Open Access Article
Open Access ArticleSynthesis of lactone-fused pyrroles by ruthenium-catalyzed 1,2-carbon migration-cycloisomerization†
Takuma
Watanabe
,
Yuichiro
Mutoh
 * and
Shinichi
Saito
* and
Shinichi
Saito
 *
*
Department of Chemistry, Faculty of Science Tokyo University of Science, 1-3 Kagurazaka Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: ymutoh@rs.tus.ac.jp; ssaito@rs.kagu.tus.ac.jp
First published on 25th November 2019
Abstract
A ruthenium-catalyzed cycloisomerization of 3-amino-4-alkynyl-2H-chromen-2-ones via 1,2-carbon migration was developed. Various 1-arylchromeno[3,4-b]pyrrol-4(3H)-ones were synthesized in good to excellent yields. The reaction was applied to the formal total synthesis of marine natural products Ningalin B and Lamellarin H. The efficient synthesis of γ-butyrolactone-fused pyrrole derivatives was also achieved.
Introduction
1-Arylchromeno[3,4-b]pyrrol-4(3H)-one skeleton is found in natural products such as Ningalin B, Lamellarin H, and related compounds (Fig. 1).1 These alkaloids are isolated from marine organisms and known to exhibit biological activities1 such as cytotoxicity,2 MDR reversal activity,2 HIV-1 integrase inhibition,3 and antitumor activity.4 Due to their promising pharmacological potentials, these pyrrole-containing natural products have been synthesized by various strategies over the years.1,5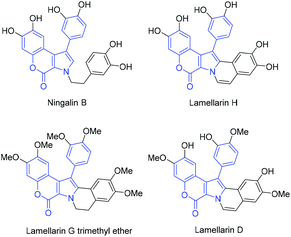 | ||
| Fig. 1 Representative natural products and analogue containing 1-arylchromeno[3,4-b]pyrrol-4(3H)-one skeleton. | ||
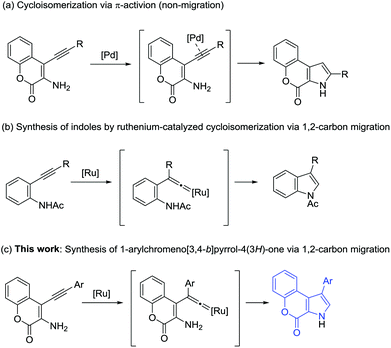
Recent examples for the synthesis of the chromeno[3,4-b]pyrrol-4(3H)-one skeleton include palladium-catalyzed cycloisomerization,6 one-pot multistep synthesis from 4-chloro-3-nitrocoumarin,7 the functionalization of 2,3-diarylpyrrole,8 the cyclization of 3-nitrocoumarin and papaverine,9 and so on. Among these synthetic methods, the palladium-catalyzed cycloisomerization of 3-amino-4-alkynyl-2H-chromen-2-ones is a straightforward and powerful method (Scheme 1a).6 The reaction proceeds via intramolecular nucleophilic amination of the π-activated alkyne, and 2-substituted chromeno[3,4-b]pyrrol-4(3H)-ones were isolated.
We have recently developed ruthenium-catalyzed cycloisomerization reactions that involve the vinylidene rearrangement of internal alkynes by 1,2-carbon migration and cyclization.10–13 For example, in the presence of a cationic ruthenium catalyst, various 2-alkynylanilides were converted into the 3-substituted indoles in high yields (Scheme 1b).11 The mode of the reaction is different from other metal-catalyzed cycloisomerization of 2-alkynylanilines, where no 1,2-carbon migration was involved, and 2-substituted indoles were isolated.14 In that study, we reported one example that 1-phenylchromeno[3,4-b]pyrrol-4(3H)-one can be synthesized by applying the reaction. Considering the importance of the chromeno[3,4-b]pyrrol-4(3H)-one skeleton in medicinal chemistry and recent active studies related to the synthesis of Ningalin and Lamellarin derivatives, the development of a new and general method for the synthesis of chromeno[3,4-b]pyrrol-4(3H)-one derivatives would be highly desirable. In this paper, we report a ruthenium-catalyzed cycloisomerization of 3-amino-4-alkynyl-2H-chromen-2-ones that leads to various 1-arylchromeno[3,4-b]pyrrol-4(3H)-ones via 1,2-carbon migration (Scheme 1c). This new methodology enabled the formal total synthesis of Ningalin B and Lamellarin H. Moreover, we describe the synthesis of rare γ-lactone-fused pyrrole derivatives by a similar ruthenium-catalyzed 1,2-carbon migration/cyclization strategy.
