex cyclic structure from a linear substrate. The ring-closing metathesis (RCM) of dienes and enynes revolutionised the way in which cycloalkenes are assembled, and has been extremely useful in modern organic synthesis.5 Herein, we report that 1,11-dien-6-ynes can undergo both cycloisomerisation and tandem RCM reactions catalysed by ruthenium complexes. Notably, these reactions are used to prepare 1,1′-biindenes from 1,2-bis(2-allylphenyl)ethynes.
Results and discussion
When 1,2-bis(2-allylphenyl)ethyne (1a)6 was heated at 60 °C in EtOH in the presence of 5 mol% CpRuCl(PPh3)2 for 24 h, it cycloisomerised to afford 2,2′-dimethyl-3H,3′H-1,1′-biindene (2a) in 21% yield (Table 1, entry 1). The use of Cp*RuCl(PPh3)2 improved the yield of 2a to 45% (entry 2), while the reaction in the presence of Cp*RuCl(cod) afforded 2a in 34% yield (entry 3). The use of a cationic ruthenium catalyst generated in situ from Cp*RuCl(cod) and NaPF6 gave 2a in 55% yield (entry 4); indeed, preformed cationic [Cp*Ru(MeCN)3]PF6 exhibited comparable activity (entry 5). The effect of the phosphine ligand was next examined; 2a was formed in 38% yield when the Cp*RuCl(cod)–BINAP catalyst system was used (entry 6); however, use of P(C6F5)3 increased the yield of 2a to 61%, together with a 24% yield of the [2 + 2 + 2] cycloadduct 3a (entry 7).7,8 The reaction performed in MeOH in the presence of Cp*RuCl(cod)–P(C6F5)3 furnished 2a in 85% isolated yield without any noticeable amount of 3a (entry 8). A similar result was obtained when the reaction was performed at 40 °C (entry 9). Interestingly, the reaction delivered cycloadduct 3a as the major product when performed in i-PrOH (entries 10 and 11).9| Entry | Ru catalyst | Ligand (mol%) | Additive | Solvent | Temp. (°C) | Time (h) | Yieldb (%) of 2a | Yieldb (%) of 3a |
|---|---|---|---|---|---|---|---|---|
a Reaction conditions: 1a (0.050 mmol), ruthenium catalyst (2.5 μmol, 5 mol%), ligand (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1 P = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), solvent (0.5 mL, 0.1 M).
b Isolated yield. 2), solvent (0.5 mL, 0.1 M).
b Isolated yield.
|
||||||||
| 1 | CpRuCl(PPh3)2 | — | — | EtOH | 60 | 24 | 21 | |
| 2 | Cp*RuCl(PPh3)2 | — | — | EtOH | 60 | 4 | 45 | |
| 3 | Cp*RuCl(cod) | — | — | EtOH | 60 | 24 | 34 | |
| 4 | Cp*RuCl(cod) | — | NaPF6 | EtOH | 60 | 24 | 55 | |
| 5 | [Cp*Ru(MeCN)3]PF6 | — | — | EtOH | 60 | 12 | 56 | |
| 6 | Cp*RuCl(cod) | rac-BINAP (5) | — | EtOH | 60 | 24 | 38 | |
| 7 | Cp*RuCl(cod) | P(C6F5)3 (10) | — | EtOH | 60 | 24 | 61 | 24 |
| 8 | Cp*RuCl(cod) | P(C6F5)3 (10) | — | MeOH | 60 | 24 | 85 | |
| 9 | Cp*RuCl(cod) | P(C6F5)3 (10) | — | MeOH | 40 | 24 | 87 | |
| 10 | Cp*RuCl(cod) | P(C6F5)3 (10) | — | i-PrOH | 60 | 24 | 11 | 55 |
| 11 | Cp*RuCl(cod) | P(C6F5)3 (10) | i-PrOH | 40 | 24 | 14 | 75 | |
With the optimised reaction conditions in hand, various diallyl diphenylacetylenes 1b–l bearing substituents on their benzene rings were subjected to the ruthenium-catalysed cycloisomerisation conditions (Table 2). The reaction of 1,2-bis(2-allyl-4-methylphenyl)ethyne (1b) afforded tetramethylbiindene 2b in 67% yield (entry 1), whereas symmetrical dienynes 1c and 1d bearing methyl or methoxy groups the 5 positions of their benzene rings afforded 2c and 2d, respectively, in good yields (entries 2 and 3). In contrast, the reactions of chloro- and trifluoromethyl-substituted dienynes 1e and 1f formed the [2 + 2 + 2] cycloadducts 3 as major products under the standard conditions (1e: 2e 24% + 3e 41%; 1f: 3f 87%). The cycloisomerisation products from 1e and 1f were obtained as the major products in yields of 44% and 29%, respectively, when the reaction was performed with [Cp*Ru(MeCN)3]PF6 (entries 4 and 5). The naphthalene derivative 1g was also converted into the corresponding product 2g (entry 6), while unsymmetrically substituted biindenes 2h–l were similarly prepared by cycloisomerising dienynes 1h–l (entries 7–11).
