Development of C-type lectin-oriented surfaces for high avidity glycoconjugates: towards mimicking multivalent interactions on the cell surface†
Abstract
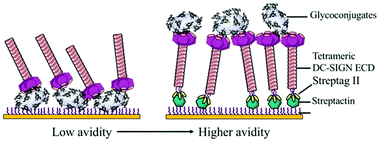
Multivalent interactions between complex carbohydrates and oligomeric C-type lectins govern a wide range of immune responses. Up to date, standard SPR (surface plasmon resonance) competitive assays have largely been to evaluate binding properties from monosaccharide units (low affinity, mM) to multivalent elemental antagonists (moderate affinity, μM). Herein, we report typical case-studies of SPR competitive assays showing that they underestimate the potency of glycoclusters to inhibit the interaction between DC-SIGN and immobilized glycoconjugates. This paper describes the design and implementation of a SPR direct interaction over DC-SIGN oriented surfaces, extendable to other C-type lectin surfaces as such Langerin. This setup provides an overview of intrinsic avidity generation emanating simultaneously from multivalent glycoclusters and from DC-SIGN tetramers organized in nanoclusters at the cell membrane. To do so, covalent biospecific capture of DC-SIGN via StreptagII/StrepTactin interaction preserves tetrameric DC-SIGN, accessibility and topology of its active sites, that would have been dissociated using standard EDC–NHS procedure under acidic conditions. From the tested glycoclusters libraries, we demonstrated that the scaffold architecture, the valency and the glycomimetic-based ligand are crucial to reach nanomolar affinities for DC-SIGN. The glycocluster 3·D illustrates the tightest binding partner in this set for a DC-SIGN surface (KD = 18 nM). Moreover, the selectivity at monovalent scale of glycomimetic D can be easily analyzed at multivalent scale comparing its binding over different C-type lectin immobilized surfaces. This approach may give rise to novel insights into the multivalent binding mechanisms responsible for avidity and make a major contribution to the full characterization of the binding potency of promising specific and multivalent immodulators.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...