Results and discussion
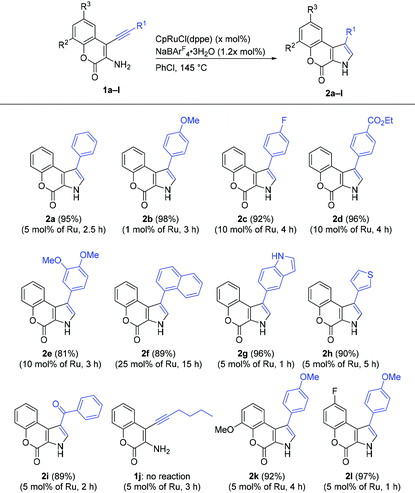
We investigated the scope and limitation of the reaction using various 3-amino-4-alkynyl-2H-chromen-2-one derivatives (1, Table 1). As previously reported, when a mixture of aminocoumarin 1a, [CpRuCl(dppe)] (Cp = η5-C5H5−; dppe = Ph2PCH2CH2PPh2) (5 mol%), and NaBArF4·3H2O (ArF = 3,5-(CF3)2C6H3) (6 mol%) was stirred at 145 °C for 2.5 h in chlorobenzene, the desired 1,2-aryl migration product 3-phenylpyrrolocoumarin (2a) was formed in 95% yield.10,15 Isomeric 2-substituted pyrrole was not observed in the reaction mixture. Although the reaction of 1a at 130 °C completed in 16 h at the same catalyst loading, the yield of 2a was lower (82%). [IndRuCl(dppe)] (Ind = η5-C9H7−) and [CpFeCl(dppe)] were inactive catalysts for this reaction, and 2a was not formed.We studied the impact of the aryl groups bound to the ethynyl group (R1) on the reaction. Under the similar reaction conditions described above, 4-methoxyphenyl pyrrole 2b was obtained in 98% yield with lower catalyst loading. Electron-withdrawing groups were tolerated for this reaction, and pyrrolocoumarins 2c (92% yield) and 2d (96% yield) were synthesized in the presence of 10 mol% [CpRuCl(dppe)]. The low reactivity of the alkynes with electron-withdrawing groups (1c and 1d) compared to the substrate with electron-donating group (1b) was consistent with our previous studies on the ruthenium-catalyzed cycloisomerizations via 1,2-carbon migration10,11 and probably attributed to the rates of the formation of the disubstituted ruthenium vinylidene complex.16 Pyrrolocoumarin 2e with 3,4-dimethoxyphenyl group was also formed from 1e in 81% yield. When the reaction of sterically congested amionocoumarin 1f bearing 1-naphthyl group at the alkyne terminus was examined in the presence of 5 mol% of the ruthenium catalyst, the progress of the reaction was sluggish. In the presence of an increased amount (25 mol%) of [CpRuCl(dppe)], however, the corresponding product 2f was isolated in 89% yield. The compatibility of the substrates with heteroaryl groups was also evaluated, and 5-indolyl derivative 2g (96% yield) and thiophen-3-yl derivative 2h (90% yield) were synthesized cleanly.
The scope of the reaction was further studied by introducing non-aromatic substituents as R1. The reactivity of benzoyl aminocoumarin 1i was similar to that of aryl-substituted aminocoumarins, and the corresponding pyrrole 2i was prepared in 89% yield. The reaction of 1j (R1 = Bu) did not proceed, and the starting material was recovered. The low reactivity of 1j is in contrast to our previous result: the cycloisomerization of a 2-hexynylaniline derivative generated the corresponding 3-butylindole derivative.10 The reaction of 1j in the presence of [CpRuCl(dppbz)] (dppbz = 1,2-bis(diphenylphosphino)benzene), which is a more effective catalyst for the synthesis of alkylated indoles,10,17 also afforded no desired product. We assume that the decreased rate for the alkyne-to-vinylidene rearrangement of electron-deficient and alkyl-substituted acetylene16,18 could be the reason for this unsuccessful result.