| Entry | Dienyne 1 | Product 2 | Yielda (%) |
|---|---|---|---|
| a Isolated yield (average of two runs). b 5 mol% [Cp*Ru(MeCN)3]PF6 was used as catalyst. c Reaction was performed at 60 °C. d The crude reaction mixtures contained byproducts such as 3. e Reaction was performed at 80 °C in MeOH (0.05 M). | |||
| 1 |

|

|
67 |

|
|
||
| 2 | 1c (R = Me) | 2c | 72 |
| 3 | 1d (R = OMe) | 2d | 63 |
| 4b,c,d | 1e (R = Cl) | 2e | 44 |
| 5b,d | 1f (R = CF3) | 2f | 29 |
| 6e |

|

|
39 |
| 7 |

|
|
90 |

|

|
||
| 8 | 1i (R = Me) | 2i | 86 |
| 9 | 1j (R = OMe) | 2j | 84 |
| 10d | 1k (R = Cl) | 2k | 55 |
| 11d | 1l (R = CF3) | 2l | 45 |
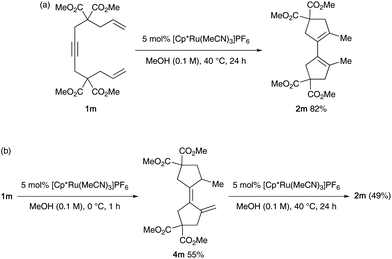
The cycloisomerisation conditions were successfully applied to dienyne 1m devoid of o-phenylene tethers, which led to the formation of 1,1′-bicyclopentene 2m in 82% yield in the presence of [Cp*Ru(MeCN)3]PF6 (Scheme 1, (a)). The alternative cycloisomerisation product 4m was obtained in 55% yield when 1m was reacted at 0 °C (Scheme 1, (b)).10,11 Heating 4m in the presence of the ruthenium catalyst gave 2m in 49% yield, but no isomerisation was observed in the absence of the ruthenium catalyst. Based on these results as well as previous studies, we conclude that 2-methylene-1,1′-bi-(cyclopentylidene) 4m is the initial cycloisomerisation product, and that 4m is also catalytically isomerised to 2m by the ruthenium catalyst.
