Enhanced ion binding by the benzocrown receptor and a carbonyl of the aminonaphthalimide fluorophore in water-soluble logic gates†‡
Abstract
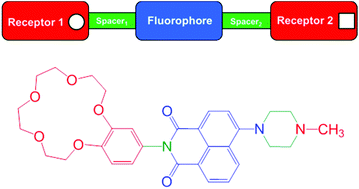
Two fluorescent logic gates 1 and 2 were designed and synthesised with a ‘receptor1-spacer1-fluorophore-spacer2-receptor2’ format. The molecules comprise of an aminonaphthalimide fluorophore, methylpiperazine and either benzo-15-crown-5 or benzo-18-crown-6. Model 3, with a weakly binding 3,4-dimethoxyphenyl moiety, was also synthesised. The compounds were studied both in 1 : 1 (v/v) methanol/water and water by UV-visible absorption and steady-state fluorescence spectroscopy. The green fluorescence of 1–3 is modulated by photoinduced electron transfer (PET) and internal charge transfer (ICT) mechanisms, and by solvent polarity. In 1 : 1 (v/v) methanol/water, logic gates 1 and 2 emit with Φf = 0.21 and 0.28, and bind with pβNa+ = 1.6 and pβK+ = 2.6, respectively, and pβH+ = 7.4 ± 0.1. In water, logic gates 1 and 2 emit with Φf = 0.14 and 0.26, and bind with pβNa+ = 0.86 and pβK+ = 1.6, respectively, and pβH+ = 8.1 ± 0.1. The measured pβNa+ are significantly lower than reported for analogous classic anthracene-based Na+, H+-driven AND logic gates indicating a stronger Na+ binding interaction, which is attributed to direct interaction with one carbonyl moiety within the aminonaphthalimide. Supporting evidence is provided by DFT calculations. Furthermore, we illustrate an example of logic function modulation by a change in solvent polarity. In 1 : 1 (v/v) methanol/water, molecules 1 and 2 function as Na+, H+ and K+, H+-driven AND logic gates. In water, the molecules function as single input H+-driven YES logic gates, while consideration as two-input devices, 1 and 2 function as AND-INH-OR logic arrays.
- This article is part of the themed collections: The Mechanics of Supramolecular Chemistry and Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...