The effect of the substituents introduced to R2 and R3 on the coumarin moiety was explored. A methoxypyrrolcoumarin (2k, R2 = OMe, R3 = H) as well as a fluoropyrrolocoumarin (2l, R2 = H, R3 = F) were synthesized in high yields.
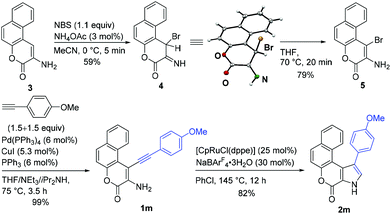
We further extended our study to the synthesis of a benzofused pyrrolocoumarin derivative.19 The synthesis and ruthenium-catalyzed cycloisomerization of a benzochromenone derivative 1m was summarized in Scheme 2. When we tried to synthesize 5 by the bromination of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 with NBS in the presence of a catalytic amount of NH4OAc at 0 °C,21 an unexpected product 4 was isolated in 59% yield. Since the corresponding bromide was not isolated in the reaction of the bicyclic 3-amino-2H-chromen-2-one under similar reaction conditions,21 we assume that the presence of the C–H bond in the proximity of the bromine atom inhibited the isomerization reaction of 4 at 0 °C. Due to the steric hindrance, the rate of the conversion of 4 to 5 should be lower compared to those of other aminocoumarins. The isomerization of 4 smoothly proceeded at elevated temperature (70 °C), and compound 5 was isolated in 79% yield. Subsequently, 1m was synthesized in 99% yield by Sonogashira reaction of 5 with 4-methoxyphenylacetylene under modified Stoddart's conditions.22 The reactivity of 1m was similar to that of sterically congested substrate 1f: the desired product 2m was isolated in 82% yield when a larger amount (25 mol%) of the catalyst was employed.
20 with NBS in the presence of a catalytic amount of NH4OAc at 0 °C,21 an unexpected product 4 was isolated in 59% yield. Since the corresponding bromide was not isolated in the reaction of the bicyclic 3-amino-2H-chromen-2-one under similar reaction conditions,21 we assume that the presence of the C–H bond in the proximity of the bromine atom inhibited the isomerization reaction of 4 at 0 °C. Due to the steric hindrance, the rate of the conversion of 4 to 5 should be lower compared to those of other aminocoumarins. The isomerization of 4 smoothly proceeded at elevated temperature (70 °C), and compound 5 was isolated in 79% yield. Subsequently, 1m was synthesized in 99% yield by Sonogashira reaction of 5 with 4-methoxyphenylacetylene under modified Stoddart's conditions.22 The reactivity of 1m was similar to that of sterically congested substrate 1f: the desired product 2m was isolated in 82% yield when a larger amount (25 mol%) of the catalyst was employed.
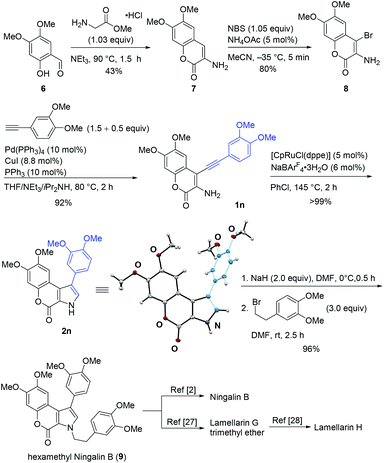
Next, we applied this reaction to the formal total synthesis of Ningalin B and Lamellarin H (Scheme 3). When 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23,24 was treated with methyl aminoacetate hydrochloride,25 aminocoumarin 7 was obtained in 43% yield. The bromination of 7 proceeded at −35 °C to afford 8 in 80% yield. No α-bromoimine, which was similar to 4, was isolated. Compound 1n was synthesized in 92% yield by Sonogashira reaction. To our delight, 2n was obtained in quantitative yield in the presence of 5 mol% of the ruthenium catalyst. The structure of 2n was confirmed by an X-ray diffraction analysis.26 With the key intermediate (2n) in hand, hexamethyl Ningalin B (9) was synthesized in 96% yield by alkylation of 2n.7 The demethylation of 9 to Ningalin B was reported by Boger and co-workers.2 Compound 9 is also the intermediate of Lamellarins, and the conversion of 9 into Lamellarin G trimethyl ether27 and subsequent transformation to Lamellarin H were reported.28 Therefore, we achieved the formal total synthesis of natural products, Ningalin B and Lamellarin H.