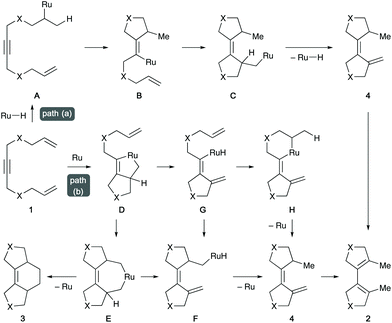
Two possible reaction pathways can be proposed for the ruthenium-catalysed cycloisomerisation of 1,11-dien-6-yne 1 (Scheme 2). Path (a) involves the formation of a hydroruthenium species from the catalyst and MeOH,12 a Markovnikov hydroruthenation to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 1 to form A, consecutive carboruthenation (through B to C), β-hydride elimination that releases 4, and the final double bond isomerisation of 4 to afford product 2. On the other hand, in path (b), dienyne 1 first undergoes oxidative cyclisation on ruthenium to generate the ruthenacyclopentene species D. The unreacted alkene moiety in D then inserts into the Ru–C(sp2) bond to give the ruthenacycloheptene intermediate E. Subsequent β-hydride elimination (to form F) followed by reductive elimination yields 4, which then isomerises to 2 catalysed by a hydroruthenium species. Alternatively, β-hydride elimination from D generates alkenylruthenium hydride G, which also leads to 4 through intramolecular carboruthenation (to F) or hydroruthenation (to H). Reductive elimination from intermediate E is possible, which gives rise to the [2 + 2 + 2] cycloadduct 3.
C bond of 1 to form A, consecutive carboruthenation (through B to C), β-hydride elimination that releases 4, and the final double bond isomerisation of 4 to afford product 2. On the other hand, in path (b), dienyne 1 first undergoes oxidative cyclisation on ruthenium to generate the ruthenacyclopentene species D. The unreacted alkene moiety in D then inserts into the Ru–C(sp2) bond to give the ruthenacycloheptene intermediate E. Subsequent β-hydride elimination (to form F) followed by reductive elimination yields 4, which then isomerises to 2 catalysed by a hydroruthenium species. Alternatively, β-hydride elimination from D generates alkenylruthenium hydride G, which also leads to 4 through intramolecular carboruthenation (to F) or hydroruthenation (to H). Reductive elimination from intermediate E is possible, which gives rise to the [2 + 2 + 2] cycloadduct 3.
Dienyne 1n or 1o, in which one allyl group is replaced with a crotyl or a methallyl group, was found to be unreactive toward cycloisomerisation, which reveals that the reaction is limited to dienynes with unsubstituted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds (Chart 1). Moreover, 1,2-bis[2-(vinyloxy)phenyl]ethyne (1p) also failed to react, and a complex mixture of products was obtained when unsymmetrical dienyne 1q, bearing malonate and o-phenylene tethers, was reacted.13
C double bonds (Chart 1). Moreover, 1,2-bis[2-(vinyloxy)phenyl]ethyne (1p) also failed to react, and a complex mixture of products was obtained when unsymmetrical dienyne 1q, bearing malonate and o-phenylene tethers, was reacted.13
We have been interested in the catalytic syntheses of silole derivatives14 and the cycloisomerisation of bis-silicon-bridged 1r was envisaged as a method for the synthesis of a bi(1-silaindene).15 However, the reaction of 1r under conditions similar to those described above led to a totally different outcome: 1,1′,2,2′-tetrahydro-4,4′-bi(1-silanaphthalene) 5 was obtained in 43% yield as the sole product after full conversion of 1r (Scheme 3). The silanaphthalene 5 may have formed through a stitching reaction mediated by a hydroruthenium species in a manner analogous to the path (a) in Scheme 2, but with initial anti-Markovnikov hydroruthenation.
Tandem ring-closing metathesis (RCM) of 1,11-dien-6-ynes that form 1,1′-bicyclopentene derivatives has previously been studied,16 but those of 1,2-bis(2-allylphenyl)ethynes have, to the best of our knowledge, never been examined. If allowed, this reaction provides a route to 3,3′H-1,1′-biindenes that lack substituents at their 2 and 2′ positions, which is complementary to the cycloisomerisation of 1. Tandem RCM of 1a in the presence of the Hoveyda–Grubbs 2nd generation catalyst at 100 °C in toluene (0.1 M) afforded the desired biindene 6a in 60% yield (Table 3, entry 1). A lower concentration of 1a resulted in an improved yield of 6a, and 0.02 M was found to be optimal for the present reaction (entries 2 and 3). Other Grubbs catalysts were not suitable for this transformation (entries 4 and 5), while the reaction with 3 mol% catalyst gave a similar result (entry 6). As for the reaction temperature, 100 °C was found to be the best among those examined for the RCM of 1 (entries 6–8).