23,24 was treated with methyl aminoacetate hydrochloride,25 aminocoumarin 7 was obtained in 43% yield. The bromination of 7 proceeded at −35 °C to afford 8 in 80% yield. No α-bromoimine, which was similar to 4, was isolated. Compound 1n was synthesized in 92% yield by Sonogashira reaction. To our delight, 2n was obtained in quantitative yield in the presence of 5 mol% of the ruthenium catalyst. The structure of 2n was confirmed by an X-ray diffraction analysis.26 With the key intermediate (2n) in hand, hexamethyl Ningalin B (9) was synthesized in 96% yield by alkylation of 2n.7 The demethylation of 9 to Ningalin B was reported by Boger and co-workers.2 Compound 9 is also the intermediate of Lamellarins, and the conversion of 9 into Lamellarin G trimethyl ether27 and subsequent transformation to Lamellarin H were reported.28 Therefore, we achieved the formal total synthesis of natural products, Ningalin B and Lamellarin H.
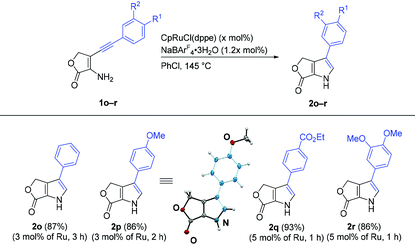
Furthermore, we examined the synthesis of γ-butyrolactone-fused pyrroles to exemplify the application of this reaction (Table 2). In spite of the simplicity of the structure, only a couple of γ-butyrolactone-fused pyrroles has been reported in the literature,29,30 and a general method for the synthesis of these compounds has not been established. We successfully synthesized a series of γ-butyrolactone-fused pyrroles by the ruthenium-catalyzed cycloisomerization of 3-amino-4-arylethynylfuranones. Under the established reaction conditions described for the reaction of 3-amino-4-alkynyl-2H-chromen-2-one, the desired γ-butyrolactone-fused pyrroles 2o and 2p were isolated in 87% and 86% yields, respectively. The molecular structure of 2p was confirmed by a single-crystal X-ray diffraction analysis. The reaction of aminobutenolide 1q with an ethoxycarbonyl group also afford the corresponding pyrrole 2q in high yield. The reaction of 1r with 3,4-dimethoxyphenyl group proceeded cleanly, and 2r was obtained in 86% yield.
Conclusions
We have developed a synthetic method for various 1-arylchromeno[3,4-b]pyrrol-4(3H)-ones by ruthenium-catalyzed cycloisomerization of 3-amino-4-ethynyl-2H-chromen-2-ones via 1,2-carbon migration. The formal total synthesis of Ningalin B and Lamellarin H was achieved by employing this reaction. Moreover, a general method for the synthesis of uncommon γ-butyrolactone-fused pyrroles was established. Our studies will contribute to the development of a new method for the synthesis of heavily substituted pyrrole derivatives.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported in part by JSPS KAKENHI Grant Number JP19J14232 (T. W.), as well as Taisho Pharmaceutical Co., Ltd. Award in Synthetic Organic Chemistry, Japan (Y. M.), the JGC-S Scholarship Foundation (Y. M.), and the Promotion Expenses for Mid-term Research Strategic Plan by Tokyo University of Science.Notes and references
- H. Fan, J. Peng, M. T. Hamann and J.-F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS
.
- D. L. Boger, D. R. Soenen, C. W. Boyce, M. P. Hendrick and Q. Jin, J. Org. Chem., 2000, 65, 2479 CrossRef CAS
.