| Entry | Grubbs catalyst (mol%) | Conc. (M) | Temp. (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | Hoveyda–Grubbs 2nd cat. (5) | 0.1 | 100 | 0.5 | 60 |
| 2 | Hoveyda–Grubbs 2nd cat. (5) | 0.04 | 100 | 1 | 67 |
| 3 | Hoveyda–Grubbs 2nd cat. (5) | 0.02 | 100 | 3 | 77 |
| 4 | Grubbs 2nd cat. (5) | 0.02 | 100 | 6 | 44 |
| 5 | Stewart–Grubbs cat. (5) | 0.02 | 100 | 6 | 26 |
| 6 | Hoveyda–Grubbs cat. 2nd (3) | 0.02 | 100 | 6 | 82 |
| 7 | Hoveyda–Grubbs cat. 2nd (3) | 0.02 | 90 | 6 | 67 |
| 8 | Hoveyda–Grubbs cat. 2nd (3) | 0.02 | 110 | 6 | 74 |
Various diallyl diphenylacetylenes 1b–l, which were successfully cycloisomerised (vide supra), were examined under the RCM conditions (Table 4). Symmetrical (1b–g) and unsymmetrical (1h–l) dienynes were converted through tandem RCM into biindenes 6b–l in yields ranging from 63% to 96%. Furthermore, dienynes 1o–q, which failed to cycloisomerise, also reacted to afford the corresponding metathesis products 4o–q, respectively, in good yields. However, the attempted tandem RCM of the bis-silicon-bridged 1r resulted in no conversion under various metathesis conditions.
Conclusions
In conclusion, we developed ruthenium-catalysed cycloisomerisation and tandem-RCM methods for the synthesis of bicyclic conjugated dienes, in which two rings (cycloalkenes) are constructed. Cycloisomerisation of 1,11-dien-6-ynes afforded 2,2′-dimethyl-[1,1′-bi(cyclopentene)] derivatives catalysed by Cp*Ru. On the other hand, 2,2′-unsubsituted bicyclopentenes were prepared through the tandem RCM of 1,11-dien-6-ynes with the Hoveyda–Grubbs catalyst.17Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS, Japan (Grant-in-Aid for Scientific Research (C) No. 16K05783).Notes and references
- P. Nicolet, J.-Y. Sanchez, A. Benaboura and M. J. M. Abadie, Synthesis, 1987, 202–203 CrossRef CAS.
- (a) T. R. Kelly and P. Meghani, J. Org. Chem., 1990, 55, 3684–3688 CrossRef CAS; (b) R. C. Kerber and B. Waldbaum, Organometallics, 1995, 14, 4742–4754 CrossRef CAS; (c) B. R. Waldbaum and R. C. Kerber, Inorg. Chim. Acta, 1999, 291, 109–126 CrossRef CAS; (d) D. Tews and P. E. Gaede, Organometallics, 2001, 20, 3869–3875 CrossRef CAS.
- X.-w. Yang, J.-h. Shen, C.-s. Da, H.-s. Wang, W. Su, D.-x. Liu, R. Wang, M. C. K. Choi and A. S. C. Chan, Tetrahedron Lett., 2001, 42, 6573–6575 CrossRef CAS.
- For recent reviews on transition-metal-catalysed cycloisomerisations, see: (a) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954–1993 CrossRef CAS PubMed; (b) I. D. G. Watson and F. D. Toste, Chem. Sci., 2012, 3, 2899–2919 RSC; (c) Y. Yamamoto, Chem. Rev., 2012, 112, 4736–4769 CrossRef CAS PubMed; (d) A. Marinetti, H. Jullien and A. Voituriez, Chem. Soc. Rev., 2012, 41, 4884–4908 RSC; (e) D.-H. Zhang, Z. Zhang and M. Shi, Chem. Commun., 2012, 48, 10271–10279 RSC; (f) D. P. Day and P. W. H. Chan, Adv. Synth. Catal., 2016, 358, 1368–1384 CrossRef CAS; (g) C. I. Stathakizis and A. L. Zografos, Nat. Prod. Rep., 2016, 33, 1093–1117 RSC; (h) Y. Hu, M. Bai, Y. Yang and Q. Zhou, Org. Chem. Front., 2017, 4, 2256–2275 RSC.