- C. P. Ridley, M. V. R. Reddy, G. Rocha, F. D. Bushman and D. J. Faulkner, Bioorg. Med. Chem., 2002, 10, 3285 CrossRef CAS
.
- D. Pla, A. Marchal, C. A. Olsen, A. Francesch, C. Cuevas, F. Albericio and M. Álvarez, J. Med. Chem., 2006, 49, 3257 CrossRef CAS
.
- For reviews, see:
(a) T. Fukuda, F. Ishibashim and M. Iwao, Heterocycles, 2011, 83, 491 CrossRef CAS
; (b) D. Imbri, J. Tauber and T. Opatz, Mar. Drugs, 2014, 12, 6142 CrossRef CAS
.
-
(a) L. Chen and M.-H. Xu, Adv. Synth. Catal., 2009, 351, 2005 CrossRef CAS
; (b) K. C. Mujumdar, N. De and B. Roy, Synthesis, 2010, 4207 CrossRef
; (c) Z. Wang, X. Xing, L. Xue, F. Gao and L. Fang, Org. Biomol. Chem., 2013, 11, 7334 RSC
.
- C.-K. Wu, Z. Weng and D.-Y. Yang, Org. Lett., 2019, 13, 5225 CrossRef
.
- R. Mei, S.-K. Zhang and L. Ackermann, Synlett, 2017, 28, 1715 CrossRef CAS
.
- K. B. Manjappa, J.-M. Lin and D.-Y. Yang, J. Org. Chem., 2017, 82, 7648 CrossRef CAS PubMed
.
- T. Watanabe, Y. Mutoh and S. Saito, J. Am. Chem. Soc., 2017, 139, 7749 CrossRef CAS
.
- T. Watanabe, H. Abe, Y. Mutoh and S. Saito, Chem. – Eur. J., 2018, 24, 11545 CrossRef CAS
.
- For recent reviews on catalytic processes that involve vinylidene intermediates, see:
(a) J. A. Varela, C. González-Rodríguez and C. Saá, Top. Organomet. Chem., 2014, 237 CrossRef
; (b) S. W. Roh, K. Choi and C. Lee, Chem. Rev., 2019, 119, 4293 CrossRef CAS PubMed
.
- For stoichiometric internal alkyne-to-vinylidene rearrangements, see:
(a) P. J. King, S. A. R. Knox, M. S. Legge, A. G. Orpen, J. N. Wilkinson and E. A. Hill, J. Chem. Soc., Dalton Trans., 2000, 1547 RSC
; (b) M. J. Shaw, S. W. Bryant and N. Rath, Eur. J. Inorg. Chem., 2007, 3943 CrossRef CAS
; (c) Y. Ikeda, T. Yamaguchi, K. Kanao, K. Kimura, S. Kamimura, Y. Mutoh, Y. Tanabe and Y. Ishii, J. Am. Chem. Soc., 2008, 130, 16856 CrossRef CAS PubMed
; (d) Y. Mutoh, Y. Ikeda, Y. Kimura and Y. Ishii, Chem. Lett., 2009, 38, 534 CrossRef CAS
; (e) E. Bustelo, I. de los Rios, M. C. Puerta and P. Valerga, Organometallics, 2010, 29, 1740 CrossRef
; (f) Y. Mutoh, K. Imai, Y. Kimura, Y. Ikeda and Y. Ishii, Organometallics, 2011, 30, 204 CrossRef CAS
; (g) V. K. Singh, E. Bustelo, I. de los Ríos, I. Macías-Arce, M. C. Puerta, P. Valerga, M. A. Ortuño, G. Ujaque and A. Lledós, Organometallics, 2011, 30, 4014 CrossRef CAS
; (h) Y. Mutoh, Y. Kimura, Y. Ikeda, N. Tsuchida, K. Takano and Y. Ishii, Organometallics, 2012, 31, 5150 CrossRef CAS
; (i) M. Otsuka, N. Tsuchida, Y. Ikeda, Y. Kimura, Y. Mutoh, Y. Ishii and K. Takano, J. Am. Chem. Soc., 2012, 134, 17746 CrossRef CAS
; (j) Y. Ikeda, Y. Mutoh, K. Imai, N. Tsuchida, K. Takano and Y. Ishii, Organometallics, 2013, 32, 4353 CrossRef CAS
; (k) F. E. Fernández, M. C. Puerta and P. Valerga, Inorg. Chem., 2013, 52, 6502 CrossRef
; (l) M. Otsuka, N. Tsuchida, Y. Ikeda, N. Lambert, R. Nakamura, Y. Mutoh, Y. Ishii and K. Takano, Organometallics, 2015, 34, 3934 CrossRef CAS
; (m) Y. Ikeda, S. Kodama, N. Tsuchida and Y. Ishii, Dalton Trans., 2015, 44, 17448 RSC
; (n) T. Kuwabara, S. Takamori, S. Kishi, T. Watanabe, Y. Ikeda, S. Kodama, Y. Minami, T. Hiyama and Y. Ishii, Synlett, 2018, 29, 727 CrossRef CAS
; (o) T. Kuwabara, K. Sakajiri, Y. Oyama, S. Kodama and Y. Ishii, Organometallics, 2019, 38, 1560 CrossRef CAS
.