- Handbook of Metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, 2003, vol. 2 Search PubMed.
- Dienynes 1 were easily prepared by the Stille coupling of 1,2-bis(2-bromophenyl)ethynes with allyltributylstannane.
- For ruthenium-catalysed [2 + 2 + 2] cycloaddition of 1,11-dien-6-ynes, see: (a) D. Tanaka, Y. Sato and M. Mori, J. Am. Chem. Soc., 2007, 129, 7730–7731 CrossRef CAS PubMed. For the reaction catalysed by rhodium complexes, see: (b) H. Sagae, K. Noguchi, M. Hirano and K. Tanaka, Chem. Commun., 2008, 3804–3806 RSC.
- Results with other phosphine ligands (2a/1a + 3a): PPh3 (12%/30%); P(c-Hex)3 (8%/38%); P(OPh)3 (18%/69%); DPPE (6%/30%); DPPBZ (25%/62%).
- The formation of a ruthenium hydride might be suppressed in i-PrOH in our case; hence the reaction is directed to proceed via the path (b) in Scheme 2. The results in other solvents are as follows: t-BuOH (2a not detected, 3a 11%); no reaction in (CF3)2CHOH.
- A similar cycloisomerisation catalysed by a rhodium(I) complex has been reported. D. S. Perekalin, N. V. Shvydkiy, Y. V. Nelyubina and A. R. Kudinov, Chem. – Eur. J., 2015, 21, 16344–16348 CrossRef CAS PubMed . See also A. A. Suleymanov, D. V. Vasilyev, V. V. Novikov, Y. V. Nelyubina and D. S. Perekalin, Beilstein J. Org. Chem., 2017, 13, 639–643 CrossRef PubMed.
- The corresponding double bond isomers were also detected when 1k and 1l were reacted in the presence of [Cp*Ru(MeCN)3]PF6.
- Y. Yamamoto, Y. Nakagai, N. Ohkoshi and K. Itoh, J. Am. Chem. Soc., 2001, 123, 6372–6380 CrossRef CAS PubMed.
- See the ESI† for other unsuccessful cycloisomerisation substrates.
- (a) T. Matsuda, Y. Suda and Y. Fujisaki, Synlett, 2011, 813–816 CrossRef CAS; (b) T. Matsuda, Y. Yamaguchi, M. Shigeno, S. Sato and M. Murakami, Chem. Commun., 2011, 47, 8697–8699 RSC; (c) T. Matsuda and Y. Ichioka, Org. Biomol. Chem., 2012, 10, 3175–3177 RSC.
- S. Yamaguchi, C. Xu, H. Yamada and A. Wakamiya, J. Organomet. Chem., 2005, 690, 5365–5377 CrossRef CAS.
- (a) S.-H. Kim, W. J. Zuercher, N. B. Bowden and R. H. Grubbs, J. Org. Chem., 1996, 61, 1073–1081 CrossRef CAS; (b) H. Clavier, A. Correa, E. C. Escudero-Adán, J. Benet-Buchholz, L. Cavallo and S. P. Nolan, Chem. – Eur. J., 2009, 15, 10244–10254 CrossRef CAS PubMed.
- For a related study that employs dienes, see: D. Sémeril, C. Bruneau and P. H. Dixneuf, Helv. Chim. Acta, 2001, 84, 3335–3341 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterisation data for new compounds. See DOI: 10.1039/d0ob00179a |
| This journal is © The Royal Society of Chemistry 2020 |