- For selected examples, see:
(a) D. Pflästerer, P. Dolbundalchok, S. Rafique, M. Rudolph, F. Rominger and A. S. K. Hashmi, Adv. Synth. Catal., 2013, 355, 1383 CrossRef
; (b) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794 CrossRef CAS PubMed
; (c) X. Tian, L. Song, M. Rudolph, F. Rominger and A. S. K. Hashmi, Org. Lett., 2019, 21, 4327 CrossRef CAS
.
- The use of distilled chlorobenzene as the solvent is important to obtain reproducible results. We did not use silver salts in place of NaBArF4 in this study, since silver salts were less effective in a closely related reaction which involves alkyne-to-vinylidene rearrangement. See, ref. 13d.
- It has been reported that the rate of the 1,2-carbon migration decreases in the reaction of an electron-deficient aromatic alkyne or an aliphatic alkyne. See, ref. 13d and f.
- The importance of dppbz ligand is unclear at this stage.
- Compared to phenylacetylene, 4-alkynylchromen-2-one would be an electron-deficient alkyne.
- The synthesis of a closely related compound was reported. See ref. 9.
- For details, see the ESI.†.
-
(a) S. B. Paul, K. C. Majumdar, S. Anwar and S. Choudhury, Synlett, 2015, 26, 1039 CrossRef CAS
; (b) B. Das, K. Venkateswarlu, A. Majhi, V. Siddaiah and K. R. Reddy, J. Mol. Catal. A: Chem., 2007, 267, 30 CrossRef CAS
.
- Y.-L. Zhao, L. Liu, W. Zhang, C.-H. Sue, Q. Li, O. Š. Miljanić, O. M. Yaghi and J. F. Stoddart, Chem. – Eur. J., 2009, 15, 13356 CrossRef CAS PubMed
.
- D. H. Dethe, S. Mahapatra and S. K. Sau, Org. Lett., 2018, 20, 2766 CrossRef CAS PubMed
.
- S. Blumberg and S. F. Martin, Tetrahedron, 2018, 74, 4981 CrossRef CAS
.
- H.-C. Song, Z.-L. Xu, Y.-W. Chen, J.-H. Yao, J. S. Bradshaw, P. B. Savage and R. M. Izatt, J. Heterocycl. Chem., 2003, 40, 475 CrossRef CAS
.
- Although the synthesis of 2n was recently reported, the spectroscopic data of 2n synthesized by us does not match those reported previously in ref. 7.
- M. Iwao, T. Takeuchi, N. Fujikawa, T. Fukuda and F. Ishibashi, Tetrahedron Lett., 2003, 44, 4443 CrossRef CAS
.
- V. Kumar, A. Awasthi, A. Salam and T. Khan, J. Org. Chem., 2019, 84, 11596 CrossRef CAS
.
- P. DeShong, D. A. Kell and D. R. Sidler, J. Org. Chem., 1985, 50, 2309 CrossRef CAS
.
- D. L. Boger and M. Patel, J. Org. Chem., 1988, 53, 1405 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. CCDC 1961068–1961070. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ob02363a |
| This journal is © The Royal Society of Chemistry 2020 |